Simple Summary
Respiratory diseases in cattle are multifactorial diseases caused by many pathogenic viruses and bacteria that cause economic losses both directly and indirectly. The objectives of this study were to assess the dead or culling and reproductive performances in heifer calves with different isolated viruses of bovine respiratory disease. We collected nasal swabs from calves that had respiratory signs in Chiang Mai Province, Thailand, and data with respect to age, farm type, and respiratory signs were recorded on the date of sample collection; thereafter, the data on the dead or culling, mortality, and reproductive performance of the BRD calves were collected. This study shows that the virus that causes the respiratory disease mixes with bovine viral diarrhoea virus (BVDV), influenza C (ICV), and influenza D virus (IDV). The calves with mucous secretion and at a younger age had higher risks of culling or death. On the other hand, the calves with IDV had a lower risk of culling. For the reproductive efficiency of BRD calves, we did not find any relationship between individual virus exposure and reproductive performance.
Abstract
Both influenza C (ICV) and influenza D (IDV) viruses were recently included as bovine respiratory disease (BRD) causes, but their role in BRD has not been evaluated. Therefore, the mortality and reproductive performances of BRD calves with different isolated viruses were determined in this study. Data on 152 BRD calves with bovine viral diarrhoea virus (BVDV), bovine respiratory syncytial virus (BRSV), bovine coronavirus (BCoV), bovine parainfluenza virus 3 (BPIV-3), ICV, or IDV from nasal swab samples using real-time rt-PCR were used. The general data and respiratory signs were recorded immediately, and thereafter, the data on dead or culling calves due to BRD and reproductive performance were collected. The percentages of the BRD calves were 71.7%, 52.6%, 40.8%, 10.5%, 68.4%, and 65.8% for BVDV, BRSV, BCoV, BPIV-3, ICV, and IDV, respectively. Mucous secretion (OR = 4.27) and age ≤ 6 months (OR =14.97) had higher risks of mortality than those with serous secretion and older age. The calves with IDV had lower risks of culling than those without IDV (OR = 0.19). This study shows that most viral infections in BRD calves are a combination of viruses with BVDV, ICV, and IDV. In addition, IDV might have a role in reducing the severity of BRD calves.
1. Introduction
The longer raising of heifers causes unforeseen cost losses related to investment, the value of the animal, the opportunity cost of other management operations, and costs incurred by heifers that have ineffective reproductive systems, which commonly range from USD 1700 to USD 2400 [1]. Bovine respiratory disease (BRD) is a multifactorial disorder caused by many viral and bacterial infectious pathogens affecting the respiratory system [2]. The productivity impairment caused by BRD in dairy heifers includes decreased growth rates, increased culling rate, increased age at first service and first calving, and subsequently poor performance and reduced milk production in the first lactation [3,4,5].
The most common viral pathogens of BRD are bovine viral diarrhoea virus (BVDV), bovine coronavirus (BCoV), bovine respiratory syncytial virus (BRSV), bovine herpesvirus-1 (BHV-1), bovine parainfluenza virus 3 (BPIV-3), influenza C virus (ICV), and influenza D virus (IDV) [6,7,8]. BVDV causes chronic and persistent infection by establishing itself in immuno-privileged sites, such as the respiratory tract, ovaries, and testes, which affects reproductive performance in mature cattle [9,10]. BPIV-3 causing asymptomatic to severe pneumonia can predispose the animal to other viruses and bacterial infections, and the subsequent infections are important components of enzootic pneumonia and BRD in calves and heifers [11]. Both influenza C and influenza D viruses have been recently included as BRD causes in North America [7,12,13], Europe [14,15], and East Asia [16,17]. Although infection with ICV and IDV causes only mild respiratory disease in infected calves, the impact on the economy and productivity of dairy cows is in doubt.
The effective control of BRD requires a combination of definitive diagnosis, efficacious BRD vaccines, appropriate treatment, and proper management practice [18]. In many tropical countries, including Thailand, control and eradication programs related to BRD have not been officially introduced to dairy farmers. In a report by the Bureau of Biotechnology on Livestock production from 2019 to 2021, the index of reproductive efficiency in replacement heifers in Thailand remained low, and the age of the first service was from 23.9 to 24.2 months, the age of first calving was from 33.6 to 33.8 months, and the first service conception rate was 26.8% [19], which might be due to tropical crossbred heifers reaching puberty later than purebred heifers in temperate regions [20]. However, information on the BRD-causing virus affecting the performances of heifers in this area is limited, and BRD might be an additional cause of low reproductive performance.
Therefore, the mortality of BRD calves in outbreak farms, i.e., the calves aged less than one year that exhibited respiratory signs related to the different viruses isolated, was determined in this study. In addition, the relationship between viruses and reproductive performance using days to first insemination and days to pregnancy was evaluated.
2. Materials and Methods
2.1. Farm Selection, Samples, and Data Collection
All animal care and sample collections were approved by the Institutional Animal Care and Use Committee at the Faculty of Veterinary Medicine, Chiang Mai University (approval no. S3/2565).
