Determination of Ram (Ovis aries) Sperm DNA Damage Due to Oxidative Stress: 8-OHdG Immunodetection Assay vs. SCSA®
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Media
2.2. Animal Ethics and Sperm Collections
2.3. Experimental Design
2.3.1. 8-OHdG Immunodetection Assay
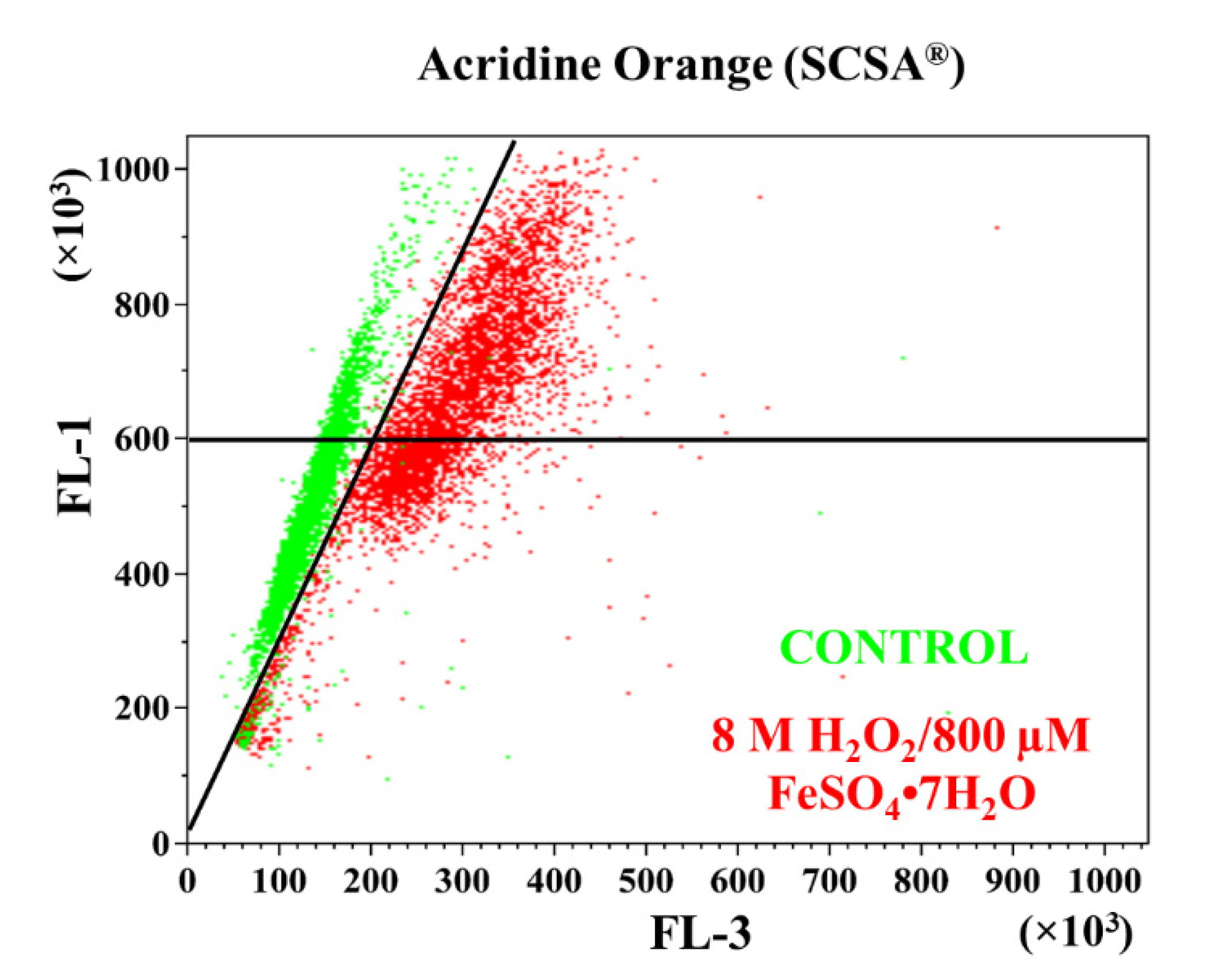
2.3.2. Sperm Chromatin Structure Assay (SCSA®)
2.4. Flow Cytometry Analysis
2.5. Statistical Analysis
3. Results
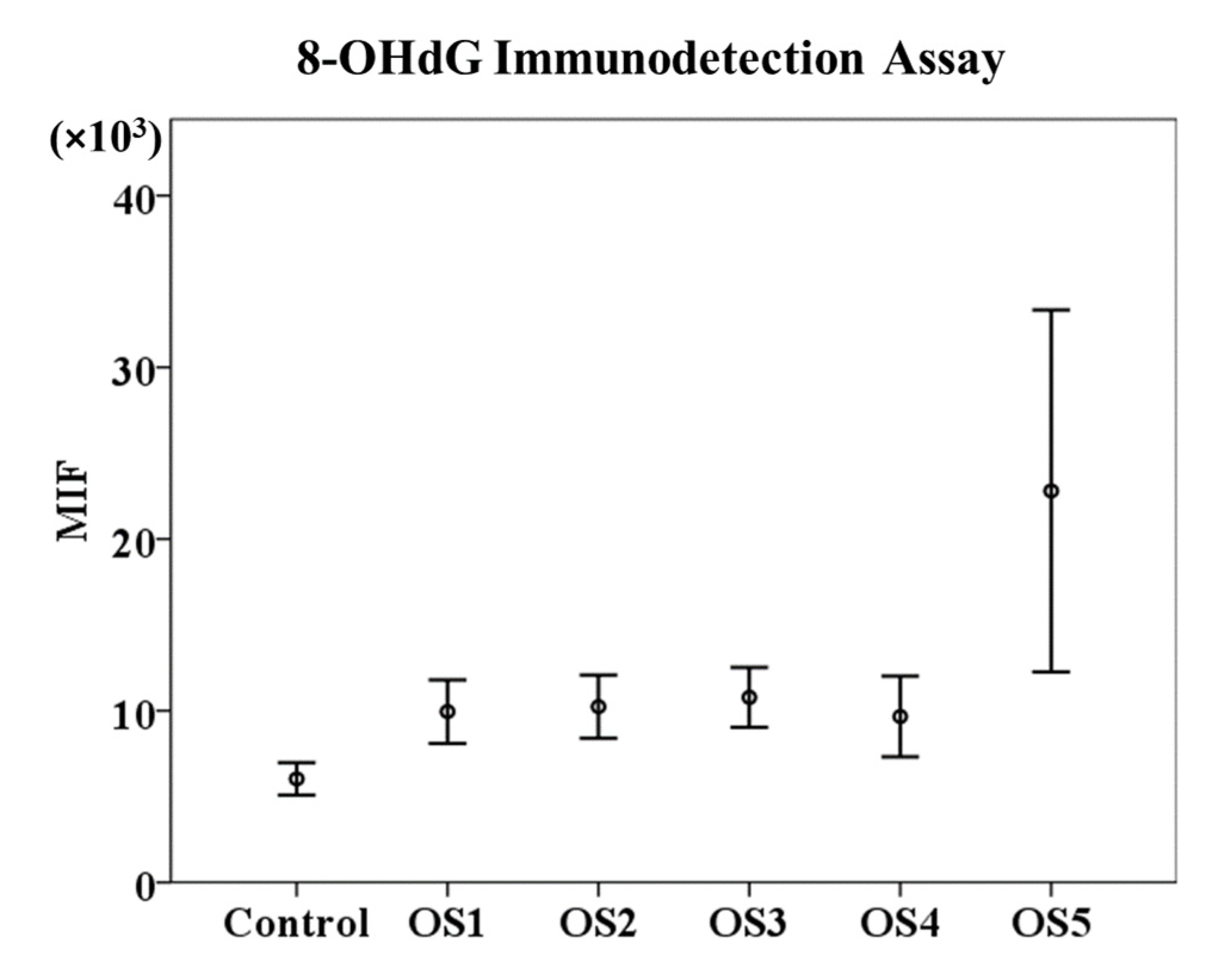
3.1. 8-OHdG Immunodetection Assay
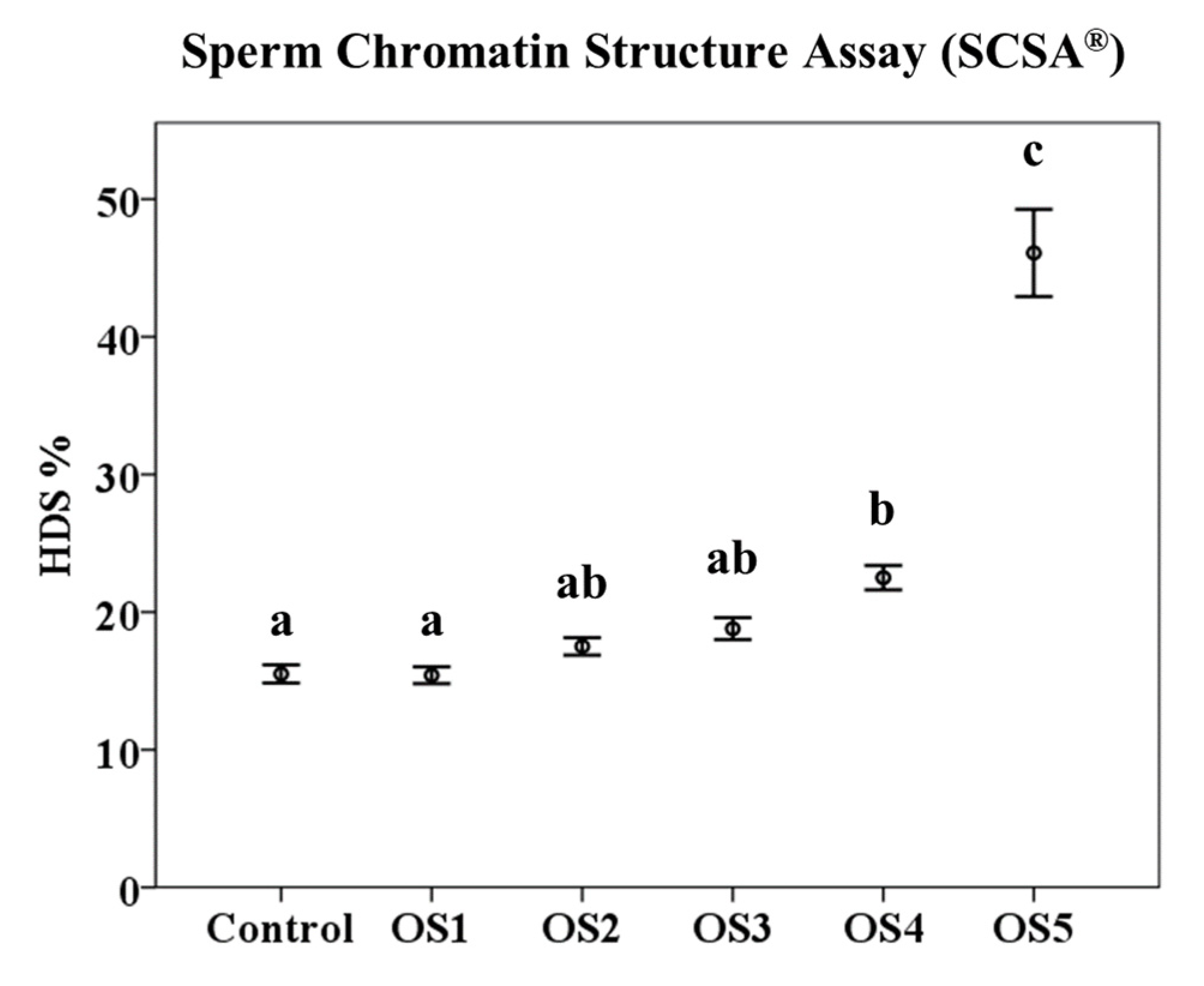
3.2. Sperm Chromatin Structure Assay (SCSA®)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drevet, J.R.; Aitken, R.J. Oxidative Damage to Sperm DNA: Attack and Defense. Adv. Exp. Med. Biol. 2019, 1166, 107–117. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Brinkworth, M.; Iles, D. Paternal DNA packaging in spermatozoa: More than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 2010, 139, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Noblanc, A.; Damon-Soubeyrand, C.; Karrich, B.; Henry-Berger, J.; Cadet, R.; Saez, F.; Guiton, R.; Janny, L.; Pons-Rejraji, H.; Alvarez, J.G.; et al. DNA oxidative damage in mammalian spermatozoa: Where and why is the male nucleus affected? Free Radic. Biol. Med. 2013, 65, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm chromatin structure assay: Its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sabanegh, E.; Mahfouz, R.; Gupta, S.; Thiyagarajan, A.; Agarwal, A. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology 2010, 76, 1380–1386. [Google Scholar] [CrossRef]
- Fernandez, J.L.; Muriel, L.; Goyanes, V.; Segrelles, E.; Gosalvez, J.; Enciso, M.; LaFromboise, M.; De Jonge, C. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil. Steril. 2005, 84, 833–842. [Google Scholar] [CrossRef]
- Simon, L.; Carrell, D.T. Sperm DNA damage measured by comet assay. Methods Mol. Biol. 2013, 927, 137–146. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; García-Peiró, A.; Fernández-Encinas, A.; Abad, C.; Amengual, M.J.; Prada, E.; Navarro, J.; Benet, J. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 2013, 1, 715–722. [Google Scholar] [CrossRef]
