Roles of Ghrelin and Leptin in Body Mass Regulation under Food Restriction Based on the AMPK Pathway in the Red-Backed Vole, Eothenomys miletus, from Kunming and Dali Regions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Experimental Design
2.2. Measurement of Body Mass and Food Intake
2.3. Measurement of RMR
2.4. Determination of Leptin, Total Ghrelin Concentration, AMPK Activity, Malonyl CoA Activity, and CPT-1 Activity
2.5. Determination of Neuropeptide Gene Expression in the Hypothalamus
2.6. Carcass Mass, Body Fat Mass and Content, Body Composition, and Digestive Tract Morphology Determination
2.7. Data Analysis
3. Results
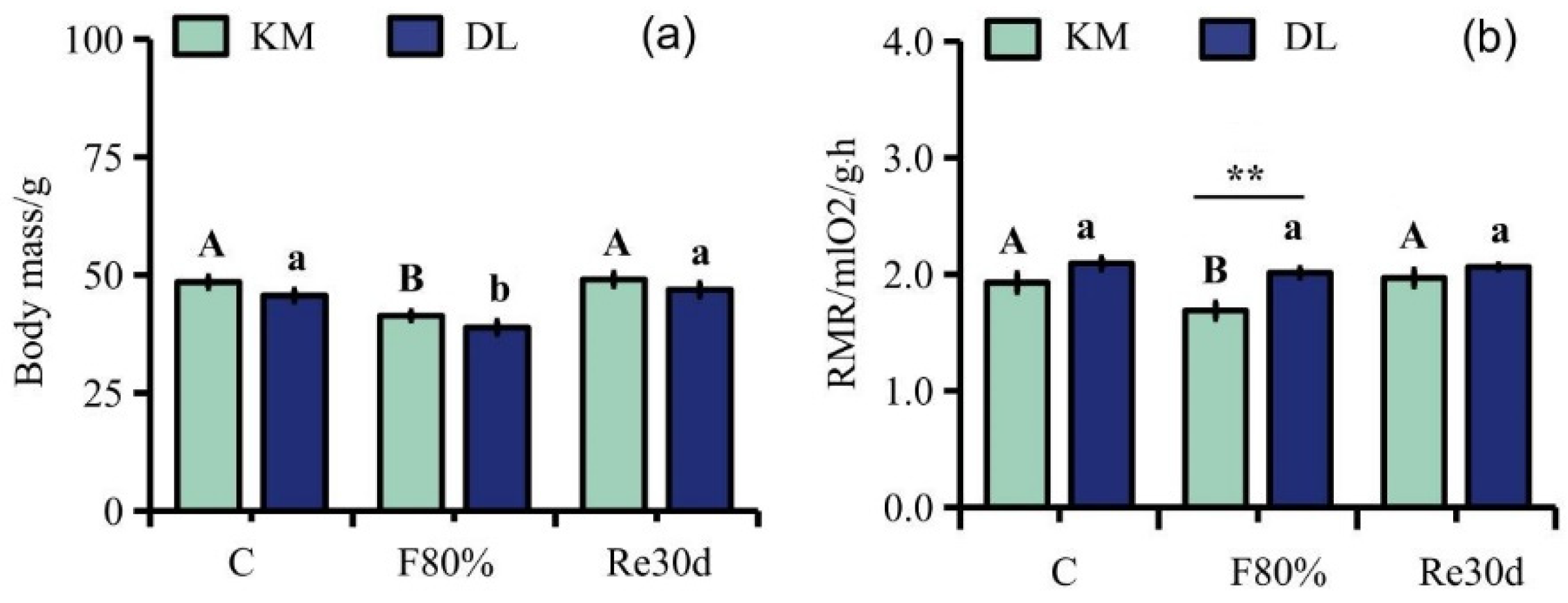
3.1. Body Mass, RMR, and Body Composition
3.2. Changes in Neuropeptide Gene Expressions in the Hypothalamus
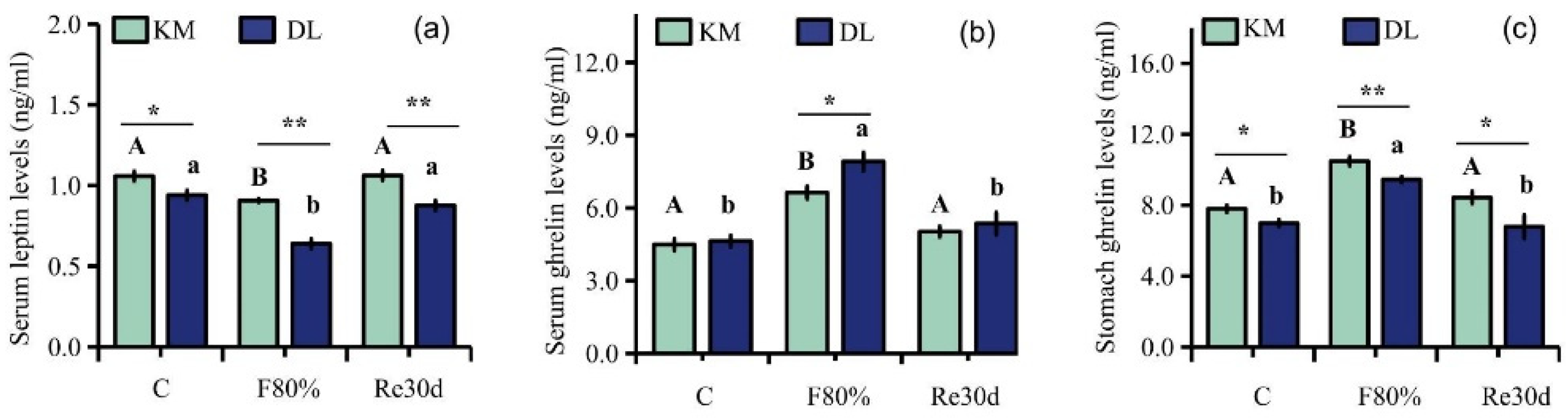
3.3. Changes in Serum Leptin, Serum Ghrelin, and Stomach Ghrelin Concentration
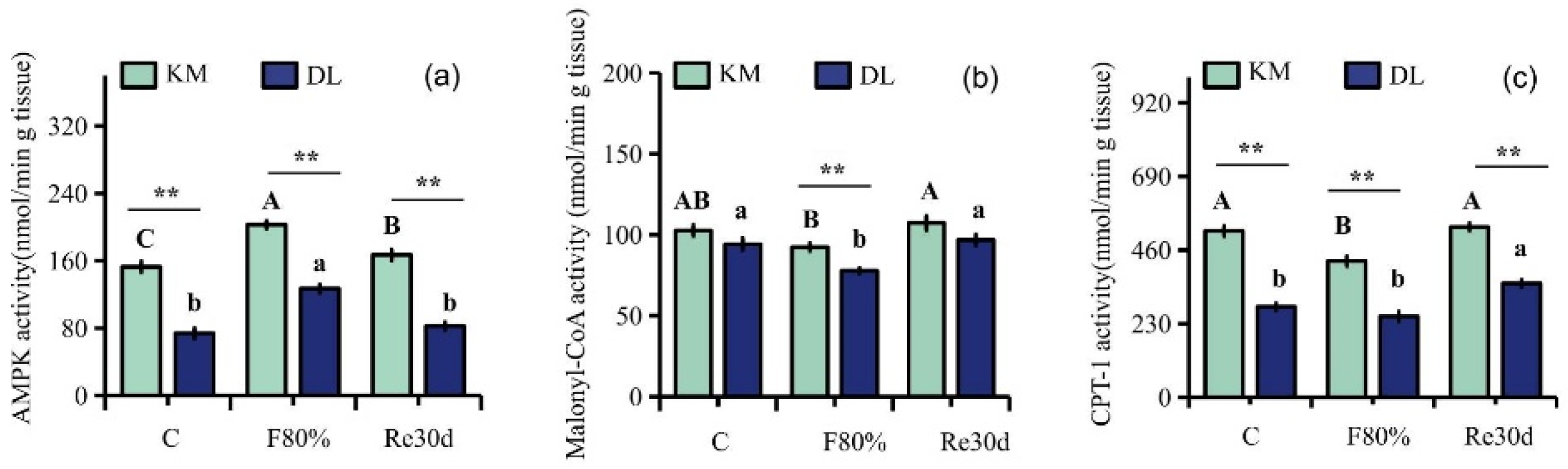
3.4. Changes in AMPK, Malonyl CoA, and CPT-1 Activities in the Hypothalamus
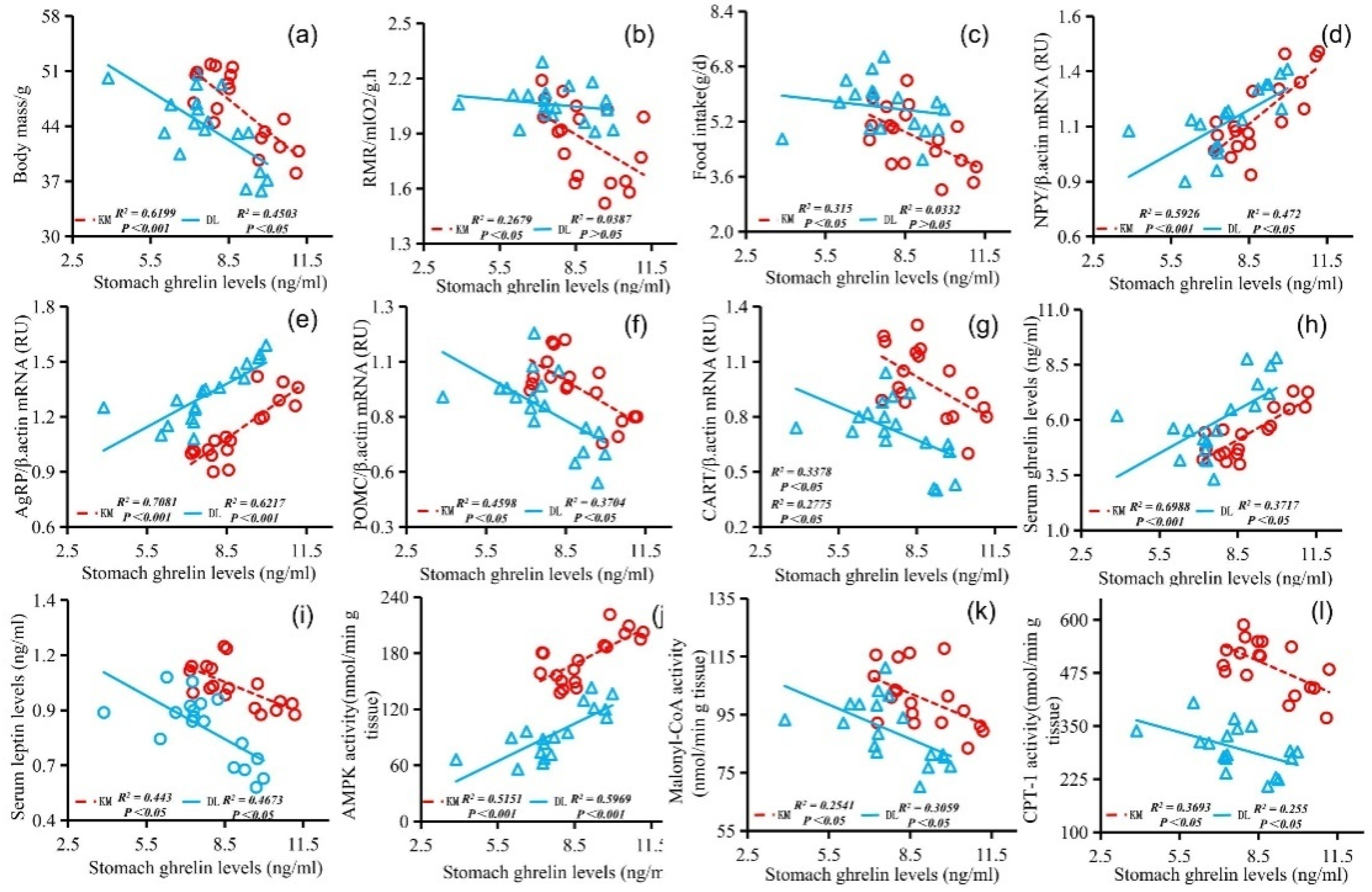
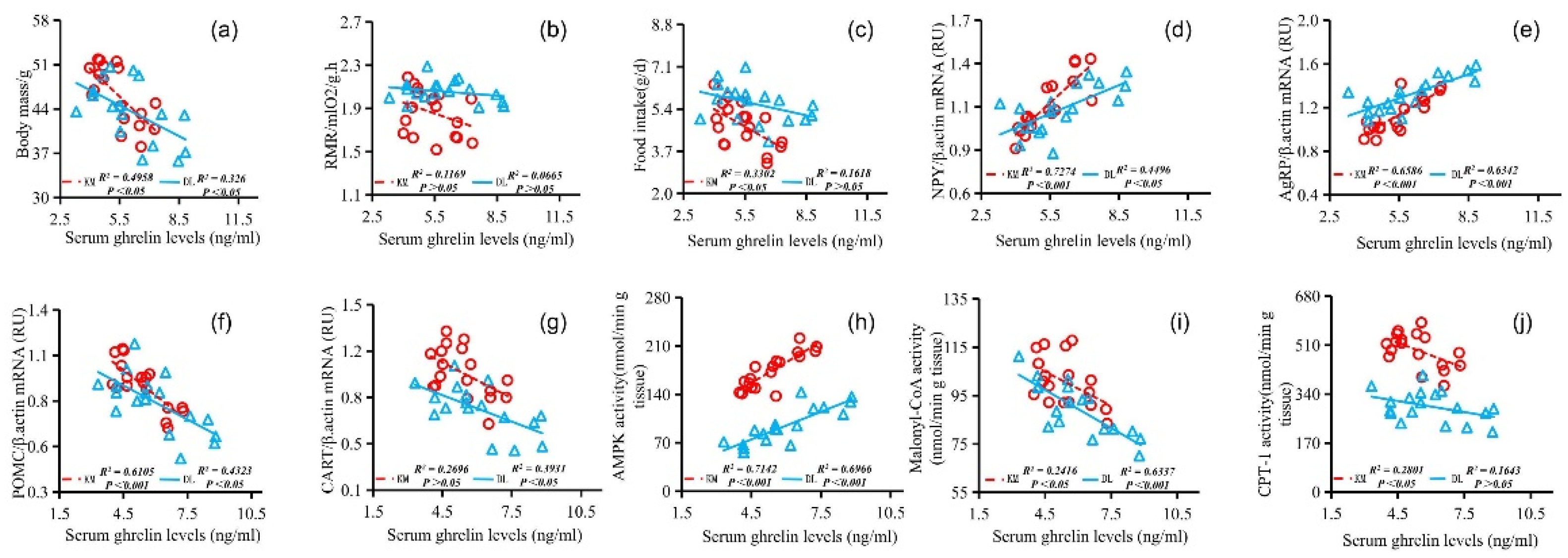
3.5. Correlation Analysis
4. Discussion
4.1. Body Mass, RMR, and Body Composition
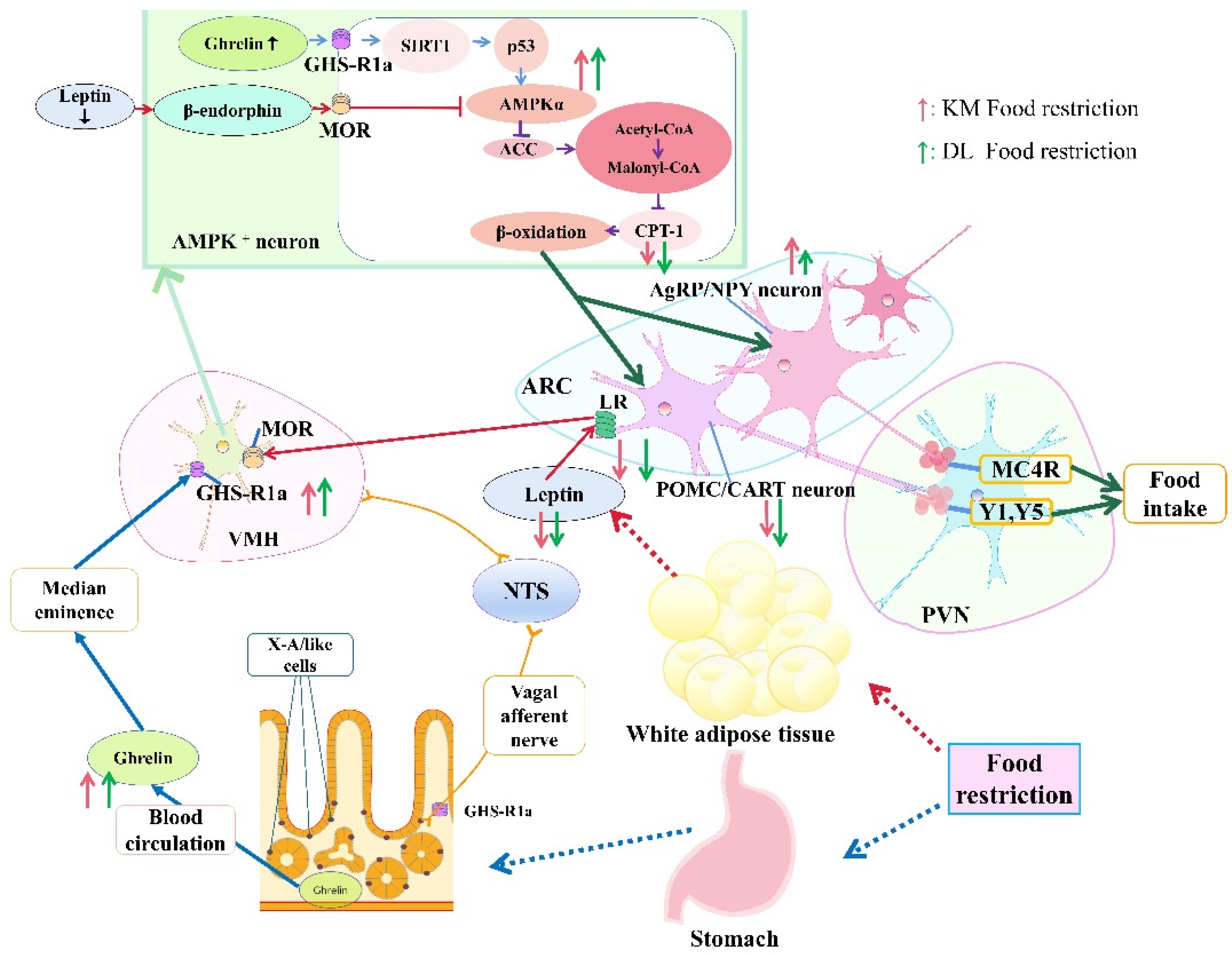
4.2. Ghrelin, Leptin, AMPK Activity, Malonyl CoA, CPT-1, and Hypothalamic Neuropeptide Expression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caumul, R.; Polly, P.D. Phylogenetic and environmental components of morphological variation: Skull, mandible, and molar shape in Marmots (Marmota, Rodentia). Evolution 2005, 59, 2460–2472. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.K.; Leibler, S. Benefits of phenotypic plasticity for population growth in varying environments. Proc. Natl. Acad. Sci. USA 2018, 115, 12745–12750. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Song, S.Y.; Liu, X.Y.; Yang, M. Phenotypic changes in the metabolic profile and adiponectin activity during seasonal fattening and hibernation in female Daurian ground squirrels (Spermophilus dauricus). Integr. Zool. 2022, 17, 297–310. [Google Scholar] [CrossRef]
- Kelly, S.A.; Panhuis, T.M.; Stoehr, A.M. Phenotypic plasticity: Molecular mechanisms and adaptive significance. Compr. Physiol. 2012, 2, 1417–1439. [Google Scholar]
- Sommer, R.J. Phenotypic plasticity: From theory and genetics to current and future challenge. Genetics 2020, 215, 1–13. [Google Scholar] [CrossRef]
- Hou, D.M.; Ren, X.Y.; Hao, Z.; Zhu, W.L. Comparative study on phenotypic differences in Eothenomys miletus under food restriction and refeeding between Xianggelila and Jianchuan from Hengduan Mountain regions. Indian J. Anim. Res. 2020, 54, 835–840. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferré, P.; Birnbaum, M.J.; et al. AMP–kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef]
