Wild Boars (Sus scrofa, L. 1758) from Castile and Leon Region (Spain): A Histopathology Survey
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Statistical Analysis
3. Results
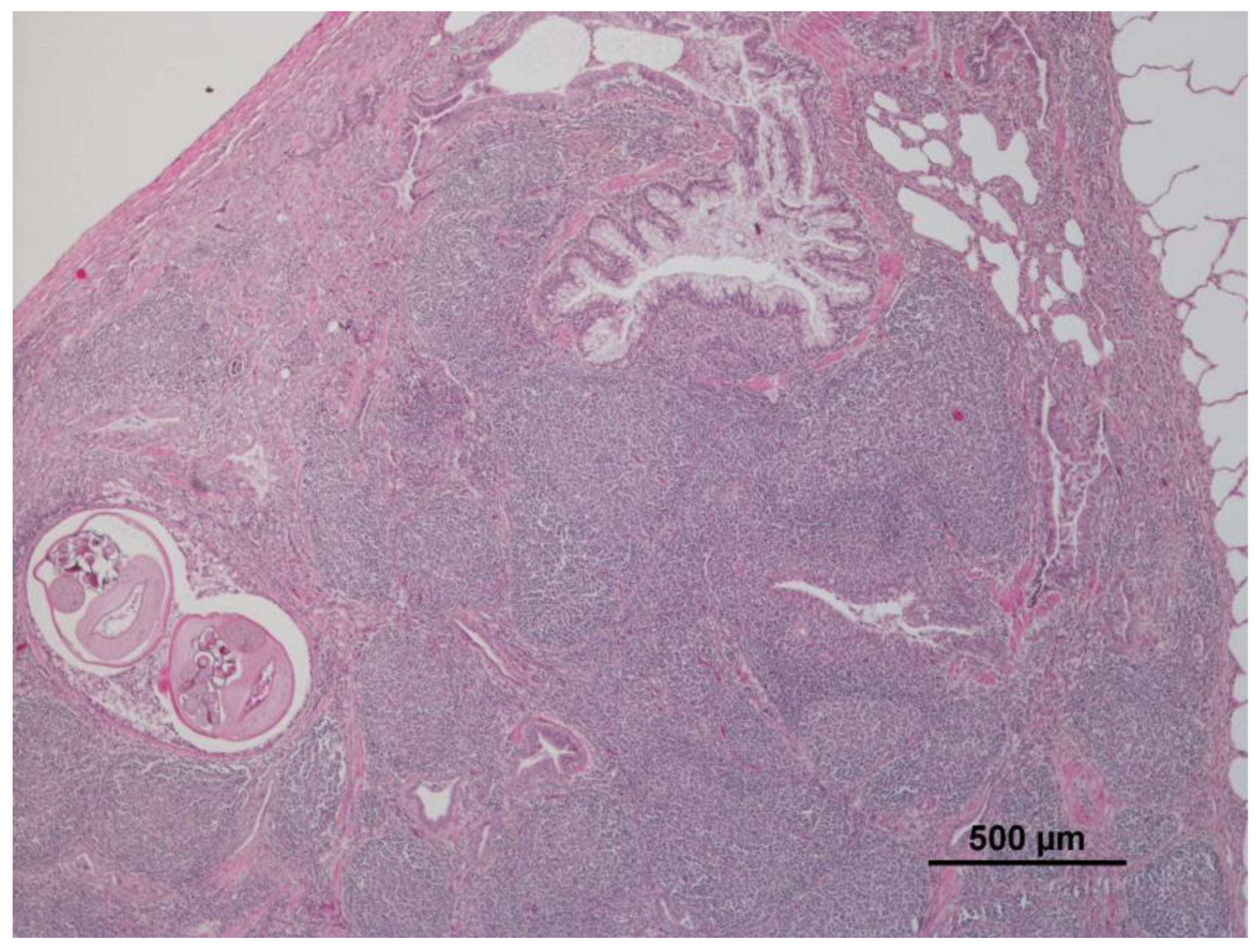
3.1. Lung
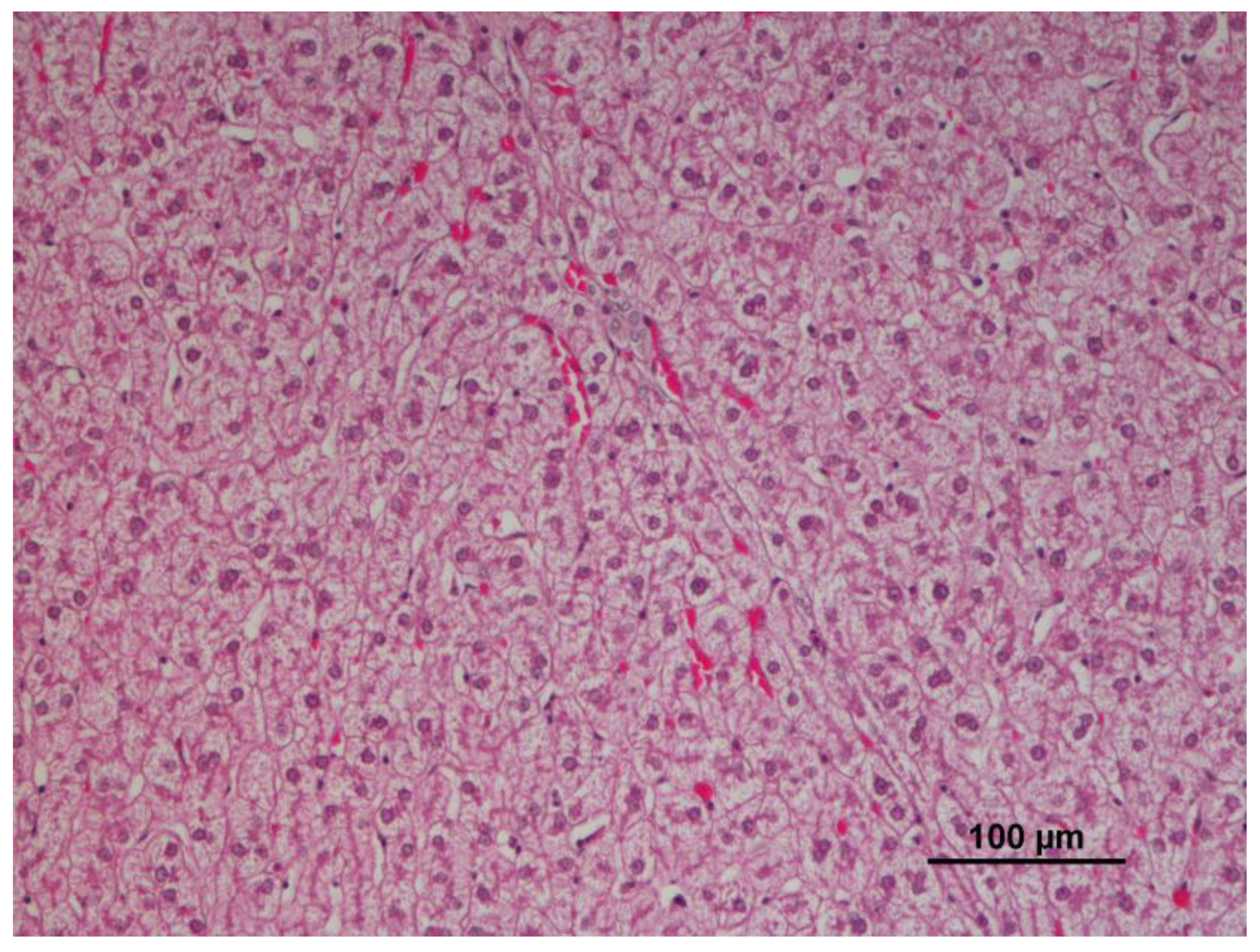
3.2. Liver
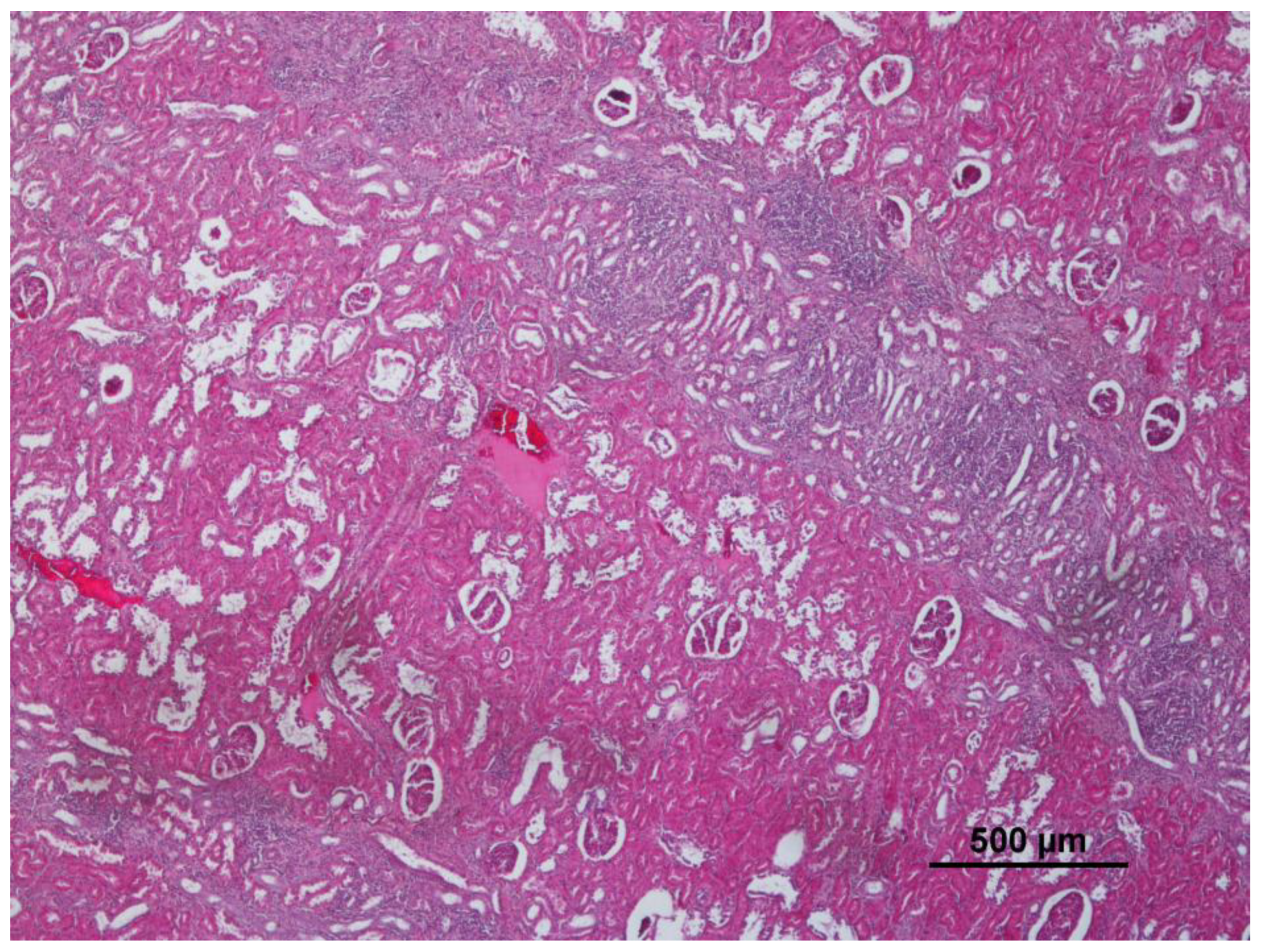
3.3. Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Location of the Sampled Wild Boars
| Province | Location | N |
|---|---|---|
| Zamora | Granucillo | 12 |
| Friera de Valverde | 10 | |
| Ferreruela | 9 | |
| Melgar de Tera | 7 | |
| Perilla de Castro | 6 | |
| Vegalatrave | 3 | |
| Argañin | 2 | |
| Montamarta | 2 | |
| Dehesa Pozos/Tabara | 1 | |
| Palencia | San Cebrian de Campos | 14 |
References
- Fredriksson-Ahomaa, M. Wild Boar: A Reservoir of Foodborne Zoonoses. Foodborne Pathog. Dis. 2019, 16, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Mendoza, M.; Marreros, N.; Boadella, M.; Gortázar, C.; Menéndez, S.; de Juan, L.; Bezos, J.; Romero, B.; Copano, M.F.; Amado, J.; et al. Wild Boar Tuberculosis in Iberian Atlantic Spain: A Different Picture from Mediterranean Habitats. BMC Vet. Res. 2013, 9, 176. [Google Scholar] [CrossRef]
- Meng, X.J.; Lindsay, D.S.; Sriranganathan, N. Wild Boars as Sources for Infectious Diseases in Livestock and Humans. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.R.; Bowen, R.A.; Bosco-Lauth, A.M. Zoonotic Pathogens from Feral Swine That Pose a Significant Threat to Public Health. Transbound. Emerg. Dis. 2018, 65, 649–659. [Google Scholar] [CrossRef]
- Gortázar, C.; Fernández-Calle, L.M.; Collazos-Martínez, J.A.; Mínguez-González, O.; Acevedo, P. Animal Tuberculosis Maintenance at Low Abundance of Suitable Wildlife Reservoir Hosts: A Case Study in Northern Spain. Prev. Vet. Med. 2017, 146, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Gavier-Widén, D.; Chambers, M.; Gortázar, C.; Delahay, R.; Cromie, R.; Lindén, A. Mycobacteria Infections. In Infectious Diseases of Wild Mammals and Birds in Europe; Wiley-Blackwell: Sussex, UK, 2012. [Google Scholar]