This study was conducted from December 2020 to June 2021 using smallholder dairy farms with outbreaks of respiratory syndromes in calves aged 1 month to 1 year old in Maeon, Doiloh, and Chaiprakan Districts, Chiang Mai Province, Thailand. The farms which voluntarily participated in the study had 10 to 40 milking cows with mostly crossbred Holstein Friesian, and all the farms participated in the veterinary herd health management program of the Department of Livestock Development, Ministry of Agriculture and Cooperatives for more than five years. The BRD calves were included in this study if they exhibited respiratory signs, such as sneezing, coughing, fever (body temperature > 103°F), and serous or mucous nasal secretion. The outbreak of BRD on the farm was determined when there were more than two BRD calves at the same time. After an outbreak, the farmers informed a researcher (D.S.) and asked her to collect nasal secretion samples from the BRD calves using nasal cavity swabs. Subsequently, the swab samples were inserted into a transport tube containing viral transport media, kept on ice, and transported to the laboratory of the Faculty of Veterinary Medicine, Chiangmai University, within 24 h; then, they were kept at −80 °C until use. During the six months after sample collection, the participating farmers were responsible for reporting the mortality of the BRD calves, including the calves that died or were culled for reasons associated with BRD. The data on age, farm type, and respiratory signs were recorded on the date of sample collection. In May 2022, the follow-up data on mortality and the reproductive performance of the BRD calves were collected from the cow history sheets of the farms.
2.2. RNA Extraction and cDNA Synthesis
Viral RNA was extracted from a separate aliquot of each sample using a NucleoSpin® RNA virus kit and cDNA synthesis by using a ReverTra Ace® qPCR RT master mix, following the manufacturer’s instructions. The cDNA amplification reaction system was set at 20 μL, including 5 × RT Master Mix 4 at μL, and RNA template at 2 µg, and nuclease-free water was added to make the final volume 20 μL. The cDNA synthesis cycle was programmed as follows: 37 °C for 15 min, 50 °C for 5 min, 98 °C for 5 min, and finally held at 4 °C. The cDNA product was stored at −20 °C for use in the real-time rt-PCR.
2.3. Real-Time RT-PCR for Virus Detection
The cDNA product of each sample was tested for bovine viral diarrhoea virus (BVDV), bovine respiratory syncytial virus (BRSV), bovine parainfluenza-3 virus (BPIV-3), bovine coronavirus (BCoV), influenza C virus (ICV), and influenza D virus (IDV) by performing real-time rt-PCR. Real-time rt-PCR assays were performed on a 7500 Fast real-time PCR System (Applied Biosystems, Foster City, CA, USA) using a SensiFAST™ SYBR® Hi-ROX Kit according to the manufacturer’s instructions. Briefly, the reaction system used for PCR amplification was 20 μL, consisting of 2x SensiFAST SYBR® Hi-ROX Mix 10 μL, 10 μM of each primer, 1 μL of template cDNA, and RNAse-free water at 20 μL. Primers were taken from a recent publication (Table 1). For BVDV, cycling conditions were 95 °C for 2 min, 40 cycles of 95 °C for 5 s, and 55 °C for 30 s. For BCoV and ICV, the cycling conditions were 95 °C for 2 min and 40 cycles of 95 °C for 5 s and 62 C for 30 s. For BRSV, the cycling conditions were 95 °C for 2 min, and 40 cycles of 95 °C for 5 s and 59 °C for 30 s. For BPIV-3, the cycling conditions were 95 °C for 2 min and 40 cycles of 95 °C for 5 s and 55 °C for 62 s. For IDV, the cycling conditions were 95 °C for 2 min and 40 cycles of 95 °C for 5 s, and 64 °C for 30 s. Testing positive for any respiratory viral diseases was determined when their CT values were 1–40, and the CT values of more than 40 were determined as negative [21].

Table 1.
Sequences of the primers used to detect viral pathogens in this study.
2.4. Statistical Analysis
The data on cattle characteristics were described by numbers and percentages. The BRD calves without any detection of the defined viruses were excluded due to unknown causes of diseases. The ages of the calves with BRD were categorised into ≤ 6 months and 7–12 months. The associations between the viruses, including BVDV, BCoV, BRSV, BPIV-3, ICV, and IDV, and different percentages of calves with fever, types of secretion (serous or mucous), and diarrhoea in the viral diseases were determined using Fisher’s Exact Chi-square test.
Mortality was determined when the BRD calves died or were culled from their severe illness for reasons associated with BRD within six months after infection. The associations of ages, fever, mucous secretion, diarrhoea, and viruses with mortality were described using Fisher’s Exact Chi-square test. The variables with p < 0.20 were selected for further analyses by using a generalised estimating equation, a method for modelling clustered binary data, using the Genmod procedure (SAS Institute Inc., Cary, NC, USA). The cattle nested in the same farm were considered as the repeated factors. To define the factors associated with mortality, variables with p < 0.1 were entered and subsequently stayed in the final model.
The reproductive performances of the BRD calves were determined using Kaplan–Meier survival statistics. The ages to the first artificial insemination (AI) and the ages to pregnancy were survival times in which the BRD calves that were not AI or pregnant within two years were censored cases, and their survival times were 730. For the other censored cases, the age at the end of follow-up, such as the age at the culling date or the age at the last AI but not pregnant, was the survival time. The estimated median values of the reproductive performances between the cattle with and without various viral diseases were described and compared using the generalised Wilcoxon test. The significance level and the tendency were defined at p < 0.05 and p < 0.10, respectively.
