Simple Summary
Salmonella spp. is a major foodborne pathogen responsible for salmonellosis in animals and humans. The gastrointestinal tracts of reptiles and amphibians have been described as a source of Salmonella transmission, and the main outbreaks that have been reported occurred after contact with infected animals as pets or after the ingestion of their meat. The aim of this study was to determine the presence of 24 virulence genes and characterize the genotypic antibiotic resistance profile in Salmonella strains isolated from Caiman crocodilus fuscus obtained in situ (natural habitat) in Prado, Tolima, (Colombia) in a previous study. Fifteen Salmonella strains were obtained from Caiman crocodilus fuscus, where all the strains have 3 out of 26 resistances genes and 19 out of 24 virulence genes. The results indicate that Colombian babilla (Caiman crocodilus fuscus) may have a role as a carrier of multidrug resistant bacteria.
Abstract
Salmonella enterica is a pathogen capable of colonizing various environments, including the intestinal tract of different animals such as mammals, birds, and reptiles, which can act as carriers. S. enterica infection induces different clinical diseases, gastroenteritis being the most common, which in some cases, can evolve to septicemia and meningitis. Reptiles and amphibians have been reported as a reservoir of Salmonella, and transmission of the pathogen to humans has been documented. This study aimed to determine the presence of virulence genes and characterize the genotypic antibiotic resistance profile in Salmonella strains isolated from Caiman crocodilus fuscus obtained in situ (natural habitat) in Prado, Tolima, Colombia in a previous study and stored in a strain bank in our laboratory. Fifteen Salmonella strains were evaluated through endpoint PCR to determine the presence of resistance genes and virulence genes. The genes blaTEM, strB, and sul1 were detected in all the strains that confer resistance to ampicillin, streptomycin, and sulfamethoxazole, as well as the virulence genes invA, pefA, prgH, spaN, tolC, sipB, sitC, pagC, msgA, spiA, sopB, sifA, lpfA, csgA, hilA, orgA, iroN, avrA, and sivH, indicating the possible role of babilla (Caiman crocodilus fuscus) as a carrier of multidrug-resistant bacteria.
1. Introduction
The most global biodiversity is concentrated in seventeen countries referred to as megadiverse, and Colombia is one of the richest countries regarding biological diversity worldwide [1]. However, human activities causing landscape-scale loss, disruption to natural habitats, and pollution generate changes in biological resources, increasing interactions between humans and wildlife. A great variety of reptile species carrying pathogens of interest to human health have gained popularity as pets, becoming possible vectors of antibiotic-resistant bacteria in the environment [2,3,4].
Salmonella enterica is a pathogen capable of colonizing various environments, including the intestinal tract of different animals, such as mammals, birds, and reptiles including lizards, snakes, and chelonians, which can be bacteria’s carriers for human contagion [5]. Currently, three species are recognized for the genus Salmonella: S. bongori, S. enterica, and S. subterranean. They are subdivided into subspecies and serotypes [6,7]. Regarding S. bongori, 22 serotypes associated with cold-blooded animals have been found, and in some cases, these infect humans and cause disease [8,9]. Nowadays, 2700 serotypes of S. enterica have been described, S. enterica subsp. enterica being the most frequently isolated from warm-blooded and cold-blooded animals. It is associated with 99% of Salmonella infections in humans and animals [10,11].
S. enterica infection induces different clinical diseases depending on the host immune response [12]. Gastroenteritis is the most common disease caused mainly by Typhimurium and Enteritidis serotypes, which, in some cases, can evolve into septicemia and meningitis, mainly in immunocompromised patients, pregnant women, and elderly adults [13]. In addition, the symptoms in humans vary depending on the host immunity and virulence factors of the strain that play a crucial role in systemic infections due to the activation during the interaction between the bacteria and the hostile environment of the gastrointestinal tract of the host [14]. It is estimated that Salmonella causes 1.35 million cases of illness and 420 deaths in the United States per year [15]. Vegetable-type foods and food of animal origins, such as pig, chicken, cow, and lamb meat, and eggs or milk, are considered the main source of Salmonella infections in humans, since production animals are in contact with wild animals, insects, or contaminated food, allowing the disease to be classified as a disease of zoonotic origin [16,17,18].
