Global Warming and Long-Distance Spread of Invasive Discoglossus pictus (Amphibia, Alytidae): Conservation Implications for Protected Amphibians in the Iberian Peninsula
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
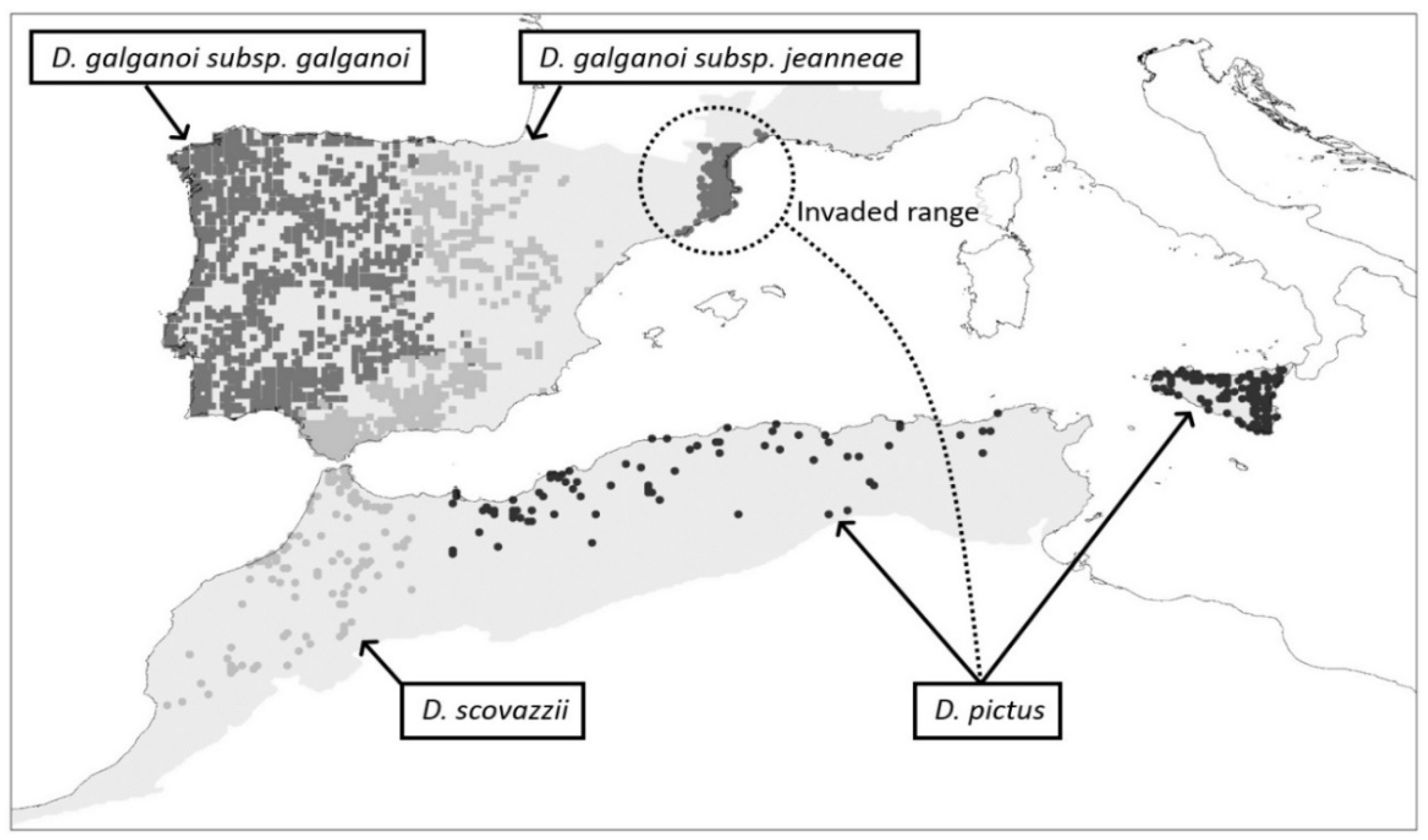
2.1. Study Area
2.2. Species Data
2.3. Environmental Data
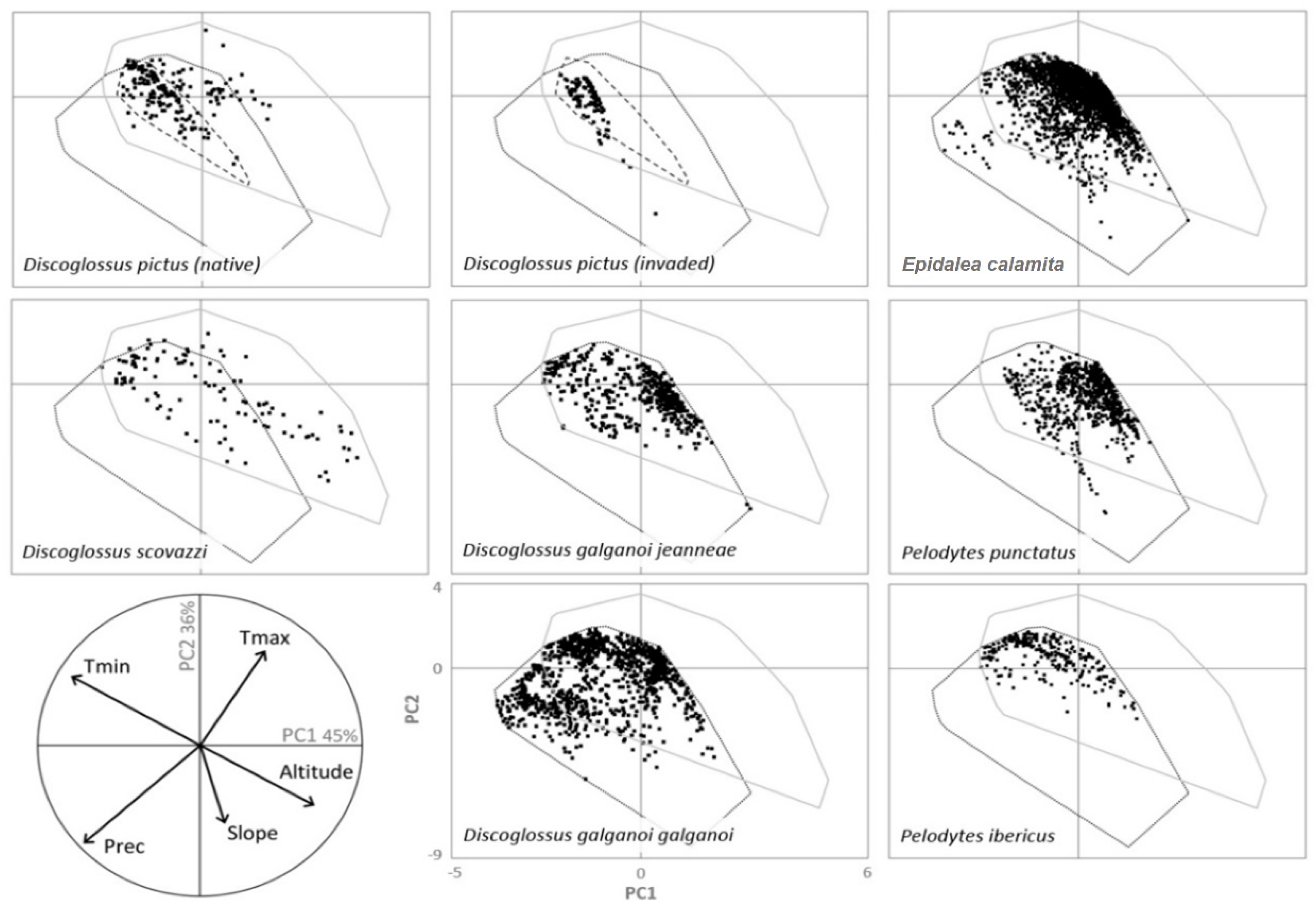
2.4. Niche Overlap Analysis
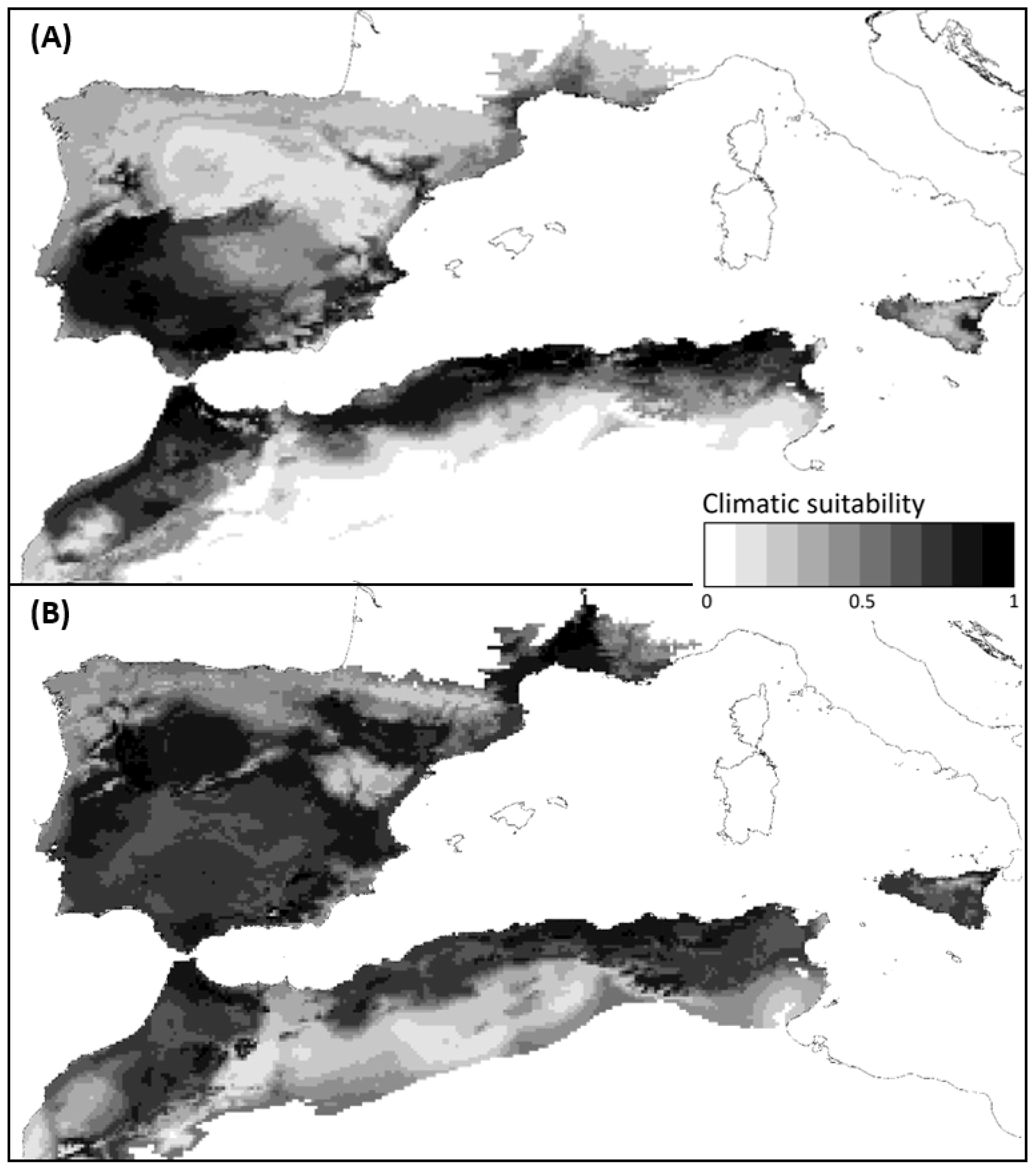
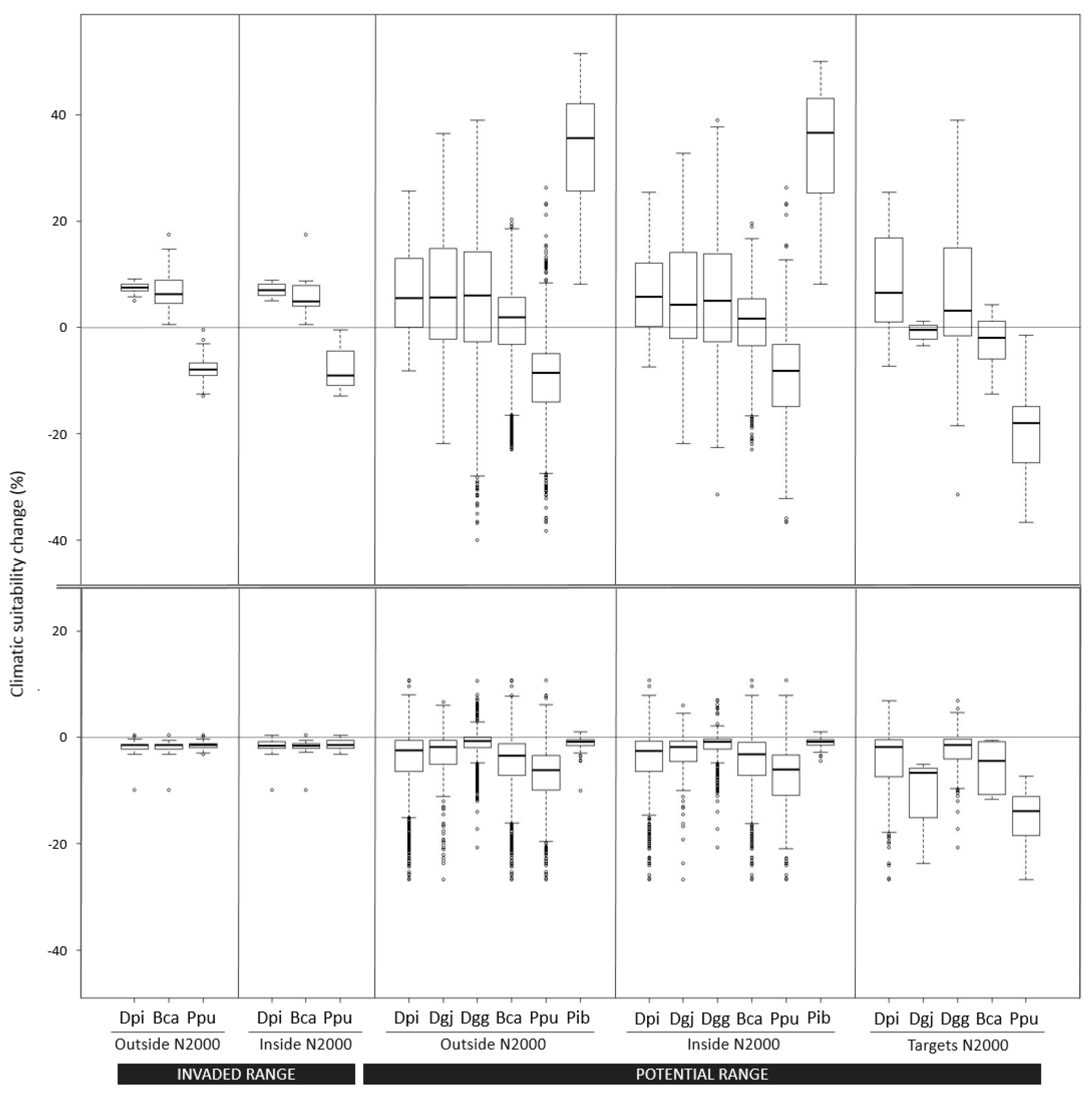
2.5. Climatic Suitability Changes
3. Results
3.1. Niche Overlap Analysis
3.2. Long-Term Climatic Suitability Changes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellard, C.; Leroy, B.; Thuiller, W.; Rysman, J.-F.; Courchamp, F. Major drivers of invasion risks throughout the world. Ecosphere 2016, 7, e01214. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark-Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Sax, D.F.; Stachowicz, J.J.; Brown, J.H.; Bruno, J.F.; Dawson, M.N.; Gaines, S.D.; Grosberg, R.K.; Hastings, A.; Holt, R.D.; Mayfield, M.M.; et al. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007, 22, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Dodd, C.K.; Seigel, R.A. Relocation, Repatriation, and Translocation of Amphibians and Reptiles: Are They Conservation Strategies That Work? Herpetologica 1991, 47, 336–350. [Google Scholar]