3. Results
Of the total of 168 BRD calves, 16 (9.5%) were excluded due to undefined viral causes of disease. The percentages of the BRD calves were 71.7%, 52.6%, 40.8%, 10.5%, 68.4%, and 65.8% for BVDV, BRSV, BCoV, BPIV-3, ICV, and IDV, respectively. Only 22 BRD calves (14.5%) had only one infection, including BVDV (10/109) and BRSV (5/80), which had the highest single infection rates. The values of the highest combination infection prevalence were 12.5% (n = 19) for BVDV + BRSV + BCoV + ICV + IDV, 9.9% (n = 15) for BVDV + BRSV + ICV + IDV, 8.6% (n = 13) for both BVDV + BCoV + ICV + IDV and BVDV + ICV + IDV, and 7.2% (n = 11) for ICV + IDV. Differences in the percentages of respiratory signs of the viral diseases are shown in Table 2, including fever (7.9%), diarrhoea (55.3%), and mucous secretion (67.8%).

Table 2.
Comparison of percentages of signs in the viral disease of bovine respiratory disease (BRD) calves, the <1-year calves with respiratory syndromes. * and ** indicate a difference between with (Yes) and without the disease (No) at p < 0.1 and p < 0.05, respectively.
The BRD calves with BVDV or BCoV had diarrhoea more than those without both diseases. The BRD calves with BVDV tended to have a higher proportion of the sign of the presence of mucous nasal secretion than those without BVDV (p = 0.056). In addition, the BRD calves with BCoV had a closed tendency of a higher percentage of mucous secretion than those without BCoV (p = 0.11). The percentages of pairwise diseases occurring together are shown in Table 3. Influenza D virus occurrence had chances of coinfection with either ICV (p < 0.01) or BCoV (p = 0.11). The BRD calves with BVDV were at a higher risk of being coinfected with BCoV (p = 0.10).

Table 3.
Percentages of a virus (row) to infect together with (Yes) or without (No) another virus (column). * and ** indicate associations among both virus, row, and column, at p < 0.10 and 0.05, as a tendency and significant levels, respectively.
After infection, 18 BRD calves (11.8%) died or were culled within six months after incurring the disease, and the days ranged from 18 to 177 days with an average of 96.8 days. All BRD calves died or were culled from severe weakness problems, including severe respiratory problems (n = 11), bloating (n = 2), and comorbidity (n = 5). Comparisons of the mortality rates of the BRD calves with different signs and their detected viruses are shown in Figure 1. The age at BRD (p < 0.05), BVDV infection (p = 0.10), ICV (p < 0.05), and IDV (p < 0.05) were associated with culling rates. The calves aged ≤ 6 months had higher culling rates than older calves (Figure 1A), and the calves with BVDV tended to be culled more than those without BVDV (Figure 1B). In contrast, the calves with ICV and IDV had lower mortality rates (Figure 1B).
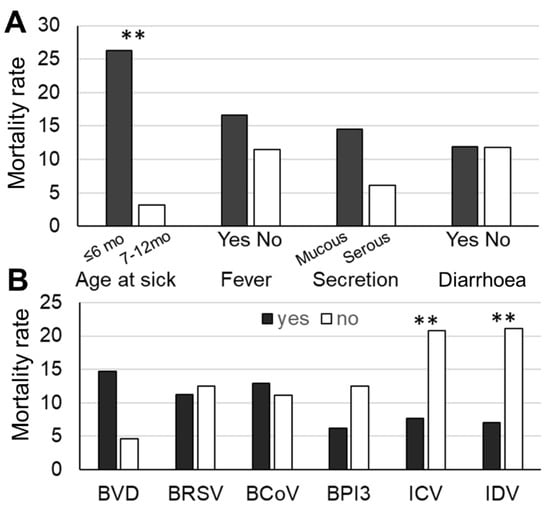
Figure 1.
Comparisons of mortality rates of bovine respiratory disease (BRD) calves between with and without factors (A) and virus detection (B). ** indicate different culling rates at p < 0.05, respectively. BVDV: bovine viral diarrhoea virus; BRSV: bovine respiratory syncytial virus; BCoV: bovine coronavirus; BPIV-3: bovine parainfluenza virus 3; ICV: influenza C virus; IDV: influenza D virus.
The final model of factors associated with the mortality of BRD calves is shown in Table 4, including the type of nasal secretion, the detection of IDV, and the age of BRD. The calves with mucous secretion (OR = 4.27) and the BRD calves ≤ 6 months of age (OR = 14.97) had a higher risk of mortality than those with serous secretion and older age. The BRD calves with IDV had a lower risk of culling compared with those without IDV (OR = 0.19).

Table 4.
Factors associated with the mortality rate of bovine respiratory disease (BRD) calves.
Results for the Kaplan–Meier analysis showed that, in terms of the median times of age, 50% of the BRD calves having the first AI and pregnancy were 572 and 710, respectively, as shown in Figure 2. The BRD calves with BVDV detection had a higher probability to have the first AI (p = 0.10) and pregnancy (p < 0.05) earlier than the calves without BVDV. The median ages of the BRD calves with and without BVDV were 572 and 521 days old for having AI and 683 and >730 days old for pregnancy, respectively. No other factor was found to be significant.
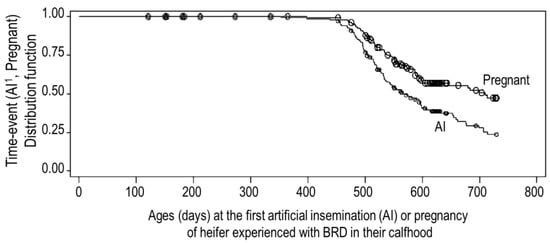
Figure 2.