- Evenson, D.P. The Sperm Chromatin Structure Assay (SCSA®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef]
- Pena, F.J.; Ortega Ferrusola, C.; Martin Munoz, P. New flow cytometry approaches in equine andrology. Theriogenology 2016, 86, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pastor, F.; Mata-Campuzano, M.; Alvarez-Rodriguez, M.; Alvarez, M.; Anel, L.; de Paz, P. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. 2010, 45 (Suppl. S2), 67–78. [Google Scholar] [CrossRef] [PubMed]
- Pena, F.J.; Ortiz Rodriguez, J.M.; Gil, M.C.; Ortega Ferrusola, C. Flow cytometry analysis of spermatozoa: Is it time for flow spermetry? Reprod. Domest. Anim. 2018, 53 (Suppl. S2), 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Masaki, J.; Agarwal, A. Sperm DNA fragmentation analysis using the TUNEL assay. Methods Mol. Biol. 2013, 927, 121–136. [Google Scholar] [CrossRef]
- Henkel, R.; Hoogendijk, C.F.; Bouic, P.J.; Kruger, T.F. TUNEL assay and SCSA determine different aspects of sperm DNA damage. Andrologia 2010, 42, 305–313. [Google Scholar] [CrossRef]
- Evenson, D.; Jost, L. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci. 2000, 22, 169–189. [Google Scholar] [CrossRef]
- Smith, T.B.; Dun, M.D.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J. Cell Sci. 2013, 126, 1488–1497. [Google Scholar] [CrossRef]
- Kasai, H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res. 1997, 387, 147–163. [Google Scholar] [CrossRef]
- Jeng, H.A.; Pan, C.H.; Chao, M.R.; Lin, W.Y. Sperm DNA oxidative damage and DNA adducts. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2015, 794, 75–82. [Google Scholar] [CrossRef]
- Vorilhon, S.; Brugnon, F.; Kocer, A.; Dollet, S.; Bourgne, C.; Berger, M.; Janny, L.; Pereira, B.; Aitken, R.J.; Moazamian, A.; et al. Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum. Reprod. 2018, 33, 553–562. [Google Scholar] [CrossRef]
- De Iuliis, G.N.; Thomson, L.K.; Mitchell, L.A.; Finnie, J.M.; Koppers, A.J.; Hedges, A.; Nixon, B.; Aitken, R.J. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol. Reprod. 