- Kraus, F. Impacts from Invasive Reptiles and Amphibians. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 75–97. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Liu, Z.; Tingley, R.; Kraus, F.; Guo, Z.; Li, Y. Congener diversity, topographic heterogeneity and human-assisted dispersal predict spread rates of alien herpetofauna at a global scale. Ecol. Lett. 2014, 17, 821–829. [Google Scholar] [CrossRef]
- Guijarro, L. La invasión silenciosa. Ambienta (Revista del Ministerio de Medio Ambiente) 2001, 1, 33–37. [Google Scholar]
- Wintrebert, P. Présence à Banyuls-sur-Mer (Pyrénées-Orientales) du Discoglossus pictus Otth. Bull. Soc. Zool. Fr. 1908, 33, 54. [Google Scholar]
- Fradet, V.; Geniez, P. La répartition du Discoglosse peint Discoglossus pictus Otth, 1837 (Amphibien, Anoure, Discoglossidés) dans le Sud de la France: Note sur sa présence dans le département de l’Hérault. Bull. Soc. Herp. Fr. 2004, 109, 35–41. [Google Scholar]
- Leblois, R.; Rousset, F.; Tikel, D.; Moritz, C.; Estoup, A. Absence of evidence or isolation by distance in an expanding cane toad (Bufo marinus) population: An individual-based analysis of microsatellite genotypes. Mol. Ecol. 2000, 9, 1905–1909. [Google Scholar] [CrossRef]