Kaplan–Meier distribution function of ages at the first artificial insemination (AI) and pregnancy of the bovine respiratory disease (BRD) calves.
4. Discussion
Due to the significant economic impact of BVDV on cattle production, many countries, including Austria, Belgium, Denmark, England, Finland, Germany, Ireland, Norway, Poland, Scotland, Sweden, Switzerland, the Netherlands, the USA, and Wales, have implemented compulsory or voluntary control and/or eradication programs [27]. Monitoring with ELISA test kits showed that BVDV is a globally spreading pestivirus affecting cattle and other ruminants, for example, in Thailand [28] and China [29]. However, many countries in the world, including Thailand, do not have a national policy for the prevention and control of BVDV and other viral BRD, although BVDV was discovered in Thailand in 1997 [30]. The high number of clinical cases in our study does not fall outside our expectations. Without any BVDV management programs in Thailand, most cases of the respiratory syndromes observed in this study were likely associated with those calves that were persistently infected (PI) due to the expected BVDV infection during the first trimester of pregnancy [31]. In addition, the design of this study aimed to determine the subsequent association among the signs, viruses, mortality rate, and reproductive performance of the calves experiencing respiratory signs. Therefore, the studied population was only the BRD calves with the specified viral infection. Any application of the results of this study must be carefully considered and only extrapolated to the BRD population.
Among the viruses causing BRD, all RNA viruses, including newly defined viruses such as ICV and IDV, were included in this study [13,32]; therefore, bovine herpesvirus-1, a double-stranded DNA virus, was excluded due to the limitations of the viral genome extraction method. The BRD has many causes and complexities, but controlling BVDV is important for suppressing secondary infections from other related BRD pathogens with respect to the immune system [33]. Therefore, mixed infections were expectedly found to be more frequent (85.5%), with the highest infections observed with BVDV (71.7%), ICV (68.4%), and IDV (65.8%) in this study. The patterns of mixed viral infections in our study were BVDV + BRSV + BCoV + ICV + IDV, BVDV + BRSV + ICV + IDV, BVDV + BCoV + ICV + IDV, BVDV + ICV + IDV, and ICV + IDV. In Mexico, mixed viral pathogens, including BVDV, BRSV, and BPIV-3, were identified in over 50% of the clinically diseased lungs of calves [34]. BRSV and BCoV also highly contributed to the cause of BRD calves in this study [35,36]. Clinical respiratory disease associated with BCoV in combination with BRSV has been reported in Danish dairy calves [37], while a study in the USA by Fulton et al. also found BCoV associated with BRSV and BoHV1 infections [38]. This is the first detected ICV and IDV in dairy cattle in tropical countries, with the prevalence of ICV and IDV at 68.4%, and 65.8%, respectively. Both ICV and IDV have been first detected in BRD cattle in North America [39,40]. As found in this study, the most common viruses found to be in coinfected with ICV and IDV were BVDV [7,41,42].
The symptoms in BRD cases vary depending on the virus cause, starting from no signs, mild respiratory signs, and severe systemic signs with fever, mucous discharges, and diarrhoea. In our study, 7.9%, 55.3%, and 67.8% of the BRD calves had fevers, mucous discharge, and diarrhoea, respectively. The BRD calves with BVDV and BCoV detected were associated with diarrhoea, and BVDV was also significantly associated with mucous nasal discharge (Table 2). BVDV is clinically presented in multiple organs, including those of the respiratory, gastrointestinal, and reproductive systems [43]. Diarrhoea in calves with BCoV infection is caused by necrotic enterocolitis with a loss of villi causing malabsorption diarrhoea [44].
From this study, 11.8% of the BRD calves died or were culled by severe respiratory problems (61%), other infectious diseases (28%), and digestive problems (11%). In 2020, BRD morbidity and mortality in the USA, where the control program has been intensively applied, were 18% and 2.1%, respectively [45], but this was not the case for the uncontrolled BRDs in this study. Our study’s results were within the range of the data from the USA from 1987 to 2001, in which mortality rates, including calves that died or were culled for reasons associated with the BRDs of feedlot calves, ranged between 0.1 and 8.9 depending on their breed [46]. In 2021, the mean cumulative BRD mortality incidence in beef calves ranged between 1 and 5 depending on the maximum temperatures and wind speeds [47]. The differences in this mortality might be caused by the fact that all the animals used in this study showed clinical respiratory signs. In Table 4, three factors, including the presence of mucous nasal secretion, the detection of IDV, and the age at sickness, were related to culling in these BRD calves. In BRD, serous nasal discharge is common in the early stages, and it turns mucopurulent as the condition progresses. As the disease progresses, dyspnoea becomes more pronounced and animals may adopt a typical stance with an extended neck, drooling with open-mouth breathing, and soft coughing. Effective mucus clearances are essential for lung health, and airway disease is a consistent consequence of poor clearance, causing pneumonia [48]. One of the highest mortality causes of BRD is severe pneumonia [49]. The virus detected from the nasal swabs in this study might not actually cause severe pneumonia of BRD related to the subsequent dead heifer calves. Primary viral infection impairing host defences and predisposing to secondary bacterial infection is preeminent pathogenesis [18]. Determining the actual causes of mortality, especially related to severe pneumonia, may need to define the pathogens from the lower respiratory tracts and lung samples collected via other collection methods, for example, post-mortem lung tissue specimens [6,13,34], pharyngeal swabs [7], and bronchoalveolar lavage fluid [8]. In this study, the BRD calves with symptoms at age ≤ 6 months had more mortality rates than older calves. In addition, many studies showed that younger calves indicated by no-weaned calves [47], and those aged between 1 day and 6 months [50] were more at risk for culling or death than weaned calves and older calves.