Salmonella is naturally found in the gastrointestinal tract of reptiles and amphibians; these animals have been described as a source of Salmonella transmission, and the main outbreaks that have been reported occurred after direct or indirect contact with animals kept as pets or after the ingestion of their meat [19]. In Colombia, it has been reported that the contamination of Salmonella spp. in reptiles, such as Caiman crocodilus, is due to the fact that bacteria colonize them systemically via water, soil, contaminated food, and/or through vertical transmission as its entry route [20]. The Caiman crocodilus is a reptile that is found in the wild and was domesticated for production due to its economic value for its meat and skin [21]. Some Salmonella serotypes frequently associated with reptiles and isolated in humans are S. Poona, S. Weltevreden, S. Enteritidis, S. Java, S. Stanley, and subspecies S. enterica diarizonae, all known to have caused infection in humans and produced diarrhea in most cases [19,22].
Salmonella is considered a public health concern due to different animals that carry the bacteria and have a function as vehicles of transmission, as well as its resistance to multiple antibiotics (MDR) that hinders its treatment [4,23]. Previously, the resistance genes aadA (aminoglycosides), sul2 (sulphonamides), dfr1, dfr7 (trimethoprim), and tet(A) and tet(B) (tetracyclines), which confer resistance to antibiotics used in humans, have been reported in Salmonella isolated from different species of reptiles [24]. In waterbodies where wildlife animals live, it has been revealed the antibiotic pollution accumulated in the sediments caused by wastewater from animal farming and hospitals is a problem due to the development of bacteria’s resistance to multiple antibiotics and horizontal gene transfer [25]. Therefore, research is necessary to obtain a better understanding of the genotypic profile of the Salmonella spp. isolates in order to establish effective prevention and control strategies. The aim of this study was to determine the presence of virulence genes and characterize the genotypic antibiotic resistance profile of Salmonella strains isolated from Colombian babilla (Caiman crocodilus fuscus) in situ, located in Prado, Tolima, (Colombia).
2. Materials and Methods
2.1. Study Population and Sample Collection
The Colombian babilla (Caiman crocodilus fuscus) has a natural habitat in the Hidroprado hydroelectric dam of the Prado, Tolima region of Colombia, which is located between the central and eastern mountains of the Colombian Andes. Fifteen (n = 15) Salmonella strains, stored in our laboratory strain bank and isolated from cloacal swabs of wild Colombian babilla collected in a previous study in our laboratory, were used for characterizing the genotypic antibiotic resistance and determination of virulence genes in this study [26,27]. The strains used were serotyped in previous research as Salmonella Paratyphi B (n = 2), Salmonella Saintpaul (n = 2), Salmonella Javiana (n = 4), Salmonella Braenderup (n = 4), Salmonella Soerenga (n = 1), Salmonella Infantis (n = 1), and Salmonella Powell (n = 1) [26]. No ethical approval was required for this study because Salmonella spp. strains were from the Bacterial Strain Collection of the Laboratory of Immunology and Molecular Biology of the Universidad del Tolima.
2.2. Genomic DNA Extraction and Molecular Confirmation of Salmonella Isolates
Genomic DNA (gDNA) was extracted from fresh bacterial colonies using Invisorb® Spin Universal Kit (Stratec, Berlin, Germany). The isolated gDNA was stored at −20 °C until its analysis. All strains were confirmed as Salmonella by amplification of a 284 bp fragment of the invA gene (accession number: M90846.1) by endpoint PCR, using S. Enteritidis (ATCC 13076®) as positive control and E. coli (ATCC 25922®) as negative control (Table 1).

Table 1.
Primer sequences used for amplification of virulence genes in Salmonella spp. isolates.
2.3. Determination of Virulence Genes and Genotypic Antibiotic Resistance
Genes related to virulence factors (Table 1) and antibiotic resistance (Table 2) were screened by endpoint PCR. In the case of the virulence genes, the S. Enteritidis (ATCC 13076®) was used as a positive control and in the resistance genes, the positive controls correspond to Salmonella spp. strains previously characterized in the laboratory of immunology and molecular biology. The reactions were carried out in a ProFlex PCR System (Applied Biosystems, Carlsbad, CA, USA) using 25 μL of total reaction volume, composed by 1 μL of gDNA, 5 μL of Flexi Buffer 5x colorless GoTaq ® (Promega, Madison, WI, USA), 1 μL of dNTPs (Invitrogen, Carlsbad, CA, USA), 1 μL of each primer (forward and reverse) (Table 1 and Table 2) (10 pmol/Μl) (Macrogen, Seoul, Korea), 1 μL of MgCl2 (25 mM) (Promega, Madison, WI, USA), 0.125 μL of GoTaq Flexi DNA Polymerase (Promega, Madison, WI, USA), and 14,875 μL of nuclease-free water. The amplification conditions were an initial denaturation at 95 °C for 3 min, followed by 35 cycles of 30 s of denaturation at 95 °C, annealing step at 55 °C for 30 s, an extension step at 72 °C for 30 s, and a final extension step of 7 min at 72 °C. The annealing temperature and the extension step time were adjusted according to the primer set and length of each amplicon. The amplification products were revealed on 2% agarose gel electrophoresis (PowerPac™ HC, Bio-Rad, Hercules, CA, USA), using 100 bp DNA Ladder (NEB, Ipswich, MA, USA) and HydraGreen™ (ACTGene, Piscataway, NJ, USA) as the intercalating agent. Finally, the gels were visualized and documented using the ENDURO™ GDS gel documentation system (Labnet International, Edison, NJ, USA).