2009, 81, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Klungland, A.; Bjelland, S. Oxidative damage to purines in DNA: Role of mammalian Ogg1. DNA Repair 2007, 6, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Lord, T.; Aitken, R.J. Fertilization stimulates 8-hydroxy-2′-deoxyguanosine repair and antioxidant activity to prevent mutagenesis in the embryo. Dev. Biol. 2015, 406, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A. The Role of Genetics and Oxidative Stress in the Etiology of Male Infertility-A Unifying Hypothesis? Front. Endocrinol. 2020, 11, 581838. [Google Scholar] [CrossRef]
- Soria-Meneses, P.J.; Jurado-Campos, A.; Montoro, V.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.R. Ovine sperm DNA oxidation quantification using an 8-OHdG immunodetection assay. Reprod. Domest. Anim. 2019, 54 (Suppl. S4), 59–64. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, O.; Maroto-Morales, A.; Martinez-Pastor, F.; Garde, J.J.; Ramon, M.; Fernandez-Santos, M.R.; Esteso, M.C.; Perez-Guzman, M.D.; Soler, A.J. Sperm characteristics and in vitro fertilization ability of thawed spermatozoa from Black Manchega ram: Electroejaculation and postmortem collection. Theriogenology 2009, 72, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.; Jost, L. Sperm chromatin structure assay for fertility assessment. Curr. Protoc. Cytom. 2001, 7, 169–189. [Google Scholar] [CrossRef]
- Martinez-Pastor, F.; Fernandez-Santos, M.R.; del Olmo, E.; Dominguez-Rebolledo, A.E.; Esteso, M.C.; Montoro, V.; Garde, J.J. Mitochondrial activity and forward scatter vary in necrotic, apoptotic and membrane-intact spermatozoan subpopulations. Reprod. Fertil. Dev. 2008, 20, 547–556. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.; Christensen, R. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. Biom. Z. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; De Iuliis, G.N.; McLachlan, R.I. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; De Iuliis, G.N. Origins and consequences of DNA damage in male germ cells. Reprod. Biomed. Online 2007, 14, 727–733. [Google Scholar] [CrossRef]
- Zini, A.; Sigman, M. Are tests of sperm DNA damage clinically useful? Pros and cons. J. Androl. 2009, 30, 219–229. [Google Scholar] [CrossRef]
- Kao, S.H.; Chao, H.T.; Chen, H.W.; Hwang, T.I.S.; Liao, T.L.; Wei, Y.H. Increase of oxidative stress in human sperm with lower motility. Fertil. Steril. 2008, 89, 1183–1190. [Google Scholar] [CrossRef]