In general, cattle that have higher viral loads of IDV and ICV also have greater numbers of coinfecting viruses than controls, suggesting that ICV and IDV may be significant contributors to BRDC [7]. This study is the first to determine the clinical role of IDV in BRD calves. Interestingly, IDV was found to decrease the risk of mortality in the BRD calves in this study. This is consistent with another study that used mice to show that IDV infections appeared to protect mice from the usual clinical features of secondary Staphylococcus aureus infections. IDV-infected mice improved their weight, survival rate, and recovery times compared with those infected only with S. aureus [51]. Infection with IDV decreases the susceptibility to secondary bacterial infection, as shown in the evidence that antiviral immune responses occurred after IDV infection to protect the host from potentially fatal outcomes. In addition, Robinson and others demonstrated that determining the immune response to IDV infection beneficially affected the prevention of the severity of the influenza A virus (IAV) [52]. IDV-IAV-infected mice showed faster and stronger IFN-β responses than IAV-infected mice alone. Moreover, their results show that active IDV infection induces IFN type 1-dependent protection against IAV-associated weight loss and mortality.
From Figure 2, it can be observed that the BRD calves had overall ages to the first AI and pregnancy of 572 (19 months) and 710 (23 months), respectively, and these would subsequently calve at the age of about 30 months. A recent review of UK dairy herd data showed that the average age at first calving was 29 months (median 28 months) [53], and this seemed significantly greater than the target of 22–24 months, which is often quoted in the press [54]. In contrast, a report using 68,555 cows in Thailand shows that the ages at first service and calving at 22.9 and 32.9 months, respectively [20], were higher than those observed in our report here. The participation in the veterinary herd health management program of all the farms in this study might be the reason for the better reproductive performance compared with other farms in Thailand, but the remaining poor reproductive performance might be related to the respiratory syndrome incurred during calfhood. In the current study, the occurrence of BRD with BVDV detection was probably associated with having the first AI and pregnancy at a younger age than in heifers without BVDV. In comparison to BRD-free calves, the calves with BVDV infections had more impaired reproductive performances [55,56], especially due to their harmful impact on the developing foetus at all stages, but more severe consequences occur during early gestation [57]. As all heifers had experiences with BRD during their calfhood, the reproductive performances of BRD calves with BVDV in this study were better than that of BRD calves with other virus infections.
5. Conclusions
In conclusion, this study is the first to investigate the role of ICV and IDV in BRD calves. We found that most viral infections in BRD calves were a combination of viruses with BVDV, ICV, and IDV, and factors such as the presence of mucous nasal secretion, the detection of IDV, and age at sickness were related to culling in BRD calves. In addition, IDV might play an important role in reducing the severity of other viral or secondary bacterial infections in BRD calves. Therefore, further research on IDV is needed and should include the pathophysiology and correlations with other viruses and/or bacterial infections in BRD calves.
Author Contributions
Conceptualisation, W.S. and D.S.; methodology, W.S., P.C., T.P. and D.S.; laboratory analysis, P.C. and D.S.; formal analysis, W.S., data curation, W.S. and D.S.; writing—original draft preparation, W.S. and D.S.; writing—review and editing, W.S. and D.S.; funding acquisition, W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research and Researchers for Industries Ph.D. scholarship program (National Research Council of Thailand), grant number PHD62I0016, and Hausen Bernstein Co., Ltd.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Animal Care and Use Committee at the Faculty of Veterinary Medicine, Chiang Mai University (approval no. S3/2565).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to thank all dairy farmers and people who contributed to this study for their help in collecting samples. Center of Elephant and Wildlife Health, Chiang Mai University is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Overton, M.W.; Dhuyvetter, K.C. Symposium Review: An Abundance of Replacement Heifers: What Is the Economic Impact of Raising More than Are Needed? J. Dairy Sci. 2020, 103, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, S.A.; Van Eenennaam, A.L.; Karle, B.M.; Rossitto, P.V.; Lehenbauer, T.W.; Aly, S.S. Bovine Respiratory Disease (BRD) Cause-Specific and Overall Mortality in Preweaned Calves on California Dairies: The BRD 10K Study. J. Dairy Sci. 2019, 102, 7320–7328. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.R.; Ollivett, T.L.; Renaud, D.L.; Leslie, K.E.; LeBlanc, S.J.; Duffield, T.F.; Kelton, D.F. The Effect of Lung Consolidation, as Determined by Ultrasonography, on First-Lactation Milk Production in Holstein Dairy Calves. J. Dairy Sci. 2018, 101, 5404–5410. [Google Scholar] [CrossRef]