- Montori, A.; Llorente, G.A.; Richter-Boix, Á.; Villero, D.; Franch, M.; Garriga, N. Colonización y efectos potenciales de la especie invasora Discoglossus pictus sobre las especies nativas. Munibe 2007, 25, 14–27. [Google Scholar]
- Llorente, G.A.; Montori, A.; Pujol-Buxó, E. El sapillo pintojo mediterráneo (Discoglossus pictus) en la península ibérica. Boletín Asoc. Herpetológica Española 2015, 26, 15–19. [Google Scholar]
- Franch, M.; Llorente, G.A.; Montori, A.; Richter-Boix, A.; Carranza, S. Discovery of an Introduced Population of Discoglossus pictus Beyond its Known Distributional Range. Herpetol. Rev. 2007, 38, 356–359. [Google Scholar]
- Richter-Boix, A.; Llorente, G.A.; Montori, A. Responses to competition effects of two anuran tadpoles according to life-history traits. Oikos 2004, 106, 39–50. [Google Scholar] [CrossRef]
- Escoriza, D.; Boix, D. Assessing the potential impact of an invasive species on a Mediterranean amphibian assemblage: A morphological and ecological approach. Hydrobiologia 2012, 680, 233–245. [Google Scholar] [CrossRef]
- White, A.W.; Shine, R. The extra-limital spread of an invasive species via “stowaway” dispersal: Toad to nowhere? Anim. Conserv. 2009, 12, 38–45. [Google Scholar] [CrossRef]
- Kot, M.; Lewis, M.A.; Van Den Driessche, P. Dispersal data and the spread of invading organisms. Ecology 1996, 77, 2027–2042. [Google Scholar] [CrossRef]