Table 2.
Primer sequences used for amplification of resistance genes in Salmonella spp. isolates.
3. Results
3.1. Distribution of Virulence Genes among Salmonella
All 15 Salmonella isolates from wild Colombian babilla (Caiman crocodilus fuscus) were positive for invA (Figure 1), pefA, prgH, spaN, tolC, sipB, sitC, pagC, msgA, spiA, sopB, sifA, lpfA, csgA, hilA, orgA, iroN, avrA, and sivH virulence-associated genes (Table 3). Likewise, lpfC (n = 13; 86.7%) and cdtB (n = 11; 73.3%) genes were found in more than 73% of the Salmonella isolates. Additionally, sopE (n = 5; 33.3%) and spvB (n = 1; 6.67%) genes were found, but at a lower frequency. Furthermore, none of the isolates was positive for the sefA gene, and overall, the 15 Salmonella isolates were positive for at least ten virulence genes (Table 3).
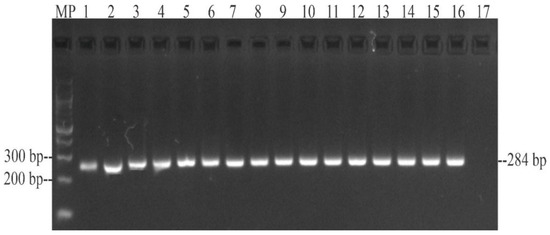
Figure 1.
PCR amplification of a 284 bp fragment from the invA gene of Salmonella isolated from wild Caiman crocodilus fuscus. MP—100 bp DNA ladder (New England Biolabs, Ipswich, MA, USA); lane 1: S. Paratyphi B; lane 2: S. Paratyphi B; lane 3: S. Saintpaul; lane 4: S. Saintpaul; lane 5: S. Javiana; lane 6: S. Javiana; lane 7: S. Javiana; lane 8: S. Javiana; lane 9: S. Braenderup; lane 10: S. Braenderup; lane 11: S. Braenderup; lane 12: S. Braenderup; lane 13: S. Soerenga; lane 14: S. Infantis; lane 15: S. Powell; lane 16: positive control S. Enteritidis ATCC 13076TM; 17: negative control E. coli ATCC 25922TM.

Table 3.
Virulence genes profiles of Salmonella spp. isolates.
3.2. Genotypic Antibiotic Resistance in Salmonella Isolates
The genes blaTEM, strB, and sul1 that confer resistance to ampicillin, streptomycin, and sulfamethoxazole, respectively, were amplified in all the strains (Table 4). Likewise, the genes conferring resistance to ceftriaxone were found in high frequency (blaCMY2: 86.7%; blaCTX-M: 80%), as was the sul2 gene (n = 12; 80%), which confers resistance to sulfamethoxazole. Furthermore, the genes dfrA1 (n = 4; 26.7%), floR (n = 1; 6.67%), and qnrD (n = 1; 6.67%) were found in low frequency. Moreover, none of the genes that encode for gentamicin, quinolones, and fluoroquinolones resistance were detected in the isolates.

Table 4.
Genotypic antibiotic resistance profiles of Salmonella spp. isolates. Black box = gene is present; box = gene is absent.