- Overton, M.W. Economics of Respiratory Disease in Dairy Replacement Heifers. Anim. Health Res. Rev. 2020, 21, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Buczinski, S.; Achard, D.; Timsit, E. Effects of Calfhood Respiratory Disease on Health and Performance of Dairy Cattle: A Systematic Review and Meta-Analysis. J. Dairy Sci. 2021, 104, 8214–8227. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.M.; More, S.J.; Sammin, D.; Casey, M.J.; McElroy, M.C.; O’Neill, R.G.; Byrne, W.J.; Earley, B.; Clegg, T.A.; Ball, H.; et al. Pathogens, Patterns of Pneumonia, and Epidemiologic Risk Factors Associated with Respiratory Disease in Recently Weaned Cattle in Ireland. J. Vet. Diagn. Invest. 2017, 29, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Nissly, R.H.; Zaman, N.; Ibrahim, P.A.S.; McDaniel, K.; Lim, L.; Kiser, J.N.; Bird, I.; Chothe, S.K.; Bhushan, G.L.; Vandegrift, K.; et al. Influenza C and D Viral Load in Cattle Correlates with Bovine Respiratory Disease (BRD): Emerging Role of Orthomyxoviruses in the Pathogenesis of BRD. Virology 2020, 551, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.H.S.; Dall Agnol, A.M.; Fritzen, J.T.T.; Lorenzetti, E.; Alfieri, A.A.; Alfieri, A.F. Microbial Diversity Involved in the Etiology of a Bovine Respiratory Disease Outbreak in a Dairy Calf Rearing Unit. Comp. Immunol. Microbiol. Infect. Dis. 2020, 71, 101494. [Google Scholar] [CrossRef]
- Voges, H.; Horner, G.W.; Rowe, S.; Wellenberg, G.J. Persistent Bovine Pestivirus Infection Localized in the Testes of an Immuno-Competent, Non-Viraemic Bull. Vet. Microbiol. 1998, 61, 165–175. [Google Scholar] [CrossRef]
- Kozasa, T.; Tajima, M.; Yasutomi, I.; Sano, K.; Ohashi, K.; Onuma, M. Relationship of Bovine Viral Diarrhea Virus Persistent Infection to Incidence of Diseases on Dairy Farms Based on Bulk Tank Milk Test by RT-PCR. Vet. Microbiol. 2005, 106, 41–47. [Google Scholar] [CrossRef]
- Ellis, J.A. Bovine Parainfluenza-3 Virus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.; Cernicchiaro, N.; Torres, S.; Li, F.; Hause, B.M. Metagenomic Characterization of the Virome Associated with Bovine Respiratory Disease in Feedlot Cattle Identified Novel Viruses and Suggests an Etiologic Role for Influenza D Virus. J. Gen. Virol. 2016, 97, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Porter, E.; Lohman, M.; Lu, N.; Peddireddi, L.; Hanzlicek, G.; Marthaler, D.; Liu, X.; Bai, J. Influenza C Virus in Cattle with Respiratory Disease, United States, 2016-2018. Emerg. Infect. Dis. 2018, 24, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Flynn, O.; Gallagher, C.; Mooney, J.; Irvine, C.; Ducatez, M.; Hause, B.; McGrath, G.; Ryan, E. Influenza D Virus in Cattle, Ireland. Emerg. Infect. Dis. 2018, 24, 389–391. [Google Scholar] [CrossRef]
- Dane, H.; Duffy, C.; Guelbenzu, M.; Hause, B.; Fee, S.; Forster, F.; McMenamy, M.J.; Lemon, K. Detection of Influenza D Virus in Bovine Respiratory Disease Samples, UK. Transbound. Emerg. Dis. 2019, 66, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Endoh, M.; Kobayashi, T.; Takenaka-Uema, A.; Chambers, J.K.; Uchida, K.; Nishihara, M.; Hause, B.; Horimoto, T. Influenza D Virus Infection in Herd of Cattle, Japan. Emerg. Infect. Dis. 2016, 22, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.-L.; Zhang, H.; Chen, S.-N.; Zhou, X.; Lin, T.; Liu, R.; Lv, D.-H.; Wen, X.-H.; Wei, W.-K.; Wang, D.; et al. Influenza D Virus in Animal Species in Guangdong Province, Southern China. Emerg. Infect. Dis. 2017, 23, 1392–1396. [Google Scholar] [CrossRef]
- Rice, J.A.; Carrasco-Medina, L.; Hodgins, D.C.; Shewen, P.E. Mannheimia Haemolytica and Bovine Respiratory Disease. Anim. Health Res. Rev. 2007, 8, 117–128. [Google Scholar] [CrossRef]
- Bureau of Biotechnology for Livestock Production, Ministry of Agriculture and Cooperatives. DLD Dairy Sire Summary 2022. Department of Livestock Development, Bangkok, Thailand; 30 April 2022. Available online: http://docimage.dld.go.th/fileroom/cabdld_bookshelf2/drawer26/general/data0000/00000088.pdf (accessed on 30 April 2022).