- Cambi, M.; Tamburrino, L.; Marchiani, S.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E.; Muratori, M. Development of a specific method to evaluate 8-hydroxy, 2-deoxyguanosine in sperm nuclei: Relationship with semen quality in a cohort of 94 subjects. Reproduction 2013, 145, 227–235. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch. Biochem. Biophys. 1986, 246, 501–514. [Google Scholar] [CrossRef]
- Kemal Duru, N.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Zribi, N.; Chakroun, N.F.; Elleuch, H.; Abdallah, F.B.; Ben Hamida, A.S.; Gargouri, J.; Fakhfakh, F.; Keskes, L.A. Sperm DNA fragmentation and oxidation are independent of malondialdheyde. Reprod. Biol. Endocrinol. 2011, 9, 47. [Google Scholar] [CrossRef]
- Serafini, R.; Varner, D.D.; Blanchard, T.L.; Teague, S.R.; LaCaze, K.; Love, C.C. Effects of seminal plasma and flash-freezing on DNA structure of stallion epididymal sperm exposed to different potentiators of DNA damage. Theriogenology 2018, 117, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ren, Z.; Fan, X.; Pan, Y.; Lv, S.; Pan, C.; Lei, A.; Zeng, W. Cysteine protects rabbit spermatozoa against reactive oxygen species-induced damages. PLoS ONE 2017, 12, e0181110. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Campos, A.; Soria-Meneses, P.J.; Arenas-Moreira, M.; Alonso-Moreno, C.; Bravo, I.; Rodríguez-Robledo, V.; Sánchez-Ajofrín, I.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.D. Vitamin E Lipid-Based Nanodevices as a Tool for Ovine Sperm Protection against Oxidative Stress: Impact on Sperm Motility. Antioxidants 2022, 11, 1988. [Google Scholar] [CrossRef] [PubMed]
- Peris-Frau, P.; Álvarez-Rodríguez, M.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Garde, J.J.; Rodriguez Martinez, H.; Soler, A.J. Comparative evaluation of DNA integrity using sperm chromatin structure assay and Sperm-Ovis-Halomax during in vitro capacitation of cryopreserved ram spermatozoa. Reprod. Domest. Anim. 2019, 54 (Suppl. S4), 46–49. [Google Scholar] [CrossRef]