- De Massis, F.; Aprea, G.; Scattolini, S.; D’Angelantonio, D.; Chiaverini, A.; Mangone, I.; Perilli, M.; Colacicco, G.; Olivieri, S.; Pomilio, F.; et al. Detection of Hepatitis E Virus (HEV) in Pigs and in the Wild Boar (Sus scrofa) Population of Chieti Province, Abruzzo Region, Italy. Appl. Microbiol. 2022, 2, 818–826. [Google Scholar] [CrossRef]
- Tsachev, I.; Baymakova, M.; Marutsov, P.; Gospodinova, K.; Kundurzhiev, T.; Petrov, V.; Pepovich, R. Seroprevalence of Hepatitis e Virus Infection among Wild Boars in Western Bulgaria. Vector-Borne Zoonotic Dis. 2021, 21, 441–445. [Google Scholar] [CrossRef]
- González-Gómez, X.; Cambeiro-Pérez, N.; Figueiredo-González, M.; Martínez-Carballo, E. Wild Boar (Sus scrofa) as Bioindicator for Environmental Exposure to Organic Pollutants. Chemosphere 2021, 268, 128848. [Google Scholar] [CrossRef]
- Fernando Portela, J. La Industria del Vino y La Viticultura en Castilla y León: Su Incidencia en el Paisaje y en el Desarrollo Rural; Universidad de Valladolid: Valladolid, Spain, 2014. [Google Scholar]
- Vicente, J.; Barasona, J.A.; Acevedo, P.; Ruiz-Fons, J.F.; Boadella, M.; Diez-Delgado, I.; Beltran-Beck, B.; González-Barrio, D.; Queirós, J.; Montoro, V.; et al. Temporal Trend of Tuberculosis in Wild Ungulates from Mediterranean Spain. Transbound. Emerg. Dis. 2013, 60, 92–103. [Google Scholar] [CrossRef]
- Gallardo, M.T.; Mateos, L.; Artieda, J.; Wesslen, L.; Ruiz, C.; García, M.A.; Galmés-Truyols, A.; Martin, A.; Hernández-Pezzi, G.; Andersson, Y.; et al. Outbreak of Trichinellosis in Spain and Sweden Due to Consumption of Wild Boar Meat Contaminated with Trichinella Britovi. Wkly. Releases (1997–2007) 2007, 12, 3154. [Google Scholar] [CrossRef]