- Buaban, S.; Duangjinda, M.; Suzuki, M.; Masuda, Y.; Sanpote, J.; Kuchida, K. Short Communication: Genetic Analysis for Fertility Traits of Heifers and Cows from Smallholder Dairy Farms in a Tropical Environment. J. Dairy Sci. 2015, 98, 4990–4998. [Google Scholar] [CrossRef]
- Charoenvisal, N.; Keawcharoen, J.; Sreta, D.; Tantawet, S.; Jittimanee, S.; Arunorat, J.; Amonsin, A.; Thanawongnuwech, R. Experimental Infection with a Thai Reassortant Swine Influenza Virus of Pandemic H1N1 Origin Induced Disease. Virol. J. 2013, 10, 88. [Google Scholar] [CrossRef]
- Mahlum, C.E.; Haugerud, S.; Shivers, J.L.; Rossow, K.D.; Goyal, S.M.; Collins, J.E.; Faaberg, K.S. Detection of Bovine Viral Diarrhea Virus by TaqMan Reverse Transcription Polymerase Chain Reaction. J. Vet. Diagn. Invest. 2002, 14, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Boxus, M.; Letellier, C.; Kerkhofs, P. Real Time RT-PCR for the Detection and Quantitation of Bovine Respiratory Syncytial Virus. J. Virol. Methods 2005, 125, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Elia, G.; Campolo, M.; Desario, C.; Mari, V.; Radogna, A.; Colaianni, M.L.; Cirone, F.; Tempesta, M.; Buonavoglia, C. Detection of Bovine Coronavirus Using a TaqMan-Based Real-Time RT-PCR Assay. J. Virol. Methods 2008, 151, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Neill, J.D.; Saliki, J.T.; Landis, C.; Burge, L.J.; Payton, M.E. Genomic and Antigenic Characterization of Bovine Parainfluenza-3 Viruses in the United States Including Modified Live Virus Vaccine (MLV) Strains and Field Strains from Cattle. Virus Res. 2017, 235, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Faccini, S.; De Mattia, A.; Chiapponi, C.; Barbieri, I.; Boniotti, M.B.; Rosignoli, C.; Franzini, G.; Moreno, A.; Foni, E.; Nigrelli, A.D. Development and Evaluation of a New Real-Time RT-PCR Assay for Detection of Proposed Influenza D Virus. J. Virol. Methods 2017, 243, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Antos, A.; Miroslaw, P.; Rola, J.; Polak, M.P. Vaccination Failure in Eradication and Control Programs for Bovine Viral Diarrhea Infection. Front. Vet. Sci. 2021, 8, 688911. [Google Scholar] [CrossRef]
- Nilnont, T.; Aiumlamai, S.; Kanistanont, K.; Inchaisri, C.; Kampa, J. Bovine Viral Diarrhea Virus (BVDV) Infection in Dairy Cattle Herds in Northeast Thailand. Trop. Anim. Health Prod. 2016, 48, 1201–1208. [Google Scholar] [CrossRef]
- Hou, P.; Zhao, G.; Wang, H.; He, H. Prevalence of Bovine Viral Diarrhea Virus in Dairy Cattle Herds in Eastern China. Trop. Anim. Health Prod. 2019, 51, 791–798. [Google Scholar] [CrossRef]
- Virakul, P.; Suadsong, S.; Suwimonteerabutr, J.; Singlor, J. Prevalence of infectious bovine rhinotracheitis (IBR) (bovine herpesvirus 1), bovine diarrhea virus (BDV), parainfluenza-3 (PI-3) and bovine respiratory syncytial (BRS) viruses in Thai dairy farms. Thai J. Vet. Med. 1997, 27, 295–314. [Google Scholar]
- Stokstad, M.; Loken, T. Pestivirus in Cattle: Experimentally Induced Persistent Infection in Calves. J. Vet. Med. Series B 2002, 49, 494–501. [Google Scholar] [CrossRef]
- Luo, J.; Ferguson, L.; Smith, D.R.; Woolums, A.R.; Epperson, W.B.; Wan, X.-F. Serological Evidence for High Prevalence of Influenza D Viruses in Cattle, Nebraska, United States, 2003–2004. Virology 2017, 501, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Pardon, B.; De Bleecker, K.; Dewulf, J.; Callens, J.; Boyen, F.; Catry, B.; Deprez, P. Prevalence of Respiratory Pathogens in Diseased, Non-Vaccinated, Routinely Medicated Veal Calves. Vet. Rec. 2011, 169, 278. [Google Scholar] [CrossRef] [PubMed]
- Juarez Barranco, F.; Trigo Tavera, F.J.; Chavez Gris, G.; Vargas Garcia, R.E. Viral participation in respiratory disease in feedlot cattle, as identified by immunohistochemistry. Vet. Mex. 2003, 34, 1–12. [Google Scholar]
- Pratelli, A.; Lucente, M.S.; Cordisco, M.; Ciccarelli, S.; Di Fonte, R.; Sposato, A.; Mari, V.; Capozza, P.; Pellegrini, F.; Carelli, G.; et al. Natural Bovine Coronavirus Infection in a Calf Persistently Infected with Bovine Viral Diarrhea Virus: Viral Shedding, Immunological Features and S Gene Variations. Animals 2021, 11, 3350. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, B.; Sun, D. Advances in Bovine Coronavirus Epidemiology. Viruses 2022, 14, 1109. [Google Scholar] [CrossRef]
- Uttenthal, A.; Jensen, N.P.; Blom, J.Y. Viral Aetiology of Enzootic Pneumonia in Danish Dairy Herds: Diagnostic Tools and Epidemiology. Vet. Rec. 1996, 139, 114–117. [Google Scholar] [CrossRef]