- Fernandez-Santos, M.R.; Soler, A.J.; Ramon, M.; Ros-Santaella, J.L.; Maroto-Morales, A.; Garcia-Alvarez, O.; Bisbal, A.; Garde, J.J.; Coloma, M.A.; Santiago-Moreno, J. Effect of post-mortem time on post-thaw characteristics of Spanish ibex (Capra pyrenaica) spermatozoa. Anim. Reprod. Sci. 2011, 129, 56–66. [Google Scholar] [CrossRef]
- Jurado-Campos, A.; Soria-Meneses, P.J.; Sánchez-Rubio, F.; Niza, E.; Bravo, I.; Alonso-Moreno, C.; Arenas-Moreira, M.; García-Álvarez, O.; Soler, A.J.; Garde, J.J.; et al. Vitamin E Delivery Systems Increase Resistance to Oxidative Stress in Red Deer Sperm Cells: Hydrogel and Nanoemulsion Carriers. Antioxidants 2021, 10, 1780. [Google Scholar] [CrossRef]
- Sánchez-Rubio, F.; Soria-Meneses, P.J.; Jurado-Campos, A.; Bartolomé-García, J.; Gómez-Rubio, V.; Soler, A.J.; Arroyo-Jimenez, M.M.; Santander-Ortega, M.J.; Plaza-Oliver, M.; Lozano, M.V.; et al. Nanotechnology in reproduction: Vitamin E nanoemulsions for reducing oxidative stress in sperm cells. Free Radic. Biol. Med. 2020, 160, 47–56. [Google Scholar] [CrossRef]
- Fernandez-Santos, M.R.; Martinez-Pastor, F.; Matias, D.; Dominguez-Rebolledo, A.E.; Esteso, M.C.; Montoro, V.; Garde, J.J. Effects of long-term chilled storage of red deer epididymides on DNA integrity and motility of thawed spermatozoa. Anim. Reprod. Sci. 2009, 111, 93–104. [Google Scholar] [CrossRef]
- Fernandez-Santos, M.R.; Martinez-Pastor, F.; Garcia-Macias, V.; Esteso, M.C.; Soler, A.J.; Paz, P.; Anel, L.; Garde, J.J. Sperm characteristics and DNA integrity of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa frozen in the presence of enzymatic and nonenzymatic antioxidants. J. Androl. 2007, 28, 294–305. [Google Scholar] [CrossRef]
- Novotny, J.; Aziz, N.; Rybar, R.; Brezinova, J.; Kopecka, V.; Filipcikova, R.; Reruchova, M.; Oborna, I. Relationship between reactive oxygen species production in human semen and sperm DNA damage assessed by Sperm Chromatin Structure Assay. Biomed. Pap. 2013, 157, 383–386. [Google Scholar] [CrossRef]
