Subcutaneous Ticks in Wild Carnivores: Any Host-Related Differences?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
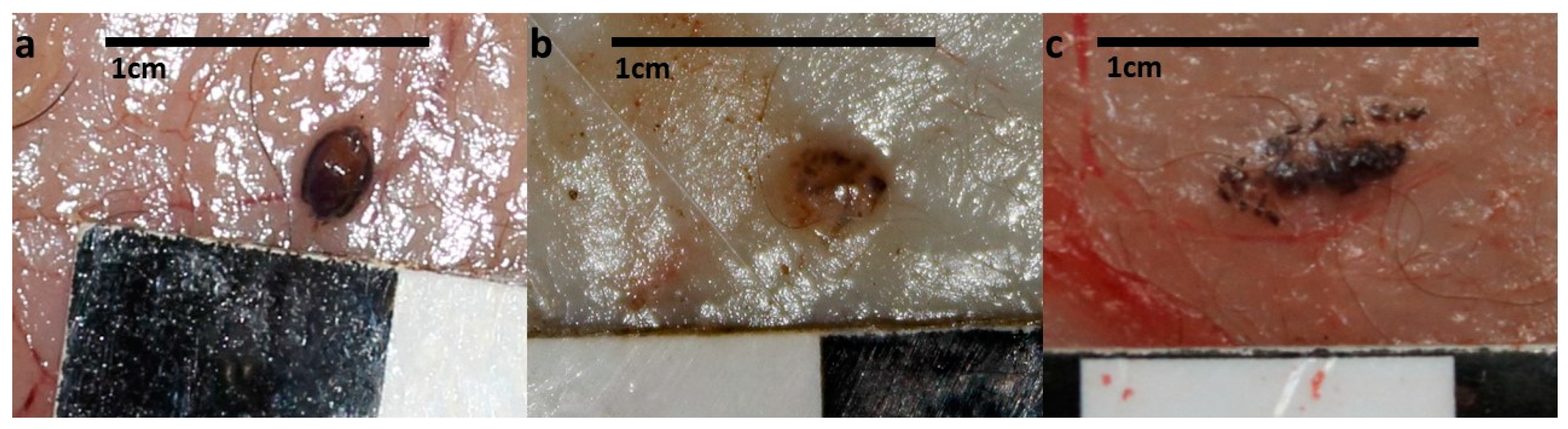
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolonin, G.V. Mammals as hosts of Ixodid ticks (Acarina, Ixodidae). Entomol. Rev. 2007, 87, 401–412. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Jansen, P.A.; Wijnen, H.J.; Coipan, E.C.; Fonville, M.; Prins, H.H.T.; Sprong, H.; Wieren, S.E. Van Cascading effects of predator activity on tick-borne disease risk. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170453. [Google Scholar] [CrossRef] [PubMed]
- Lesiczka, P.M.; Rudenko, N.; Golovchenko, M.; Juránková, J.; Daněk, O.; Modrý, D.; Hrazdilová, K. Red fox (Vulpes vulpes) play an important role in the propagation of tick-borne pathogens. Ticks Tick. Borne. Dis. 2022, 14, 102076. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Matuschka, F.R.; Spielman, A.; Mahadevan, L. How ticks get under your skin: Insertion mechanics of the feeding apparatus of Ixodes ricinus ticks. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131758. [Google Scholar] [CrossRef]
- Oliver, J.H. Biology and systematics of ticks (Acari: Ixodida). Annu. Rev. Ecol. Syst. 1989, 20, 397–430. [Google Scholar] [CrossRef]
- Wikel, S. Ticks and tick-borne pathogens at the cutaneous interface: Host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front. Microbiol. 2013, 4, 337. [Google Scholar] [CrossRef]
- Cross, C.E.; Stokes, J.V.; Alugubelly, N.; Ross, A.M.L.; Willeford, B.V.; Walker, J.D.; Varela-Stokes, A.S. Skin in the game: An assay to monitor leukocyte infiltration in dermal lesions of a guinea pig model for tick-borne rickettsiosis. Pathogens 2022, 11, 119. [Google Scholar] [CrossRef]
- D’Amico, G.; Juránková, J.; Tăbăran, F.A.; Frgelecová, L.; Forejtek, P.; Matei, I.A.; Ionică, A.M.; Hodžić, A.; Modrý, D.; Mihalca, A.D. Occurrence of ticks in the subcutaneous tissue of red foxes, Vulpes vulpes in Czech Republic and Romania. Ticks Tick. Borne. Dis. 2017, 8, 309–312. [Google Scholar] [CrossRef]
- Haut, M.; Król, N.; Obiegala, A.; Seeger, J.; Pfeffer, M. Under the skin: Ixodes ticks in the subcutaneous tissue of red foxes (Vulpes vulpes) from Germany. Parasit. Vectors 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Mechouk, N.; Deak, G.; Ionică, A.M.; Ionescu, D.T.; Chișamera, G.B.; Gherman, C.M.; Mihalca, A.D. Subcutaneous ticks: A first report in a golden jackal, and their absence in non-canid carnivores. Parasit. Vectors 2021, 14, 5. [Google Scholar] [CrossRef]
- Dwużnik, D.; Mierzejewska, E.J.; Kowalec, M.; Alsarraf, M.; Stańczak, Ł.; Opalińska, P.; Krokowska-Paluszak, M.; Górecki, G.; Bajer, A. Ectoparasites of red foxes (Vulpes vulpes) with a particular focus on ticks in subcutaneous tissues. Parasitology 2020, 147, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, A.; Wasielewski, O.; Wlodarek, J.; Ondrejkova, A.; Tryjanowski, P. First report of ticks in the subcutaneous tissue of the raccoon dog Nyctereutes procyonoides. Vet. Med. 2018, 63, 571–574. [Google Scholar] [CrossRef]
- Christensson, D.; Zakrisson, G. Others Ticks, Ixodes ricinus in the sub-cutaneous tissues of a dog and foxes. Sven. Veterinärtidning 2010, 62, 25–27. [Google Scholar]