- de Guadalupe Carriço Neves, M. Contribuição Para a Caracterização Do Parasitismo em Suínos de Raça Ibérica e Javalis Silvestres das Comunidades Autónomas da Extremadura e Castilla y León (Espanha) e dos Factores de Risco Associados; Universidade Técnica de Lisboa-Faculdade de Medicina Veterinária: Lisboa, Portugal, 2013. [Google Scholar]
- Höfle, Ú.; Vincente, J.; Fernández de Mera, I.G.; Villanúa, D.; Acevedo, P.; Ruiz-Fons, F.; Gortázar, C. Health Risks in Game Production: The Wild Boar. Galemys 2004, 16, 197–206. [Google Scholar]
- Larenas-Muñoz, F.; Sánchez-Carvajal, J.M.; Galán-Relaño, Á.; Ruedas-Torres, I.; Vera-Salmoral, E.; Gómez-Gascón, L.; Maldonado, A.; Carrasco, L.; Tarradas, C.; Luque, I.; et al. The Role of Histopathology as a Complementary Diagnostic Tool in the Monitoring of Bovine Tuberculosis. Front. Vet. Sci. 2022, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- McNamara, T.S. Wildlife Pathology Studies and How They Can Inform Public Health. ILAR J. 2016, 56, 306–311. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (OiE). Guidelines for Wildlife Disease Surveillance: An Overview; World Organisation for Animal Health (OiE): Paris, France, 2015. [Google Scholar]
- Santos, N.; Correla-Neves, M.; Ghebremichael, S.; Källenius, G.; Svenson, S.B.; Almeida, V. Epidemiology of Mycobacterium Bovis Infection in Wild Boar (Sus scrofa) from Portugal. J. Wildl. Dis. 2009, 45, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Abbondanzo, S.; Rush, W.; Bijwaard, K.; Koss, M. Nodular Lymphoid Hyperplasia of the Lung: A Clinicopathologic Study of 14 Cases. Am. J. Surg. Pathol. 2000, 24, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Panayotova-Pencheva, M.; Todorova, K.; Dakova, V. Pathomorphological Studies on Wild Boars Infected with Metastrongylus Spp., Ascarops Strongylina, and Macracanthorhynchus Hirudinaceus. J. Vet. Res. 2019, 63, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.Á.J.; Gasper, D.J.; Muciño, M.d.C.; Terio, K.A. Suidae and Tayassuidae. In Pathology of Wildlife and Zoo Animals; Terio, K.A., McAloose, D., St. Leger, J., Eds.; Elsevier academic Press: Cambridge, MA, USA, 2018; pp. 207–228. [Google Scholar]