- Araujo, M.B.; Thuiller, W.; Pearson, R.G.; Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Escoriza, D.; Boix, D. Reproductive habitat selection in alien and native populations of the genus Discoglossus. Acta Oecologica 2014, 59, 97–103. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Garriga, N.; Montori, A.; Franch, M.; San Sebastián, O.; Villero, D.; Llorente, G.A. Effects of the non-native amphibian species Discoglossus pictus on the recipient amphibian community: Niche overlap, competition and community organization. Biol. Invasions 2012, 15, 799–815. [Google Scholar] [CrossRef]
- Escoriza, D.; Ben Hassine, J.; Boix, D. Factors regulating the invasive success of an alien frog: A comparison of the ecology of the native and alien populations. Hydrobiologia 2014, 730, 127–138. [Google Scholar] [CrossRef]
- San Sebastián, O.; Navarro, J.; Llorente, G.A.; Richter-Boix, A. Trophic strategies of a non-native and a native amphibian species in shared ponds. PLoS ONE 2015, 10, e0130549. [Google Scholar] [CrossRef] [PubMed]
- San Sebastián, O.; Pujol-Buxó, E.; Garriga, N.; Richter-Boix, A.; Llorente, G.A. Differential trophic traits between invasive and native anuran tadpoles. Aquat Inv. 2015, 10, 475–484. [Google Scholar] [CrossRef]