4. Discussion
Salmonella has been isolated from different animals, such as impala, sable, leopard, and reptiles, including alligators, snakes, lizards, and turtles in captivity and as pets [4,32,33]. This bacterium is naturally found in the gastrointestinal tract of animals, and the outbreaks that have been reported occurred after direct or indirect contact with animals as pets or as meat [34]. Previously, Zając et al. [33] reported the presence of Salmonella in different reptile species (85.5%; n = 597/696) and pet reptiles, such as snakes (76.3%; n = 16/21), lizards (69%; n = 33/48), and chelonians (19%; n = 10/54) [4]. In Colombia, the serotypes of Salmonella reported by López-Cruz et al. [26] and obtained from Colombian babilla were similar to those reported in other animals, such as broiler chickens, known to be responsible for the disease in humans [30,35,36]. It has been reported that feeding with contaminated chicks and rodents is a factor that promotes the spread of Salmonella common serotypes. This fact could be related to this study because the animals were located in a dam near farms with domestic animals that, in some cases, become food for the babillas [33]. Furthermore, S. Paratyphi B and S. Braenderup have been reported in reptiles such as snakes and turtles [37]. Particularly, S. Paratyphi B is a serotype of special concern due to its ability to infect humans and induce paratyphoid fever [37,38]. In addition, S. Saintpaul has been isolated from fruits such as mangoes and vegetables such as tomatoes, which play a role in several outbreaks in humans [39,40].
In the genotypic resistance, the blaTEM gene was found in all the isolates (n = 15/15). The blaTEM gene encodes for Extended Spectrum Beta-lactamase (ESBL), a protein that produces a hydrolyzation of β-lactam antibiotics such as ampicillin [41]. This frequency is higher compared to the Salmonella strains isolated from iguanas, where two out of eight carried the gene [42]. In addition, Bittner-Torrejón [43] reported that 61.7% (n = 21/34) of the strains isolated from reptiles were positive for blaTEM gene. Previously, Marin et al. [4] showed that the phenotypic resistance to ampicillin (46.7%; n = 35/75) was the third most common resistance in pet reptiles that could be transmitted due to direct or indirect contact between humans and pets. The babillas used in this study were found in the wild, where contact with humans is not common. However, the antibiotic pollution is an increasing problem that generates MDR bacteria and depends on the antibiotics used in the community; in the case of Colombia, ampicillin, an antibiotic of the penicillin group, is a common antibiotic prescribed to patients, classified in the access group of antibiotics [44].
On the other hand, strB, a gene that encodes for enzymes that inactivate streptomycin [45] was found in all the isolates (n = 15/15), which differs from other studies where this gene was reported in Salmonella associated with poultry, swine, cattle, horses, wild reptiles, wild mammals, and companion animals but with a lower frequency (34.7%; n = 67/193) [46]. Moreover, in Chile, no reptiles presented the gene [43]. In reptiles of Poland, Salmonella strains present a frequent resistance to streptomycin (25%; n = 134/533) [33] as well as reptiles in Lithuania (26%; n = 13/50) [24]. Nevertheless, in Lithuania the phenotypic resistance is mediated by other genes, such as armA and aadA, that were not evaluated in this study [24]. Likewise, the sul1 gene encoding dihydropteroate synthases, which confer sulfamethoxazole resistance [47], was detected in the totality of the strains, which differs from reports in the United States, where this gene was not present in wild reptiles [46]. Furthermore, blaCMY2 (86.7%; n = 13/15) and blaCTX-M (80%; n = 12/15) genes that confer resistance to ceftriaxone and cefotaxime through the hydrolyzation of antibiotics have been reported in Salmonella isolated from iguanas (blaCTX-M; n = 1/8), horses, wild reptiles, wild mammals, and companion animals (blaCMY2; n = 44/193) [42,46].
In our study, all 15 Salmonella strains present virulence genes related to the host invasion, host recognition, and colonization, such as invA, pefA, prgH, spaN, tolC, sipB, sitC, pagC, msgA, spiA, sopB, sifA, lpfA, csgA, hilA, orgA, iroN, avrA, and sivH (n = 19/24), which are identified as responsible for the pathogenesis of the bacteria during salmonellosis [48]. Dudek et al. [49] reported fifteen virulence genes in Salmonella strains (invA, prgH, orgA, tolC, sitC, spiA, spiB, spaN, iroN, IpfC, sifA, sopB, pagC, cdtB and msgA) isolated from reptiles (n = 15/84). Additionally, in Poland, Salmonella from wild birds (n = 64/1000) was reported to contain spiA, msgA, invA, lpfC, and sifA genes [50]. However, even though the detection of virulence genes is used as a tool to predict the virulence of the bacteria, the presence of the genes does not necessarily confer greater pathogenicity because in order to increase it, the combined expression of several genes is required [51,52].