- Peris, S.I.; Bilodeau, J.F.; Dufour, M.; Bailey, J.L. Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol. Reprod. Dev. 2007, 74, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Pirhonen, A.; Linnala-Kankkunen, A.; Maenpaa, P.H. Identification of phosphoseryl residues in protamines from mature mammalian spermatozoa. Biol. Reprod. 1994, 50, 981–986. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perreault, S.D.; Barbee, R.R.; Elstein, K.H.; Zucker, R.M.; Keefer, C.L. Interspecies differences in the stability of mammalian sperm nuclei assessed in vivo by sperm microinjection and in vitro by flow cytometry. Biol. Reprod. 1988, 39, 157–167. [Google Scholar] [CrossRef]
- Christova, Y.; James, P.S.; Jones, R. Lipid diffusion in sperm plasma membranes exposed to peroxidative injury from oxygen free radicals. Mol. Reprod. Dev. 2004, 68, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Bronson, R.; Smith, T.B.; De Iuliis, G.N. The source and significance of DNA damage in human spermatozoa; a commentary on diagnostic strategies and straw man fallacies. Mol. Hum. Reprod. 2013, 19, 475–485. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soria-Meneses, P.J.; Jurado-Campos, A.; Gómez-Rubio, V.; Sánchez-Ajofrín, I.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.d.R. Determination of Ram (Ovis aries) Sperm DNA Damage Due to Oxidative Stress: 8-OHdG Immunodetection Assay vs. SCSA®. Animals 2022, 12, 3286. https://doi.org/10.3390/ani12233286
Soria-Meneses PJ, Jurado-Campos A, Gómez-Rubio V, Sánchez-Ajofrín I, Soler AJ, Garde JJ, Fernández-Santos MdR. Determination of Ram (Ovis aries) Sperm DNA Damage Due to Oxidative Stress: 8-OHdG Immunodetection Assay vs. SCSA®. Animals. 2022; 12(23):3286. https://doi.org/10.3390/ani12233286
Chicago/Turabian StyleSoria-Meneses, Pedro Javier, Alejandro Jurado-Campos, Virgilio Gómez-Rubio, Irene Sánchez-Ajofrín, Ana Josefa Soler, José Julián Garde, and María del Rocío Fernández-Santos. 2022. "Determination of Ram (Ovis aries) Sperm DNA Damage Due to Oxidative Stress: 8-OHdG Immunodetection Assay vs. SCSA®" Animals 12, no. 23: 3286. https://doi.org/10.3390/ani12233286
APA StyleSoria-Meneses, P. J., Jurado-Campos, A., Gómez-Rubio, V., Sánchez-Ajofrín, I., Soler, A. J., Garde, J. J., & Fernández-Santos, M. d. R. (2022). Determination of Ram (Ovis aries) Sperm DNA Damage Due to Oxidative Stress: 8-OHdG Immunodetection Assay vs. SCSA®. Animals, 12(23), 3286. https://doi.org/10.3390/ani12233286