- Pujol-Buxó, E.; Riaño, G.M.; Llorente, G.A. Stable isotopes reveal mild trophic modifications in a native-invasive competitive relationship. Biol. Invasions 2018, 21, 1167–1177. [Google Scholar] [CrossRef]
- Pujol-Buxó, E.; Riaño, G.M.; Llorente, G.A. Mild segregation in the breeding preferences of an invasive anuran (Discoglossus pictus) and its main native competitor (Epidalea calamita) in ephemeral ponds. Amphibia-Reptilia 2019, 40, 425–435. [Google Scholar] [CrossRef]
- Montori, A.; San Sebastián, O.; Franch, M.; Pujol-Buxó, E.; Llorente, G.A.; Fernández-Loras, A.; Richter-Boix, A.; Bosch, J. Observations on the intensity and prevalence of Batrachochytrium dendrobatidis in sympatric and allopatric Epidalea calamita (native) and Discoglossus pictus (invasive) populations. Basic Appl. Herpetol. 2019, 33, 5–17. [Google Scholar] [CrossRef]
- Pleguezuelos, J.M.; Márquez, R.; Lizana, M. Atlas y Libro Rojo de los Anfibios y Reptiles de España, 2nd ed.; Dirección General de Conservación de la Naturaleza-Asociación Herpetológica Española: Madrid, Spain, 2002; 587p. [Google Scholar]
- DG-ENV. Natura 2000 Data—The European Network of Protected Sites. 2013. Available online: https://www.eea.europa.eu/data-and-maps/data/natura-13 (accessed on 18 August 2022).