- Moroni, B.; Rossi, L.; Meneguz, P.G.; Orusa, R.; Zoppi, S.; Robetto, S.; Marucco, F.; Tizzani, P. Dirofilaria immitis in wolves recolonizing northern Italy: Are wolves competent hosts? Parasites Vectors 2020, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Marucco, F.; Pilgrim, K.L.; Avanzinelli, E.; Schwartz, M.K.; Rossi, L. Wolf Dispersal Patterns in the Italian Alps and Implications for Wildlife Diseases Spreading. Animals 2022, 12, 1260. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, M.A.; Moroni, B.; Mendoza-Roldan, J.A.; Perrucci, S.; Cavicchio, P.; Cordon, R.; Cianfanelli, C.; Lia, R.P.; Rossi, L.; Otranto, D. Wild carnivores and Thelazia callipaeda zoonotic eyeworms: A focus on wolves. Int. J. Parasitol. Parasites Wildl. 2022, 17, 239–243. [Google Scholar] [CrossRef]
- Gipson, P.; Ballard, W.B.; Nowak, R.M.; Mech, D.L. Accuracy and Precision of Estimating Age of Gray Wolves by Tooth Wear. J. Wildl. Manag. 2000, 64, 752–758. [Google Scholar] [CrossRef]
- Cringoli, G.; Iori, A.; Rinaldi, L.; Veneziano, V.; Genchi, C. Zecche. Mappe Parassitol. 2005, 3, 177–199. [Google Scholar]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Chitimia, L.; Iustin, R.L.; Wu, C.X. Genetic characterization of ticks from southwestern Romania by sequences of mitochondrial cox 1 and nad 5 genes. Exp. Appl. Acarol. 2010, 52, 305–311. [Google Scholar] [CrossRef]
- D’Oliveira, C.; Van Der Weide, M.; Jacquiet, P.; Jongejan, F. Detection of Theileria annulata by the PCR in ticks (Acari: Ixodidae) collected from cattle in Mauritania. Exp. Appl. Acarol. 1997, 21, 279–291. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Yssouf, A.; Almeras, L.; Berenger, J.; Laroche, M.; Raoult, D.; Parola, P. Ticks and Tick-borne Diseases Identification of tick species and disseminate pathogen using hemolymph by MALDI-TOF MS. Ticks Tick. Borne. Dis. 2015, 6, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Diarra, A.Z.; Almeras, L.; Laroche, M.; Berenger, J.; Bocoum, Z.; Dabo, A.; Doumbo, O.; Kone, A.K.; Raoult, D.; Parola, P. Molecular and MALDI-TOF identification of ticks and tick-associated bacteria in Mali. PLoS Neglected Trop. Dis. 2017, 11, e0005762. [Google Scholar] [CrossRef]
- Ahamada M’madi, S.; Diarra, A.Z.; Almeras, L.; Parola, P. Identification of ticks from an old collection by MALDI-TOF MS. J. Proteomics 2022, 264, 104623. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Ferroglio, E.; Battisti, E.; Tizzani, P. Ecological niche modelling of Babesia spp. infection in wildlife experimentally evaluated in Northern Italy with reference to questing Ixodes ricinus ticks. Geospat. Health 2020, 15, 843. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Krawczyk, A.I.; Sprong, H.; Rossi, L.; Ramassa, E.; Tomassone, L. Ticks climb the mountains: Ixodid tick infestation and infection by tick-borne pathogens in the Western Alps. Ticks Tick. Borne. Dis. 2020, 11, 101489. [Google Scholar] [CrossRef]
- Perrucci, S.; Verin, R.; Mancianti, F.; Poli, A. Sarcoptic mange and other ectoparasitic infections in a red fox (Vulpes vulpes) population from central Italy. PAREPI 2016, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Turchetto, S.; Obber, F.; Rossi, L.; D’Amelio, S.; Cavallero, S.; Poli, A.; Parisi, F.; Lanfranchi, P.; Ferrari, N.; Dellamaria, D.; et al. Sarcoptic Mange in Wild Caprinae of the Alps: Could Pathology Help in Filling the Gaps in Knowledge? Front. Vet. Sci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Valldeperes, M.; Moroni, B.; Rossi, L.; López-Olvera, J.R.; Velarde, R.; Molinar Min, A.R.; Mentaberre, G.; Serrano, E.; Angelone, S.; Lavín, S.; et al. First report of interspecific transmission of sarcoptic mange from Iberian ibex to wild boar. Parasit. Vectors 2021, 14, 481. [Google Scholar] [CrossRef]