- Fulton, R.W.; Step, D.L.; Wahrmund, J.; Burge, L.J.; Payton, M.E.; Cook, B.J.; Burken, D.; Richards, C.J.; Confer, A.W. Bovine Coronavirus (BCV) Infections in Transported Commingled Beef Cattle and Sole-Source Ranch Calves. Can. J. Vet. Res. 2011, 75, 191–199. [Google Scholar]
- Collin, E.A.; Sheng, Z.; Lang, Y.; Ma, W.; Hause, B.M.; Li, F. Cocirculation of Two Distinct Genetic and Antigenic Lineages of Proposed Influenza D Virus in Cattle. J. Virol. 2015, 89, 1036–1042. [Google Scholar] [CrossRef]
- Ferguson, L.; Eckard, L.; Epperson, W.B.; Long, L.-P.; Smith, D.; Huston, C.; Genova, S.; Webby, R.; Wan, X.-F. Influenza D Virus Infection in Mississippi Beef Cattle. Virology 2015, 486, 28–34. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Pelletier, C.; Meyer, G. Influenza D Virus in Cattle, France, 2011–2014. Emerg. Infect. Dis. 2015, 21, 368. [Google Scholar] [CrossRef]
- Chiapponi, C.; Faccini, S.; De Mattia, A.; Baioni, L.; Barbieri, I.; Rosignoli, C.; Nigrelli, A.; Foni, E. Detection of Influenza D Virus among Swine and Cattle, Italy. Emerg. Infect. Dis. 2016, 22, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Turkington, H.L.; Cosby, S.L. The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments. Vaccines 2021, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, G.Y.; Choy, H.E.; Hong, Y.J.; Saif, L.J.; Jeong, J.H.; Park, S.I.; Kim, H.H.; Kim, S.K.; Shin, S.S.; et al. Dual Enteric and Respiratory Tropisms of Winter Dysentery Bovine Coronavirus in Calves. Arch. Virol. 2007, 152, 1885–1900. [Google Scholar] [CrossRef] [PubMed]
- Blakebrough-Hall, C.; McMeniman, J.P.; González, L.A. An Evaluation of the Economic Effects of Bovine Respiratory Disease on Animal Performance, Carcass Traits, and Economic Outcomes in Feedlot Cattle Defined Using Four BRD Diagnosis Methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef] [PubMed]
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Bovine Respiratory Disease in Feedlot Cattle: Environmental, Genetic, and Economic Factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Wisnieski, L.C.; Amrine, D.E.; Cernicchiaro, N.; Sanderson, M.W.; Renter, D.G. Weather Conditions Associated with Death Attributed to Bovine Respiratory Disease Complex in High-Risk Auction Market-Sourced Male Beef Calves. Am. J. Vet. Res. 2021, 82, 644–652. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway Mucus Function and Dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Chang, Y.; Luo, H.; Brito, L.F.; Dong, Y.; Shi, R.; Wang, Y.; Dong, G.; Liu, L. Mortality-Culling Rates of Dairy Calves and Replacement Heifers and Its Risk Factors in Holstein Cattle. Animals 2019, 9, E730. [Google Scholar] [CrossRef]
- Brickell, J.S.; McGowan, M.M.; Pfeiffer, D.U.; Wathes, D.C. Mortality in Holstein-Friesian Calves and Replacement Heifers, in Relation to Body Weight and IGF-I Concentration, on 19 Farms in England. Animals 2009, 3, 1175–1182. [Google Scholar] [CrossRef]
- Skelton, R.M.; Shepardson, K.M.; Hatton, A.; Wilson, P.T.; Sreenivasan, C.; Yu, J.; Wang, D.; Huber, V.C.; Rynda-Apple, A. Contribution of Host Immune Responses Against Influenza D Virus Infection Toward Secondary Bacterial Infection in a Mouse Model. Viruses 2019, 11, E994. [Google Scholar] [CrossRef]
- Robinson, E.; Shepardson, K.M.; Skelton, R.M.; Hernandez, E.; Huber, V.C.; Rynda-Apple, A. Active Influenza D Coinfection Reduces Influenza A Disease Severity in Mice. J. Immunol. 2021, 206, 20. [Google Scholar]
- Eastham, N.T.; Coates, A.; Cripps, P.; Richardson, H.; Smith, R.; Oikonomou, G. Associations between Age at First Calving and Subsequent Lactation Performance in UK Holstein and Holstein-Friesian Dairy Cows. PLoS ONE 2018, 13, e0197764. [Google Scholar] [CrossRef] [PubMed]
- Steele, M. Age at First Calving in Dairy Cows: Which Months Do You Aim for to Maximise Productivity? Vet. Evid. 2020, 5, 2–22. [Google Scholar] [CrossRef]
- Cheng, Z.; Brown, L.E.; Wathes, D.C. Bovine Viral Diarrhoea Virus Infection Disrupts Uterine Interferon Stimulated Gene Regulatory Pathways During Pregnancy Recognition in Cows. Viruses 2019, 12, E1. [Google Scholar] [CrossRef]
- Rypuła, K.; Płoneczka-Janeczko, K.; Czopowicz, M.; Klimowicz-Bodys, M.D.; Shabunin, S.; Siegwalt, G. Occurrence of BVDV Infection and the Presence of Potential Risk Factors in Dairy Cattle Herds in Poland. Animals 2020, 10, E230. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, I.; Cerviño, M.; Martínez, S.; Fouz, R.; Diéguez, F.J. Bovine Viral Diarrhea Virus (BVDV) Infection: Effect on Reproductive Performance and Milk Yield in Dairy Herds. Vet. J. 2021, 277, 105747. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).