- Mateo, J.A.; Geniez, P.; Pether, J. Diversity and conservation of Algerian amphibian assemblages. Chapter 26 in Part 2. Mauritania, Morocco, Algeria, Tunisia, Libya and Egypt in Vol. 11. Conservation and Decline of Amphibians: Eastern Hemisphere of the series Amphibian Biology. Basic Appl. Herpetol. 2013, 11, 51–83. [Google Scholar] [CrossRef]
- Sindaco, R.; Doria, G.; Razzetti, E.; Bernini, F. Atlante degli Anfibi e dei Rettili d’Italia/Atlas of Italian Amphibians and Reptiles; Societas Herpetologica Italica: Turin, Italy, 2009; 789p. [Google Scholar]
- Bons, J.; Geniez, P. Amphibiens et Reptiles du Maroc (Sahara Occidental Compris). Atlas Biogéographique; Asociación Herpetológica Española: Barcelona, Spain, 1996; 319p. [Google Scholar]
- Lescure, J.; Massary, J.C. Atlas des Amphibiens et Reptiles de France; Muséum National d’Histoire Naturelle: Paris, France, 2012; 272p. [Google Scholar]
- AHE. Base de Datos de Anfibios y Reptiles de ESPAÑA [WWW Document]. Asoc. Herpetológica Española. 2016. Available online: http://siare.herpetologica.es (accessed on 18 May 2016).
- Loureiro, A.; Ferrand de Almeida, N.; Carretero, M.A.; Paulo, O.S. Atlas dos Anfibios e Répteis de Portugal; Instituto da Conservaçao da Natureza: Lisboa, Portugal, 2008; 257p. [Google Scholar]
- Díaz-Rodríguez, J.; Gehara, M.; Márquez, R.; Vences, M.; Gonçalves, H.; Sequeira, F.; Martínez-Solano, I.; Tejedo, M. Integration of molecular, bioacoustical and morphological data reveals two new cryptic species of Pelodytes (Anura, Pelodytidae) from the Iberian Peninsula. Zootaxa 2017, 4243, 1–41. [Google Scholar] [CrossRef]
- Sillero, N. Modelling suitable areas for Hyla meridionalis under current and future hypothetical expansion scenarios. Amphibia-Reptilia 2010, 31, 37–50. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; Core Writing Team: Geneva, Switzerland, 2014. [Google Scholar]
- Naujokaitis-Lewis, I.R.; Curtis, J.M.R.; Tischendorf, L.; Badzinski, D.; Lindsay, K.; Fortin, M.J. Uncertainties in coupled species distribution-metapopulation dynamics models for risk assessments under climate change. Divers. Distrib. 2013, 19, 541–554. [Google Scholar] [CrossRef]
- Regos, A.; D’Amen, M.; Titeux, N.; Herrando, S.; Guisan, A.; Brotons, L. Predicting the future effectiveness of protected areas for bird conservation in Mediterranean ecosystems under climate change and novel fire regime scenarios. Divers. Distrib. 2015, 22, 83–96. [Google Scholar] [CrossRef]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Dufour, A. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Peng, R.D. Gpclib: General Polygon Clipping Library for R. 2020. Available online: https://cran.r-project.org/web/packages/gpclib/index.html (accessed on 15 December 2020).