5. Conclusions
The presence of several resistance genes in Salmonella strains isolated from Colombian babilla (Caiman crocodilus fuscus) could describe this reptile as a carrier of multidrug-resistant bacteria that has genotypic resistance genes, such as blaTEM, strB, and sul1 genes; likewise, blaCMY2 and blaCTX-M genes were found in 80% of the isolates. In addition, Salmonella isolates from wild babilla are carriers of a wide number of virulence genes and are necessary to develop more investigations of wild reptiles on this topic.
Author Contributions
Conceptualization, R.R.-H., C.M.-R. and I.S.R.-B.; methodology, R.R.-H., M.P.H.-S., J.D.O.-M. and C.M.-R.; validation, R.R.-H., M.P.H.-S. and J.D.O.-M.; formal analysis, R.R.-H.; investigation, R.R.-H. and M.P.H.-S.; resources, R.R.-H. and I.S.R.-B.; data curation, R.R.-H. and M.P.H.-S.; writing—original draft preparation, R.R.-H., M.P.H.-S. and J.D.O.-M.; writing—review and editing, R.R.-H., M.P.H.-S., J.D.O.-M. and I.S.R.-B.; supervision, R.R.-H. and I.S.R.-B.; project administration, I.S.R.-B.; funding acquisition, R.R.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Office of the University of Tolima, funding number 13-2022 and the Laboratory of Immunology and Molecular Biology at Veterinary Medicine Faculty.
Institutional Review Board Statement
This study was approved by the Research and Technologic development office at the University of Tolima.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors want to thank to Martín Eduardo López Cruz, Noel Verjan, and Luz Clemencia Fandiño de Rubio for their previous work.
Conflicts of Interest
The authors declare no conflict of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Paknia, O.; Koch, A. Lack of Well-Maintained Natural History Collections and Taxonomists in Megadiverse Developing Countries Hampers Global Biodiversity Exploration. Org. Divers. Evol. 2015, 15, 619–629. [Google Scholar] [CrossRef]
- Harley, C.D. Climate Change, Keystone Predation, and Biodiversity Loss. Science 2011, 334, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- La Tela, I.; Peruzy, M.F.; D’Alessio, N.; Di Nocera, F.; Casalinuovo, F.; Carullo, M.R.; Cardinale, D.; Cristiano, D.; Capuano, F. Serotyping and Evaluation of Antimicrobial Resistance of Salmonella Strains Detected in Wildlife and Natural Environments in Southern Italy. Antibiotics 2021, 10, 353. [Google Scholar] [CrossRef]
- Marin, C.; Lorenzo-Rebenaque, L.; Laso, O.; Villora-Gonzalez, J.; Vega, S. Pet Reptiles: A Potential Source of Transmission of Multidrug-Resistant Salmonella. Front. Vet. Sci. 2021, 7, 613718. [Google Scholar] [CrossRef] [PubMed]
- Tomastikova, Z.; Barazorda, S.; Knotek, Z.; Karpiskova, R. Prevalence and Characteristics of Salmonella Species Isolated from Captive Reptiles in the Czech Republic. Vet. Med. 2017, 62, 456–469. [Google Scholar] [CrossRef]
- Chen, H.M.; Wang, Y.; Su, L.H.; Chiu, C.H. Nontyphoid Salmonella Infection: Microbiology, Clinical Features, and Antimicrobial Therapy. Pediatr. Neonatol. 2013, 54, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Eng, S.K.; Pusparajah, P.; Ab Mutalib, N.S.; Ser, H.L.; Chan, K.G.; Lee, L.H. Salmonella: A Review on Pathogenesis, Epidemiology and Antibiotic Resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Fookes, M.; Schroeder, G.N.; Langridge, G.C.; Blondel, C.J.; Mammina, C.; Connor, T.R.; Seth-Smith, H.; Vernikos, G.S.; Robinson, K.S.; Sanders, M.; et al. Salmonella bongori Provides Insights into the Evolution of the Salmonellae. PLoS Pathog. 2011, 7, e1002191. [Google Scholar] [CrossRef]
- Percival, S.L.; Williams, D.W. Salmonella. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M., Williams, D., Chalmers, R., Gray, N., Eds.; Academic Press: Boston, MA, USA, 2014; Chapter 10; pp. 209–222. [Google Scholar]
- Rady, M.; Ezz-El-Din, N.A.; Mohamed, K.F.; Nasef, S.; Samir, A.; Elfeil, W.K. Correlation Between ESβL Salmonella Serovars Isolated from Broilers and their Virulence Genes. J. Hell. Vet. Med. Soc. 2020, 71, 2163–2170. [Google Scholar] [CrossRef]
- Jajere, S.M. A Review of Salmonella enterica with Particular Focus on the Pathogenicity and Virulence Factors, Host Specificity and Adaptation and Antimicrobial Resistance Including Multidrug Resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef]
- Kurtz, J.R.; Goggins, J.A.; McLachlan, J.B. Salmonella Infection: Interplay Between the Bacteria and Host Immune System. Immunol. Lett. 2017, 190, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Fardsanei, F.; Nikkhahi, F.; Bakhshi, B.; Salehi, T.Z.; Tamai, I.A.; Soltan-Dallal, M.M. Molecular Characterization of Salmonella enterica Serotype Enteritidis Isolates from Food and Human Samples by Serotyping, Antimicrobial Resistance, Plasmid Profiling, (GTG)5-PCR and ERIC-PCR. New Microbes New Infect. 2016, 14, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Webber, B.; Borges, K.A.; Furian, T.Q.; Rizzo, N.N.; Tondo, E.C.; Santos, L.R.; Rodrigues, L.; do Nascimento, V. Detection of Virulence Genes in Salmonella Heidelberg Isolated from Chicken Carcasses. Rev. Inst. Med. Trop. São Paulo 2019, 61, e36. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Salmonella for Health Professionals. Available online: https://www.cdc.gov/salmonella/general/technical.html (accessed on 20 January 2022).