- Biondo, N.; Takeuti, K.L.; Montes, J.H.; de Almeida, L.L.; de Andrade, C.P.; Zlotowski, P.; Driemeier, D.; de Barcellos, D.E.S.N. Bacterial Pneumonia in Captive Wild Boars in Southern Brazil-Etiological and Pathological Causes. Acta Sci. Vet. 2021, 49. [Google Scholar] [CrossRef]
- DeMuth, W.E.; Smith, J.M. Pulmonary Contusion. Am. J. Surg. 1965, 109, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Sehl, J.; Pikalo, J.; Schäfer, A.; Franzke, K.; Pannhorst, K.; Elnagar, A.; Blohm, U.; Blome, S.; Breithaupt, A. Comparative Pathology of Domestic Pigs and Wild Boar Infected with the Moderately Virulent African Swine Fever Virus Strain “Estonia 2014”. Pathogens 2020, 9, 662. [Google Scholar] [CrossRef]
- Pérez-García, A.; Hurtado-Carneiro, V.; Herrero-De-Dios, C.; Dongil, P.; García-Mauriño, J.E.; Sánchez, M.D.; Sanz, C.; Álvarez, E. Storage and Utilization of Glycogen by Mouse Liver during Adaptation to Nutritional Changes Are Glp-1 and Pask Dependent. Nutrients 2021, 13, 2552. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Lo, C.F.; Ritchie, B.; Harms, C.A.; Rotstein, D.S.; Han, S.; Hassan, S.M.; Lehner, A.F.; Buchweitz, J.P.; Thayer, V.G.; et al. Anthropogenic Contaminants and Histopathological Findings in Stranded Cetaceans in the Southeastern United States, 2012–2018. Front. Mar. Sci. 2020, 7, 630. [Google Scholar] [CrossRef]
- Gupta, P.K. Herbicides and Fungicides. In Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014; pp. 409–431. [Google Scholar] [CrossRef]
- Carabias Martínez, R.; Rodríguez Gonzalo, E.; Fernández Laespada, M.E.; Sánchez San Román, F.J. Evaluation of Surface- and Ground-Water Pollution Due to Herbicides in Agricultural Areas of Zamora and Salamanca (Spain). J. Chromatogr. A 2000, 869, 471–480. [Google Scholar] [CrossRef]