- Andersson, U.; Filipsson, K.; Abbott, C.R.; Woods, A.; Smith, K.; Bloom, S.R.; Carling, D.; Small, C.J. AMP–activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004, 279, 12005–12008. [Google Scholar] [CrossRef]
- López, M.; Lage, R.; Saha, A.K.; Pérez-Tilve, D.; Vázquez, M.J.; Varela, L.; Sangiao-Alvarellos, S.; Tovar, S.; Raghay, K.; Rodríguez-Cuenca, S.; et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008, 7, 389–399. [Google Scholar] [CrossRef]
- Briski, K.P.; Mandal, S.K.; Bheemanapally, K.; Ibrahim, M.M.H. Effects of acute versus recurrent insulin–induced hypoglycemia on ventromedial hypothalamic nucleus metabolic–sensory neuron AMPK activity: Impact of alpha1–adrenergic receptor signaling. Brain Res. Bull. 2020, 157, 41–50. [Google Scholar] [CrossRef]
- Shimizu, H.; Arima, H.; Watanabe, M.; Goto, M.; Banno, R.; Sato, I.; Ozaki, N.; Nagasaki, H.; Oiso, Y. Glucocorticoids increase neuropeptide Y and agouti–related peptide gene expression via adenosine monophosphate–activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology 2008, 149, 4544–4553. [Google Scholar] [CrossRef][Green Version]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; et al. Adiponectin stimulates AMP–Activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007, 6, 55–68. [Google Scholar] [CrossRef]
- Andrews, Z.B.; Liu, Z.W.; Walllingford, N.; Erion, D.M.; Borok, E.; Friedman, J.M.; Tschöp, M.H.; Shanabrough, M.; Cline, G.; Shulman, G.I.; et al. UCP2 mediates Ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 2008, 454, 846–851. [Google Scholar] [CrossRef]
- Schaeffer, M.; Langlet, F.; Lafont, C.; Molino, F.; Hodson, D.J.; Roux, T.; Lamarque, L.; Verdié, P.; Bourrier, E.; Dehouck, B.; et al. Rapid sensing of circulating ghrelin by hypothalamic appetite–modifying neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.S.; Ruch, T.R.; Hsiao, P.Y.; Hwang, Y.; Zhang, P.; Dai, L.; Huang, C.R.L.; Berndsen, C.E.; Kim, M.S.; Pandey, A.; et al. Architectural organization of the metabolic regulatory enzyme ghrelin o–acyltransferase. J. Biol. Chem. 2013, 288, 32211–32228. [Google Scholar] [CrossRef]
- Zhang, F.; Basinski, M.B.; Beals, J.M.; Briggs, S.L.; Churgay, L.M.; Clawson, D.K.; DiMarchi, R.D.; Furman, T.C.; Hale, J.E.; Hsiung, H.M.; et al. Crystalstructure of the obese protein leptin–E100. Nature 1997, 387, 206–209. [Google Scholar] [CrossRef]