- Lynch, M.F.; Tauxe, R.V.; Hedberg, C.W. The Growing Burden of Foodborne Outbreaks Due to Contaminated Fresh Produce: Risks and Opportunities. Epidemiol. Infect. 2009, 137, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.N.; Madsen, J.J.; Rahbek, C.; Lodal, J.; Jespersen, J.B.; Jørgensen, J.C.; Dietz, H.; Chriél, M.; Baggesen, D.L. Transmission of Salmonella Between Wildlife and Meat-Production Animals in Denmark. J. Appl. Microbiol. 2008, 105, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Mouttotou, N.; Ahmad, S.; Kamran, Z.; Koutoulis, K.C. Prevalence, Risks and Antibiotic resistance of Salmonella in poultry production chain. In Current Topics in Salmonella and Salmonellosis; Mares, M., Ed.; IntechOpen: London, UK, 2017; pp. 215–234. [Google Scholar]
- Mermin, J.; Hutwagner, L.; Vugia, D.; Shallow, S.; Daily, P.; Bender, J.; Koehler, J.; Marcus, R.; Angulo, F.J. Reptiles, Amphibians, and Human Salmonella Infection: A Population-Based, Case-Control Study. Clin. Infect. Dis. 2004, 38, S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Pachón, D.; Adriana, P.V.; Moreno, C. Aislamiento y Serotipificación de Salmonella sp. en Estanques con Crocodylus intermedius y Testudines Cautivos en Villavicencio-Colombia. Rev. MVZ Córdoba. 2011, 16, 2564–2575. [Google Scholar] [CrossRef]
- Marioni, B.; Barão, J.; Botero, R.; Muniz, F.; Campos, Z.; Da Silveira, R.; Magnusson, W.; Villamarín, F. Science and Conservation of Amazonian crocodilians: A Historical Review. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1056–1067. [Google Scholar] [CrossRef]
- Bjelland, A.M.; Sandvik, L.M.; Skarstein, M.M.; Svendal, L.; Debenham, J.J. Prevalence of Salmonella Serovars Isolated from Reptiles in Norwegian zoos. Acta Vet. Scand. 2020, 62, 3. [Google Scholar] [CrossRef]
- European Food Safety Authority & European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2018/2019. EFSA J. 2021, 19, e06490. [Google Scholar]
- Merkevičienė, L.; Butrimaitė-Ambrozevičienė, Č.; Paškevičius, G.; Pikūnienė, A.; Virgailis, M.; Dailidavičienė, J.; Daukšienė, A.; Šiugždinienė, R.; Ruzauskas, M. Serological Variety and Antimicrobial Resistance in Salmonella Isolated from Reptiles. Biology 2022, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tan, L.; Zhang, L.; Tian, W.; Ma, L. A Review of the Distribution of Antibiotics in Water in Different Regions of China and Current Antibiotic Degradation Pathways. Front. Environ. Sci. 2021, 9, 692298. [Google Scholar] [CrossRef]
- López-Cruz, M.E. Aislamiento e Identificación de Salmonella spp. de Babillas (Caiman crocodilus fuscus) en su Hábitat Natural (represa Hidroprado), Departamento del Tolima. Undergraduate Program, Bachelor’s Thesis, University of Tolima, Ibagué, Tolima, Colombia, 2018. [Google Scholar]
- Mooijman, K.A.; Pielaat, A.; Kuijpers, A.F. Validation of EN ISO 6579-1-Microbiology of the Food Chain-Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella-Part 1 Detection of Salmonella spp. Int. J. Food Microbiol. 2019, 288, 3–12. [Google Scholar] [CrossRef]
- Skyberg, J.A.; Logue, C.M.; Nolan, L.K. Virulence Genotyping of Salmonella spp. with Multiplex PCR. Avian Dis. 2006, 50, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Chuanchuen, R.; Padungtod, P. Antimicrobial Resistance Genes in Salmonella enterica Isolates from Poultry and Swine in Thailand. J. Vet. Med. Sci. 2009, 71, 1349–1355. [Google Scholar] [CrossRef]