- Kesik, H.K.; Kilinc, S.G.; Celik, F.; Simsek, S.; Ahmed, H. A Case-Study of the Molecular Diagnosis of Echinococcus Multilocularis in Wild Boar with Comments on Its Public Health Significance in Turkey. J. Parasitol. 2020, 106, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, B.; Mansouri, M.; Noorpisheh-Ghadimi, S.; Abdolahi-Khabisi, S.; Doshmanziari, A. Molecular Evaluation of a Case of Fasciola Hepatica in Wild Boar in Southwestern Iran: A Case Report. Iran J. Parasitol. 2018, 13, 149. [Google Scholar] [PubMed]
- Samuel, W.M.; Pybus, M.J.; Kocan, A.A. Infectious Diseases of Wild Mammals, 3rd ed.; Williams, E.S., Barker, I.K., Eds.; Iowa State University Press: Ames, IA, USA, 2001; ISBN 081382978X. [Google Scholar]
- Nyindo, M.; Lukambagire, A.H. Fascioliasis: An Ongoing Zoonotic Trematode Infection. BioMed Res. Int. 2015, 2015, 786195. [Google Scholar] [CrossRef]
- Bendall, R.P.; Barlow, M.; Betson, M.; Stothard, J.R.; Nejsum, P. Zoonotic Ascariasis, United Kingdom. Emerg. Infect. Dis. 2011, 17, 1964. [Google Scholar] [CrossRef]
- Jeandron, A.; Rinaldi, L.; Abdyldaieva, G.; Usubalieva, J.; Steinmann, P.; Cringoli, G.; Utzinger, J. Human Infections with Dicrocoelium Dendriticum in Kyrgyzstan: The Tip of the Iceberg? J. Parasitol. 2011, 97, 1170–1172. [Google Scholar] [CrossRef]
- Vieira-Pinto, M.; Morais, L.; Caleja, C.; Themudo, P.; Aranha, J.; Torres, C.; Igrejas, G.; Poeta, P.; Martins, C. Salmonella Spp. in Wild Boar (Sus scrofa): A Public and Animal Health Concern. In Game Meat Hygiene in Focus; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 131–136. [Google Scholar] [CrossRef]
- Rivera, L.S.; Rivera, I.F.; Toro, D.H.; Gutierrez, J.; Acosta, E. Eosinophilic Liver Infiltration. ACG Case Rep. J. 2015, 3, 63. [Google Scholar] [CrossRef]