- Stark, R.; Ashley, S.E.; Andrews, Z.B. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol. Cell. Endocrinol. 2013, 366, 215–223. [Google Scholar] [CrossRef]
- Kohno, D.; Sone, H.; Minokoshi, Y.; Yada, T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochemical & Biophysical Res. Commun. 2008, 366, 388–392. [Google Scholar]
- Gao, S.; Kinzig, K.P.; Aja, S.; Scott, K.A.; Keung, W.; Kelly, S.; Strynadka, K.; Chohnan, S.; Smith, W.W.; Tamashiro, K.L.; et al. Leptin activates hypothalamic acetyl–CoA carboxylase to inhibit food intake. Proc. Natl. Acad. Sci. USA 2007, 104, 17358–17363. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, M.J.; Lane, M.D. Hypothalamic malonyl–CoA and CPT-1c in the treatment of obesity. FEBS J. 2011, 278, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chaturvedi, C.M. Neuroendocrine mechanism of food intake and energy regulation in Japanese quail under differential simulated photoperiodic conditions: Involvement of hypothalamic neuropeptides, AMPK, insulin and adiponectin receptors. J. Photochem. Photobiol. B Biol. Off. J. Eur. Soc. Photobiol. 2018, 185, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Jia, T.; Lian, X.; Wang, Z.K. Effects of cold acclimation on body mass, serum leptin level, energy metabolism and thermognesis in Eothenomys miletus in Hengduan Mountains region. J. Therm. Biol. 2010, 35, 41–46. [Google Scholar] [CrossRef]
- Ren, X.Y.; Liu, C.Y.; Hou, D.M.; Zhu, W.L. Effects of short–term fasting and refeeding on hypothalamic neuropeptides expressions and behavior in Eothenomys miletus from different regions. J. Biol. 2020, 37, 66–70. [Google Scholar]
- Zhu, W.L.; Jia, T.; Lian, X.; Wang, Z.K. Evaporative water loss and energy metabolic in two small mammals voles (Eothenomys miletus) and mice (Apodemus chevrieri), in Hengduan mountains region. J. Therm. Biol. 2008, 33, 324–331. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, D.H. Regulation of body weight and thermogenesis in seasonally acclimatized Brandt’s voles (Microtus brandti). Horm. Behav. 2005, 48, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Li, J.; Lu, S.Y. Changes of leptin—hypothalamic neuropeptide Y zxis during the development of type 2 diabetes in rats. Chin. Gen. Pract. 2014, 17, 4329–4332. [Google Scholar]