- Thuiller, W. BIOMOD—Optimizing predictions of species distributions and projecting potential future shifts under global change. Glob. Chang. Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence: Absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Lobo, J.M. Assessing the effects of pseudo-absences on predictive distribution model performance. Ecol. Modell. 2008, 210, 478–486. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. Bioscience 2001, 51, 933. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Araujo, M.B.; New, M.; Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Manel, S.; Williams, H.C.; Ormerod, S.J. Evaluating presence-absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Maiorano, L.; Falcucci, A.; Dendoncker, N.; Boitani, L.; Padoa-Schioppa, E.; Miaud, C.; Thuiller, W. Knowing the past to predict the future: Land-use change and the distribution of invasive bullfrogs. Glob. Chang. Biol. 2010, 16, 528–537. [Google Scholar] [CrossRef]
- Urban, M.C.; Phillips, B.L.; Skelly, D.K.; Shine, R. A Toad More Traveled: The Heterogeneous Invasion Dynamics of Cane Toads in Australia. Am. Nat. 2008, 171, E134–E148. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Suarez, A.V.; Gómez, C.; Pons, P.; Touyama, Y.; Wild, A.L.; Peterson, A.T. Geographical potential of Argentine ants (Linepithema humile Mayr) in the face of global climate change. Proc. Biol. Sci. 2004, 271, 2527–2535. [Google Scholar] [CrossRef]
- Van Wilgen, N.J.; Roura-Pascual, N.; Richardson, D.M. A quantitative climate-match score for risk-assessment screening of reptile and amphibian introductions. Environ. Manag. 2009, 44, 590–607. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2011, 21, 481–497. [Google Scholar] [CrossRef]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.G.; Araujo, M.B.; Pearson, R.G.G. Equilibrium of species’ distributions with climate. Ecography 2005, 28, 693–695. [Google Scholar] [CrossRef]
- Dukes, J.S.; Mooney, H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999, 14, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Lavorel, S.; Sykes, M.T.; Araujo, M.B. Using niche-based modelling to assess the impact of climate change on tree functional diversity in Europe. Divers. Distrib. 2006, 12, 49–60. [Google Scholar] [CrossRef]
- Franklin, J. Predicting the distribution of shrub species in southern California from climate and terrain-derived variables. J. Veg. Sci. 1998, 9, 733–748. [Google Scholar] [CrossRef]
- Willis, K.J.; Whittaker, R.J. Species Diversity–Scale Matters. Science 2002, 295, 1245. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species–climate impact models under climate change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Swanson, A.K.; Dobrowski, S.Z.; Finley, A.O.; Thorne, J.H.; Schwartz, M.K. Spatial regression methods capture prediction uncertainty in species distribution model projections through time. Glob. Ecol. Biogeogr. 2012, 22, 242–251. [Google Scholar] [CrossRef]
- Brook, B.W.; Sodhi, N.S.; Bradshaw, C.J.A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008, 23, 453–460. [Google Scholar] [CrossRef]
- Pearson, R.G. Climate change and the migration capacity of species. Trends Ecol. Evol. 2006, 21, 111–113. [Google Scholar] [CrossRef]
- Wiens, J.A. Spatial scaling in ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Holt, R.D.; Gaines, M.S. Analysis of Adaptation in Heterogeneous Landscapes—Implications for the Evolution of Fundamental Niches. Evol. Ecol. 1992, 6, 433–447. [Google Scholar] [CrossRef]
- Araújo, M.B.; Alagador, D.; Cabeza, M.; Nogués-Bravo, D.; Thuiller, W. Climate change threatens European conservation areas. Ecology Letters 2011, 14, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Dorcas, M.E.; Willson, J.D.; Reed, R.N.; Snow, R.W.; Rochford, M.R.; Miller, M.A.; Meshaka, W.E., Jr.; Andreadis, P.T.; Mazzotti, F.J.; Romagosa, C.M.; et al. Severe mammal declines coincide with proliferation of invasive Burmese pythons in Everglades National Park. Proc. Natl. Acad. Sci. USA 2012, 109, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.C.; Jones, C.G.; Harris, S. The need for enemy-free space: The impact of an invasive gecko on island endemics. Biol. Conserv. 2005, 125, 467–474. [Google Scholar] [CrossRef]