- Castro-Vargas, R.; Fandiño-de-Rubio, L.C.; Vega, A.; Rondón-Barragán, I.S. Phenotypic and Genotypic Resistance of Salmonella Heidelberg Isolated from One of the Largest Poultry Production Region from Colombia. Int. J. Poult. Sci. 2019, 18, 610–617. [Google Scholar] [CrossRef]
- Herrera-Sánchez, M.P.; Castro-Vargas, R.E.; Fandiño-de-Rubio, L.C.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Molecular Identification of Fluoroquinolone Resistance in Salmonella spp. Isolated from Broiler Farms and Human Samples Obtained from Two Regions in Colombia. Vet. World 2021, 14, 1767–1773. [Google Scholar] [CrossRef]
- Mubita, C.M.; Muma, B.J.; Nalubamba, K.; Pandey, G.S.; Samui, K.; Munyeme, M.; Masahiro, K.; Qiu, Y.; Saasa, N.; Hang’ombe, B.M. Characterization of Non-Typhoid Salmonellae Isolated from Domestic Animals and Wildlife from Selected Areas of Zambia. Sci. Afr. 2020, 8, e00345. [Google Scholar] [CrossRef]
- Zając, M.; Skarżyńska, M.; Lalak, A.; Kwit, R.; Śmiałowska-Węglińska, A.; Pasim, P.; Szulowski, K.; Wasyl, D. Salmonella in Captive Reptiles and Their Environment—Can We Tame the Dragon? Microorganisms 2021, 9, 1012. [Google Scholar] [CrossRef]
- Whiley, H.; Gardner, M.G.; Ross, K. A Review of Salmonella and Squamates (Lizards, Snakes and Amphisbians): Implications for Public Health. Pathogens 2017, 6, 38. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, R.; Bernal, J.F.; Cifuentes, J.F.; Fandiño-de-Rubio, L.C.; Herrera-Sánchez, M.P.; Rondón-Barragán, I.S.; Verjan-García, N. Prevalence and Molecular Characterization of Salmonella Isolated from Broiler Farms at the Tolima Region—Colombia. Animals 2021, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Hernandez, A.; Carrascal-Camacho, A.K.; Varón-García, A.; Brashears, M.M.; Sanchez-Plata, M.X. Genotypic Characterization of Antimicrobial Resistant Salmonella spp. Strains from Three Poultry Processing Plants in Colombia. Foods 2021, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Eudoxia, E.J.; Sampaio, A.T.; Mendes, B.M. Salmonella in wild animals: A public health concern. In Enterobacteria; Bhonchal, S., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Khanam, F.; Rajib, N.H.; Tonks, S.; Khalequzzaman, M.; Pollard, A.J.; Clemens, J.D.; Qadri, F.; Strataa Study Team. Case Report: Salmonella enterica serovar Paratyphi B Infection in a Febrile ILL Child During Enhanced Passive Surveillance in an Urban Slum in Mirpur, Dhaka. Am. J. Trop. Med. Hyg. 2020, 103, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Beatty, M.E.; LaPorte, T.N.; Phan, Q.; Van-Duyne, S.V.; Braden, C. A Multistate Outbreak of Salmonella enterica Serotype SaintPaul Infections Linked to Mango Consumption: A Recurrent Theme. Clin. Infect. Dis. 2004, 38, 1337–1338. [Google Scholar] [CrossRef]
- Mathew, E.N.; Muyyarikkandy, M.S.; Kuttappan, D.; Amalaradjou, M.A. Attachment of Salmonella enterica on Mangoes and Survival under Conditions Simulating Commercial Mango Packing House and Importer Facility. Front Microbiol. 2018, 9, 1519. [Google Scholar] [CrossRef] [PubMed]