- Fuglei, E.; Oritsland, N.A. Body composition, resting and running metabolic rates, and net cost of running in rats during starvation. Acta Physiol. 2010, 165, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Nagy, T.R.; Pistole, D.H. The effects of fasting on some physiological parameters in the meadow vole, Microtus pennsylvanicus. Comp. Biochem. Physiol. A Comp. Physiol. 1988, 91, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.A.; Schneide, J.E. Food hoarding is increased by food deprivation and decreased by leptin treatment in Syrian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Day, D.E.; Bartness, T.J. Fasting–induced increases in food hoarding are dependent on the foraging–effort level. Physiol. Behav. 2003, 78, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.L.; Bao, M.H.; Cao, J.; Zhao, Z.J. The nonshivering thermogenesis of brown adipose tissue and fat mobilization of striped hamsters exposed to cycles of cold and warm temperatures. Eur. Zool. J. 2022, 89, 190–203. [Google Scholar] [CrossRef]
- Bonin, M.; Jean–Pierre, T.; Côté, S.D. Contributions of digestive plasticity to the ability of white–tailed deer to cope with a low–quality diet. J. Mammal. 2016, 97, 1406–1413. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yang, K.; Yang, P.P.; Su, Y.S.; Zheng, W.H.; Liu, J.S. Food restriction decreases BMR, body and organ mass, and cellular energetics, in the Chinese Bulbul (Pycnonotus sinensis). Avian Res. 2018, 9, 39. [Google Scholar] [CrossRef]
- Bake, T.; Le-May, M.V.; Edvardsson, C.E.; Vogel, H.; Bergström, U.; Albers, M.N.; Skibicka, K.P.; Farkas, I.; Liposits, Z.; Dickson, S.L. Ghrelin receptor stimulation of the lateral parabrachial nucleus in rats increases food intake but not food motivation. Obesity 2020, 28, 1503–1511. [Google Scholar] [CrossRef]
- Zhu, W.L.; Mu, Y.; Zhang, H.; Zhang, L.; Wang, Z.K. Effects of food restriction on body mass, thermogenesis and serum leptin level in Apodemus chevrieri (Mammalia: Rodentia: Muridae). Ital. J. Zool. 2013, 80, 337–344. [Google Scholar] [CrossRef]
- Briggs, D.I.; Andrews, Z.B. Metabolic status regulates Ghrelin function on energy homeostasis. Neuroendocrinology 2011, 93, 48–57. [Google Scholar] [CrossRef]
- MacDonald, L.; Radler, M.; Paolini, A.G.; Kent, S. Calorie restriction attenuates LPS–induced sickness behavior and shifts hypothalamic signaling pathways to an antiinflammatory bias. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2011, 301, R172–R184. [Google Scholar] [CrossRef]
- Gropp, E.; Shanabrough, M.; Borok, E.; Xu, A.W.; Janoschek, R.; Buch, T.; Plum, L.; Balthasar, N.; Hampel, B.; Waisman, A.; et al. Agouti–related peptide–expressing neurons are mandatory for feeding. Nat. Neurosci. 2005, 8, 1289–1291. [Google Scholar] [CrossRef]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef]
- Claret, M.; Smith, M.A.; Batterham, R.L.; Selman, C.; Choudhury, A.I.; Fryer, L.G.; Clements, M.; Al-Qassab, H.; Heffron, H.; Xu, A.W.; et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Investig. 2007, 117, 2325–2336. [Google Scholar] [CrossRef]