- Novosel, D.; Tuboly, T.; Csagola, A.; Lorincz, M.; Cubric-Curik, V.; Jungic, A.; Curik, I.; Segalés, J.; Cortey, M.; Lipej, Z. Origin of Porcine Circovirus Type 2 (PCV2) from Swine Affected by PCV2-Associated Diseases in Croatia. Vet. Rec. 2014, 174, 431. [Google Scholar] [CrossRef]
- Segalés, J.; Allan, G.M.; Domingo, M. Porcine Circovirus Diseases. Anim. Health Res. Rev. 2005, 6, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Luge, E.; Guerra, B.; Wittschen, P.; Gruber, A.D.; Loddenkemper, C.; Schneider, T.; Lierz, M.; Ehlert, D.; Appel, B.; et al. Leptospirosis in Urban Wild Boars, Berlin, Germany. Emerg. Infect. Dis. 2007, 13, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Vale-Goncąlves, H.M.; Cabral, J.A.; Faria, M.C.; Nunes-Pereira, M.; Faria, A.S.; Veloso, O.; Vieira, M.L.; Paiva-Cardoso, N. Prevalence of Leptospira Antibodies in Wild Boars (Sus scrofa) from Northern Portugal: Risk Factor Analysis. Epidemiol. Infect. 2015, 143, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Storck, C.H.; Postic, D.; Lamaury, I.; Perez, J.M. Changes in Epidemiology of Leptospirosis in 2003–2004, a Two El Niño Southern Oscillation Period, Guadeloupe Archipelago, French West Indies. Epidemiol. Infect. 2008, 136, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Michna, S.W.; Campbell, R.S.F. Leptospirosis in Wild Animals. J. Comp. Pathol. 1970, 80, 101–106. [Google Scholar] [CrossRef]
- Boes, K.M.; Durham, A.C. Bone Marrow, Blood Cells, and the Lymphoid/Lymphatic System. In Pathologic Basis of Veterinary Disease Expert Consult; Zachary, J., Ed.; Mosby: Maryland Heights, MO, USA, 2017; pp. 724–804.e2. ISBN 9780323357975. [Google Scholar]
| Lung | Liver | Kidney | ||||||
|---|---|---|---|---|---|---|---|---|
| Lesions | N | % | Lesions | N | % | Lesions | N | % |
| Lymphoid hyperplasia | 26 | 36.1 | Cellular changes: cell swelling; hydropic change; vacuolar change | 24 | 33.3 | Non-purulent nephritis | 16 | 22.2 |