- Ojdana, D.; Sacha, P.; Wieczorek, P.; Czaban, S.; Michalska, A.; Jaworowska, J.; Jurczak, A.; Poniatowski, B.; Tryniszewska, E. The Occurrence of blaCTX-M, blaSHV, and blaTEM Genes in Extended-Spectrum β-Lactamase-Positive Strains of Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in Poland. Int. J. Antibiot. 2014, 2014, 935842. [Google Scholar] [CrossRef]
- Ríos, R.; Flores, B.; Mora-Sánchez, B.; Torres, D.; Sheleby-Elías, J.; Jirón, W.; Balcázar, J.L. Isolation of Salmonella spp. from Black Spiny-Tailed Iguana (Ctenosaura similis) Meat Commercialised in Markets of León city, Nicaragua. Vet. Med. Sci. 2022, 8, 695–699. [Google Scholar] [CrossRef]
- Bittner-Torrejón, C.A. Detección de Genes de Resistencia Antimicrobiana en Cepas de Salmonella spp. Aisladas de Reptiles en Cautiverio, Región Metropolitana, Chile. Undergraduate Program, Bachelor’s Thesis, University of Chile, Santiago de Chile, Chile, 2016. [Google Scholar]
- Machado-Alba, J.E.; Valladales-Restrepo, L.F.; Gaviria-Mendoza, A.; Machado-Duque, M.E.; Figueras, A. Patterns of Antibiotic Prescription in Colombia: Are there Differences between Capital Cities and Municipalities? Antibiotics 2020, 9, 389. [Google Scholar] [CrossRef]
- Sundin, G.W.; Bender, C.L. Dissemination of the strA-strB Streptomycin-Resistance Genes among Commensal and Pathogenic Bacteria from Humans, Animals, and Plants. Mol. Ecol. 1996, 5, 133–143. [Google Scholar] [CrossRef]
- McMillan, E.A.; Gupta, S.K.; Williams, L.E.; Jové, T.; Hiott, L.M.; Woodley, T.A.; Barrett, J.; Jackson, C.; Wasilenko, J.; Simmons, M.; et al. Antimicrobial Resistance Genes, Cassettes, and Plasmids Present in Salmonella enterica Associated with United States Food Animals. Front. Microbiol. 2019, 10, 832. [Google Scholar] [CrossRef]
- Katiyar, A.; Sharma, P.; Dahiya, S.; Singh, H.; Kapil, A.; Kaur, P. Genomic Profiling of Antimicrobial Resistance Genes in Clinical Isolates of Salmonella Typhi from Patients Infected with Typhoid Fever in India. Sci. Rep. 2020, 10, 8299. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.H.; Nayak, J.B.; Brahmbhatt, M.N.; Makwana, P.P. Virulence Genes Detection of Salmonella Serovars Isolated from Pork and Slaughterhouse Environment in Ahmedabad, Gujarat. Vet. World 2015, 8, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Dudek, B.; Książczyk, M.; Krzyżewska, E.; Rogala, K.; Kuczkowski, M.; Woźniak-Biel, A.; Korzekwa, K.; Korzeniowska-Kowal, A.; Ratajszczak, R.; Wieliczko, A.; et al. Comparison of the Phylogenetic Analysis of PFGE Profiles and the Characteristic of Virulence Genes in Clinical and Reptile Associated Salmonella Strains. BMC Vet. Res. 2019, 15, 312. [Google Scholar] [CrossRef] [PubMed]
- Krawiec, M.; Kuczkowski, M.; Kruszewicz, A.G.; Wieliczko, A. Prevalence and Genetic Characteristics of Salmonella in Free-Living Birds in Poland. BMC Vet. Res. 2015, 11, 15. [Google Scholar] [CrossRef]
- Suez, J.; Porwollik, S.; Dagan, A.; Marzel, A.; Schorr, Y.I.; Desai, P.T.; Agmon, V.; McClelland, M.; Rahav, G.; Gal-Mor, O. Virulence Gene Profiling and Pathogenicity Characterization of Non-Typhoidal Salmonella Accounted for Invasive Disease in Humans. PLoS ONE 2013, 8, e58449. [Google Scholar] [CrossRef]
- Xia, Y.; Li, H.; Shen, Y. Antimicrobial Drug Resistance in Salmonella enteritidis Isolated from Edible Snakes with Pneumonia and its Pathogenicity in Chickens. Front. Vet. Sci. 2020, 7, 463. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).