- Williams, J.; Mobarhan, S. A critical interaction: Leptin and Ghrelin. Nutr. Rev. 2010, 61, 391–393. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Zhou, S.; Owyang, C. Low–affinity CCK–A receptors are co expressed with leptin receptors in rat no dose ganglia: Implications for leptin as a regulator of short–term satiety. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G217–G227. [Google Scholar] [CrossRef]
- Heldsinger, A.; Grabauskas, G.; Wu, X.; Zhou, S.; Lu, Y.; Song, I.; Owyang, C. Ghrelin induces leptin resistance by activation of suppressor of cytokine signaling 3 expression in male rats: Implications in satiety regulation. Endocrinology 2014, 155, 3956–3969. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Leinninger, G.M.; Myers, M.G., Jr. Molecular and neural mediators of leptin action. Physiol. Behav. 2008, 94, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, M.; Duffy, P.; Simasko, S.; Ritter, R.C. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology 2010, 151, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Frontini, A.; Bertolotti, P.; Tonello, C.; Valerio, A.; Nisoli, E.; Cinti, S.; Giordano, A. Leptin–dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res. 2008, 1215, 105–115. [Google Scholar] [CrossRef]
- Date, Y.; Murakami, N.; Toshinai, K.; Matsukura, S.; Niijima, A.; Matsuo, H.; Kangawa, K.; Nakazato, M. The role of the stomachafferent vagal nerve in ghrelin–induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–1128. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, M.D.; Martínez, V.; Wang, L.; Wei, J.Y.; Taché, Y. Synergistic interaction between leptin and cholecystokinin to reduce short–term food intake in lean mice. Proc. Natl. Acad. Sci. USA 1997, 94, 10455–10460. [Google Scholar] [CrossRef] [PubMed]
- Kola, B.; Farkas, I.; Christ–Crain, M.; Wittmann, G.; Lolli, F.; Amin, F.; Harvey-White, J.; Liposits, Z.; Kunos, G.; Grossman, A.B.; et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE 2008, 3, e1797. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Hanada, R.; Hanada, T.; Aki, D.; Mashima, R.; Nishinakamura, H.; Torisu, T.; Chien, K.R.; Yasukawa, H.; Yoshimura, A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet–induced obesity. Nat. Med. 2004, 10, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Cave, B.J.; Oksanen, L.J.; Tzameli, I.; Bjorbaek, C.; Flier, J.S. Enhanced leptin sensitivity and attenuation of diet–induced obesity in mice with haploinsufficiency of Socs3. Nat. Med. 2004, 10, 734–738. [Google Scholar] [CrossRef]