- Oleaga, A.; Casais, R.; Prieto, J.M.; Gortázar, C.; Balseiro, A. Comparative pathological and immunohistochemical features of sarcoptic mange in five sympatric wildlife species in Northern Spain. Eur. J. Wildl. Res. 2012, 58, 997–1000. [Google Scholar] [CrossRef]
- Moroni, B.; Rossi, L.; Bernigaud, C.; Guillot, J. Zoonotic Episodes of Scabies: A Global Overview. Pathogens 2022, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Astorga, F.; Carver, S.; Almberg, E.S.; Sousa, G.R.; Wingfield, K.; Niedringhaus, K.D.; Van Wick, P.; Rossi, L.; Xie, Y.; Cross, P.; et al. International meeting on sarcoptic mange in wildlife, June 2018, Blacksburg, Virginia, USA. Parasites Vectors 2018, 11, 449. [Google Scholar] [CrossRef] [PubMed]

| Host Species | N | Animals Found with SCT |
|---|---|---|
| Red fox (Vulpes vulpes) | 30 | 11 |
| Wolf (Canis lupus) | 24 | 0 |
| Badger (Meles meles) | 6 | 0 |
| Stone marten (Martes foina) | 2 | 0 |
| Pine marten (Martes martes) | 1 | 0 |
| ID | Sex | No. of SCT | SCT Morphological ID | SCT Molecular ID | SCT Localization | Mange | ||
|---|---|---|---|---|---|---|---|---|
| Head Region | Thoraco-Abdominal Region | Limbs | ||||||
| 37 | M | 1 | Ixodes spp. | nd | 1 | 0 | 0 | no |
| 98 | F | 1 | Ixodes spp. | nd | 1 | 0 | 0 | no |
| 129 | F | 15 | I. ricinus (3), Ixodes spp. (12) | I. ricinus | 2 | 10 | 3 | no |
| 132 | F | 10 | I.ricinus (2), Ixodes spp. (8) | I. ricinus | 0 | 8 | 2 | no |
| 133 | M | 5 | I. ricinus (1), Ixodes spp. (4) | I. ricinus | 0 | 4 | 1 | no |
| 145 | M | 4 | Ixodes spp. | I. ricinus | 0 | 4 | 0 | yes |
| V_1 | M | 2 | I. ricinus (1), Ixodes spp. (1) | I. ricinus | 0 | 0 | 2 | no |
| 75 | F | 1 | Ixodes spp. | nd | 1 | 0 | 0 | no |
| 87 | M | 2 | Ixodes spp. | nd | 2 | 0 | 0 | yes |
| V_14 | M | 1 | Ixodes spp. | nd | 1 | 0 | 0 | no |
| V_18 | F | 9 | Ixodes spp. | I. ricinus | 0 | 9 | 0 | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroni, B.; Coenda, F.; Garcia-Vozmediano, A.; Nicoletti, A.; Pregel, P.; Mina, A.; Tomassone, L.; Rossi, L.; Scaglione, F.E. Subcutaneous Ticks in Wild Carnivores: Any Host-Related Differences? Animals 2022, 12, 3411. https://doi.org/10.3390/ani12233411
Moroni B, Coenda F, Garcia-Vozmediano A, Nicoletti A, Pregel P, Mina A, Tomassone L, Rossi L, Scaglione FE. Subcutaneous Ticks in Wild Carnivores: Any Host-Related Differences? Animals. 2022; 12(23):3411. https://doi.org/10.3390/ani12233411
Chicago/Turabian StyleMoroni, Barbara, Fabrizio Coenda, Aitor Garcia-Vozmediano, Arturo Nicoletti, Paola Pregel, Alessandra Mina, Laura Tomassone, Luca Rossi, and Frine Eleonora Scaglione. 2022. "Subcutaneous Ticks in Wild Carnivores: Any Host-Related Differences?" Animals 12, no. 23: 3411. https://doi.org/10.3390/ani12233411
APA StyleMoroni, B., Coenda, F., Garcia-Vozmediano, A., Nicoletti, A., Pregel, P., Mina, A., Tomassone, L., Rossi, L., & Scaglione, F. E. (2022). Subcutaneous Ticks in Wild Carnivores: Any Host-Related Differences? Animals, 12(23), 3411. https://doi.org/10.3390/ani12233411