- Okamoto, T.; Kuriyama, T.; Goka, K. An impact assessment of the alien lizard Plestiodon japonicus (Scincidae, Reptilia) on a threatened island population of the native lizard P. latiscutatus at an early phase of the biological invasion. Biol. Invasions 2013, 15, 2029–2037. [Google Scholar] [CrossRef]
- Garcia-Paris, M.; Jockusch, E.L. A mitochondrial DNA perspective on the evolution of Iberian Discoglossus (Amphibia: Anura). J. Zool. 1999, 248, 209–218. [Google Scholar] [CrossRef]
- Real, R.; Barbosa, A.M.; Martínez-Solano, Í.; García-París, M. Distinguishing the distributions of two cryptic frogs (Anura: Discoglossidae) using molecular data and environmental modeling. Can. J. Zool. 2005, 83, 536–545. [Google Scholar] [CrossRef]
- Veith, M.; Martens, H. What’s the part of Discoglossus pictus? Analysis of an ecological niche in a frog community. In Proceedings of the 4th Ordinary General Meeting of the Societas Europaea Herpetologica, Nijmegen, The Netherlands, 17–21 August 1987; pp. 433–436. [Google Scholar]
- Vences, M.; de Pous, P.; Nicolas, V.; Diaz-Rodriguez, J.; Donaire, D.; Hugemann, K.; Hauswaldt, J.S.; Amat, F.; Barnestein, J.A.M.; Bogaerts, S.; et al. New insights on phylogeography and distribution of painted frogs (Discoglossus) in northern Africa and the Iberian Peninsula. Amphibia-Reptilia 2014, 35, 305–320. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Wake, D.B. Declining Amphibian Populations—A Global Phenomenon. Trends Ecol. Evol. 1990, 5, 203–204. [Google Scholar] [CrossRef]
- Mendelson, J.R.; Lips, K.R.; Gagliardo, R.W.; Rabb, G.B.; Collins, J.P.; Diffendorfer, J.E.; Daszak, P.; Ibáñez D, R.; Zippel, K.C.; Lawson, D.P.; et al. Biodiversity—Confronting amphibian declines and extinctions. Science 2006, 313, 48. [Google Scholar] [CrossRef]
- Buckley, L.B.D.; Jetz, W. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B Biol. Sci. 2006, 274, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Crochet, P. No evidence of general decline in an amphibian community of Southern France. Biol. Conserv. 2004, 119, 297–304. [Google Scholar] [CrossRef]
- Ficetola, G.F.; De Bernardi, F. Amphibians in a human-dominated landscape: The community structure is related to habitat features and isolation. Biol. Conserv. 2004, 119, 219–230. [Google Scholar] [CrossRef]
- Knutson, M.G.; Richardson, W.B.; Reineke, D.M.; Gray, B.R.; Parmelee, J.R.; Weick, S.E. Agricultural Ponds Support Amphibian Populations. Ecol. Appl. 2004, 14, 669–684. [Google Scholar] [CrossRef]
- Brühl, C.A.; Schmidt, T.; Pieper, S.; Alscher, A. Terrestrial pesticide exposure of amphibians: An underestimated cause of global decline? Sci. Rep. 2013, 3, 1135. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Bickford, D.; Diesmos, A.C.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Sekercioglu, C.H.; Bradshaw, C.J.A. Measuring the Meltdown: Drivers of Global Amphibian Extinction and Decline. PLoS ONE 2008, 3, e1636. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villero, D.; Montori, A.; Llorente, G.A.; Roura-Pascual, N.; Geniez, P.; Brotons, L. Global Warming and Long-Distance Spread of Invasive Discoglossus pictus (Amphibia, Alytidae): Conservation Implications for Protected Amphibians in the Iberian Peninsula. Animals 2022, 12, 3236. https://doi.org/10.3390/ani12233236
Villero D, Montori A, Llorente GA, Roura-Pascual N, Geniez P, Brotons L. Global Warming and Long-Distance Spread of Invasive Discoglossus pictus (Amphibia, Alytidae): Conservation Implications for Protected Amphibians in the Iberian Peninsula. Animals. 2022; 12(23):3236. https://doi.org/10.3390/ani12233236
Chicago/Turabian StyleVillero, Dani, Albert Montori, Gustavo A. Llorente, Núria Roura-Pascual, Philippe Geniez, and Lluís Brotons. 2022. "Global Warming and Long-Distance Spread of Invasive Discoglossus pictus (Amphibia, Alytidae): Conservation Implications for Protected Amphibians in the Iberian Peninsula" Animals 12, no. 23: 3236. https://doi.org/10.3390/ani12233236
APA StyleVillero, D., Montori, A., Llorente, G. A., Roura-Pascual, N., Geniez, P., & Brotons, L. (2022). Global Warming and Long-Distance Spread of Invasive Discoglossus pictus (Amphibia, Alytidae): Conservation Implications for Protected Amphibians in the Iberian Peninsula. Animals, 12(23), 3236. https://doi.org/10.3390/ani12233236