| Kunming (KM) | Dali (DL) | |
|---|---|---|
| Longitude | 102°51′57″ E | 99°75′03″ E |
| Latitude | 24°52′4″ N | 26°43′95″ N |
| Altitude | 2020 m | 2590 m |
| Minimum temperature | −1 °C | −5 °C |
| Maximum temperature | 30.1 °C | 30 °C |
| Annual average temperature | 16.5 °C | 7 °C |
| Rainfall | 1026.1 mm | 657.5 mm |
| Sunshine | 198.4–343.1 h | 191.2–220.6 h |
| Vegetation type | Small leaved shrub | Evergreen broad-leaf forest |
| Primer | Oligonuncleotide Sequence (5′ to 3′) | Product Size (bp) |
|---|---|---|
| NPY (forward) | TGGACTGACCCTCGCTCTAT | 162 |
| NPY (reverse) | GTGTCTCAGGGCTGGATCTC | |
| AgRP (forward) | AGAGTTCTCAGGTCTAAGTCT | 187 |
| AgRP (reverse) | CTTGAAGAAGCGGCAGTAGCACGT | |
| POMC (forward) | CCTGTGAAGGTGTACCCAATGTC | 240 |
| POMC (reverse) | CACGTTCTTGATGATGGCGTTC | |
| CART (forward) | AGAAGAAGTACGGCCAAGTCC | 55 |
| CART (reverse) | CACACAGCTTCCCGATCC | |
| β-actin (forward) | GAGAGGGAAATCGTGCGTGAC | 170 |
| β-actin (reverse) | CATCTGCTGGAAGGTGGACA |
| Parameters | KM | DL | Statistical Summary | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-KM | F80%-KM | Re30d-KM | C-DL | F80%-DL | Re30d-DL | Region | Food Restriction | Region × Food Restriction | ||||
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | F1,30 | p | F2,30 | p | F2,30 | p | |
| Carcass wet mass (g) | 32.88 ± 1.27 | 26.24 ± 1.47 | 30.59 ± 1.95 | 29.52 ± 1.43 | 27.72 ± 1.39 | 30.59 ± 3.13 | 0.140 | 0.711 | 0.182 | 0.835 | 0.856 | 0.435 |
| Carcass dry mass (g) | 19.08 ± 1.14 | 14.45 ± 1.32 | 16.76 ± 1.96 | 15.69 ± 1.28 | 15.67 ± 1.29 | 17.41 ± 2.52 | 0.139 | 0.712 | 1.165 | 0.326 | 1.144 | 0.332 |
| Body fat mass (g) | 8.03 ± 0.47 | 3.36 ± 0.31 | 7.16 ± 0.87 | 6.28 ± 0.51 | 3.34 ± 0.29 | 7.22 ± 1.00 | 6.712 | <0.05 | 168.320 | <0.01 | 0.473 | 0.628 |
| Body fat content (%) | 0.42 ± 0.01 | 0.23 ± 0.01 | 0.43 ± 0.00 | 0.40 ± 0.00 | 0.21 ± 0.01 | 0.42 ± 0.00 | 11.298 | <0.01 | 540.402 | <0.01 | 0.705 | 0.503 |
| Parameters | KM | DL | Statistical Summary | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-KM | F80%-KM | Re30d-KM | C-DL | F80%-DL | Re30d-DL | Region | Food restriction | Region × Food Restriction | ||||
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | F1,30 | p | F2,30 | p | F2,30 | p | |
| Heart wet mass (g) | 0.25 ± 0.01 | 0.21 ± 0.02 | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.23 ± 0.02 | 0.27 ± 0.02 | 0.808 | 0.376 | 0.516 | 0.602 | 0.093 | 0.911 |
| Liver wet mass (g) | 3.15 ± 0.37 | 1.65 ± 0.16 | 2.75 ± 0.09 | 2.39 ± 0.20 | 1.42 ± 0.30 | 2.58 ± 0.12 | 2.106 | 0.158 | 4.735 | <0.05 | 0.930 | 0.406 |