| Agonic hemorrhage | 25 | 34.7 | centrolobular | 1 | 1.4 | Congestion | 11 | 15.3 |
| Verminous bronchitis | 25 | 34.7 | panlobular | 8 | 11.1 | Renal steatosis | 2 | 2.8 |
| Atelectasis | 14 | 19.4 | diffuse/not specified | 15 | 20.8 | Chronic interstitial nephritis | 2 | 2.8 |
| Congestion | 6 | 8.3 | Congestion | 12 | 16.7 | Basal membrane thickening | 2 | 2.8 |
| Emphysema | 4 | 5.6 | Increase of eosinophils | 11 | 15.3 | Hypercellular glomeruli | 2 | 2.8 |
| Alveolar proteinosis | 1 | 1.4 | Increase of other white cells | 5 | 6.9 | Cellular infiltration | 2 | 2.8 |
| Silicates | 1 | 1.4 | Hemosiderin | 4 | 5.6 | Hemosiderin | 2 | 2.8 |
| Hemosiderin | 1 | 1.4 | Granulomatous hepatitis | 1 | 1.4 | Purulent nephritis | 1 | 1.4 |
| Subacute hepatitis | 1 | 1.4 | ||||||
| Steatosis | 1 | 1.4 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jota Baptista, C.; Gonzalo-Orden, J.M.; Merino-Goyenechea, L.J.; Oliveira, P.A.; Seixas, F. Wild Boars (Sus scrofa, L. 1758) from Castile and Leon Region (Spain): A Histopathology Survey. Animals 2022, 12, 3282. https://doi.org/10.3390/ani12233282
Jota Baptista C, Gonzalo-Orden JM, Merino-Goyenechea LJ, Oliveira PA, Seixas F. Wild Boars (Sus scrofa, L. 1758) from Castile and Leon Region (Spain): A Histopathology Survey. Animals. 2022; 12(23):3282. https://doi.org/10.3390/ani12233282
Chicago/Turabian StyleJota Baptista, Catarina, José M. Gonzalo-Orden, Luís J. Merino-Goyenechea, Paula A. Oliveira, and Fernanda Seixas. 2022. "Wild Boars (Sus scrofa, L. 1758) from Castile and Leon Region (Spain): A Histopathology Survey" Animals 12, no. 23: 3282. https://doi.org/10.3390/ani12233282
APA StyleJota Baptista, C., Gonzalo-Orden, J. M., Merino-Goyenechea, L. J., Oliveira, P. A., & Seixas, F. (2022). Wild Boars (Sus scrofa, L. 1758) from Castile and Leon Region (Spain): A Histopathology Survey. Animals, 12(23), 3282. https://doi.org/10.3390/ani12233282