| Spleen wet mass (g) | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.11 ± 0.03 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.122 | 0.729 | 2.964 | 0.067 | 0.067 | 0.935 |
| Lungs wet mass (g) | 0.36 ± 0.02 | 0.27 ± 0.03 | 0.29 ± 0.01 | 0.28 ± 0.03 | 0.28 ± 0.03 | 0.29 ± 0.03 | 0.394 | 0.535 | 0.565 | 0.574 | 1.504 | 0.239 |
| Kidney wet mass (g) | 0.39 ± 0.01 | 0.37 ± 0.02 | 0.32 ± 0.03 | 0.39 ± 0.02 | 0.37 ± 0.03 | 0.39 ± 0.03 | 1.556 | 0.222 | 1.446 | 0.252 | 1.445 | 0.252 |
| Heart dry mass (g) | 0.06 ± 0.01 | 0.05 ± 0.003 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 2.968 | 0.096 | 1.117 | 0.341 | 0.369 | 0.695 |
| Liver dry mass (g) | 1.06 ± 0.15 | 0.51 ± 0.04 | 1.00 ± 0.08 | 0.73 ± 0.06 | 0.58 ± 0.03 | 0.92 ± 0.06 | 1.205 | 0.281 | 3.777 | <0.05 | 3.284 | 0.052 |
| Spleen dry mass (g) | 0.03 ± 0.002 | 0.02 ± 0.002 | 0.03 ± 0.003 | 0.03 ± 0.002 | 0.02 ± 0.002 | 0.02 ± 0.004 | 0.005 | 0.943 | 1.688 | 0.203 | 0.128 | 0.880 |
| Lungs dry mass (g) | 0.10 ± 0.01 | 0.06 ± 0.01 | 0.08 ± 0.003 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.244 | 0.652 | 0.998 | 0.381 | 2.036 | 0.149 |
| Kidney dry mass (g) | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 | 2.996 | 0.094 | 2.905 | 0.071 | 0.020 | 0.980 |
| Parameters | KM | DL | Statistical Summary | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-KM | F80%-KM | Re30d-KM | C-DL | F80%-DL | Re30d-DL | Region | Food Restriction | Region × Food Restriction | ||||
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | F1,30 | p | F2,30 | p | F2,30 | p | |
| Stomach wet mass with content (g) | 0.50 ± 0.09 | 0.46 ± 0.07 | 0.64 ± 0.09 | 0.39 ± 0.05 | 0.36 ± 0.04 | 0.46 ± 0.09 | 3.745 | 0.063 | 1.211 | 0.312 | 0.221 | 0.803 |
| Large intestine wet mass with content (g) | 0.68 ± 0.07 | 0.46 ± 0.06 | 0.58 ± 0.03 | 0.63 ± 0.08 | 0.60 ± 0.04 | 0.84 ± 0.19 | 1.262 | 0.271 | 1.258 | 0.299 | 1.415 | 0.259 |
| Small intestine wet mass with content (g) | 1.77 ± 0.05 | 1.69 ± 0.18 | 1.48 ± 0.09 | 1.68 ± 0.11 | 1.66 ± 0.09 | 1.53 ± 0.12 | 0.443 | 0.511 | 2.001 | 0.153 | 0.231 | 0.795 |
| Cecum wet mass with content (g) | 1.60 ± 0.14 | 2.30 ± 0.33 | 1.55 ± 0.25 | 1.53 ± 0.10 | 2.50 ± 0.15 | 2.05 ± 0.34 | 0.025 | 0.875 | 1.147 | 0.332 | 0.978 | 0.388 |
| Stomach wet mass with no content (g) | 0.22 ± 0.03 | 0.28 ± 0.07 | 0.19 ± 0.03 | 0.22 ± 0.02 | 0.19 ± 0.01 | 0.19 ± 0.03 | 0.125 | 0.726 | 2.168 | 0.133 | 1.190 | 0.319 |
| Large intestine wet mass with no content (g) | 0.40 ± 0.02 | 0.28 ± 0.02 | 0.40 ± 0.05 | 0.41 ± 0.05 | 0.33 ± 0.01 | 0.46 ± 0.05 | 1.435 | 0.241 | 2.001 | 0.153 | 0.306 | 0.739 |
| Small intestine wet mass with no content (g) | 1.01 ± 0.03 | 0.92 ± 0.06 | 0.99 ± 0.05 | 0.67 ± 0.07 | 0.70 ± 0.06 | 0.76 ± 0.07 | 24.210 | <0.001 | 0.467 | 0.632 | 0.492 | 0.617 |
| Cecum wet mass with no content (g) | 0.46 ± 0.01 | 0.39 ± 0.06 | 0.36 ± 0.02 | 0.44 ± 0.02 | 0.45 ± 0.03 | 0.44 ± 0.02 | 1.980 | 0.170 | 1.092 | 0.349 | 1.617 | 0.216 |
| Large intestine length (cm) | 20.77 ± 0.39 | 19.77 ± 1.12 | 21.48 ± 1.48 | 23.67 ± 0.42 | 20.10 ± 0.25 | 22.47 ± 0.67 | 6.099 | <0.05 | 0.469 | 0.631 | 1.368 | 0.271 |
| Small intestine length (cm) | 36.72 ± 1.52 | 34.77 ± 2.38 | 35.43 ± 0.81 | 36.93 ± 1.67 | 37.52 ± 1.20 | 36.13 ± 1.32 | 1.194 | 0.284 | 0.305 | 0.740 | 0.358 | 0.702 |
| Cecum length (cm) | 11.10 ± 0.93 | 9.78 ± 0.16 | 12.63 ± 0.99 | 11.18 ± 1.35 | 13.65 ± 0.87 | 12.22 ± 0.56 | 5.422 | <0.05 | 2.015 | 0.152 | 3.789 | <0.05 |
| Large intestine dry mass with content (g) | 0.08 ± 0.004 | 0.06 ± 0.004 | 0.09 ± 0.02 | 0.08 ± 0.01 | 0.07 ± 0.003 | 0.09 ± 0.02 | 0.642 | 0.429 | 0.572 | 0.571 | 0.173 | 0.842 |
| Small intestine dry mass with content (g) | 0.20 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.18 ± 0.01 | 1.735 | 0.198 | 0.361 | 0.700 | 7.383 | <0.05 |
| Cecum dry mass with content (g) | 0.08 ± 0.003 | 0.07 ± 0.01 | 0.07 ± 0.005 | 0.08 ± 0.003 | 0.09 ± 0.01 | 0.10 ± 0.01 | 6.174 | <0.05 | 0.457 | 0.638 | 3.220 | 0.055 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jia, T.; Ren, Y.; Wang, Z.; Zhu, W. Roles of Ghrelin and Leptin in Body Mass Regulation under Food Restriction Based on the AMPK Pathway in the Red-Backed Vole, Eothenomys miletus, from Kunming and Dali Regions. Animals 2022, 12, 3333. https://doi.org/10.3390/ani12233333
Liu Y, Jia T, Ren Y, Wang Z, Zhu W. Roles of Ghrelin and Leptin in Body Mass Regulation under Food Restriction Based on the AMPK Pathway in the Red-Backed Vole, Eothenomys miletus, from Kunming and Dali Regions. Animals. 2022; 12(23):3333. https://doi.org/10.3390/ani12233333
Chicago/Turabian StyleLiu, Yuting, Ting Jia, Yue Ren, Zhengkun Wang, and Wanlong Zhu. 2022. "Roles of Ghrelin and Leptin in Body Mass Regulation under Food Restriction Based on the AMPK Pathway in the Red-Backed Vole, Eothenomys miletus, from Kunming and Dali Regions" Animals 12, no. 23: 3333. https://doi.org/10.3390/ani12233333
APA StyleLiu, Y., Jia, T., Ren, Y., Wang, Z., & Zhu, W. (2022). Roles of Ghrelin and Leptin in Body Mass Regulation under Food Restriction Based on the AMPK Pathway in the Red-Backed Vole, Eothenomys miletus, from Kunming and Dali Regions. Animals, 12(23), 3333. https://doi.org/10.3390/ani12233333








