Effect of Animal Stocking Density and Habitat Enrichment on Survival and Vitality of Wild Green Shore Crabs, Carcinus maenas, Maintained in the Laboratory
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Collection
2.2. Experimental Design
2.3. Vitality Indices
2.4. Statistical Analysis
3. Results
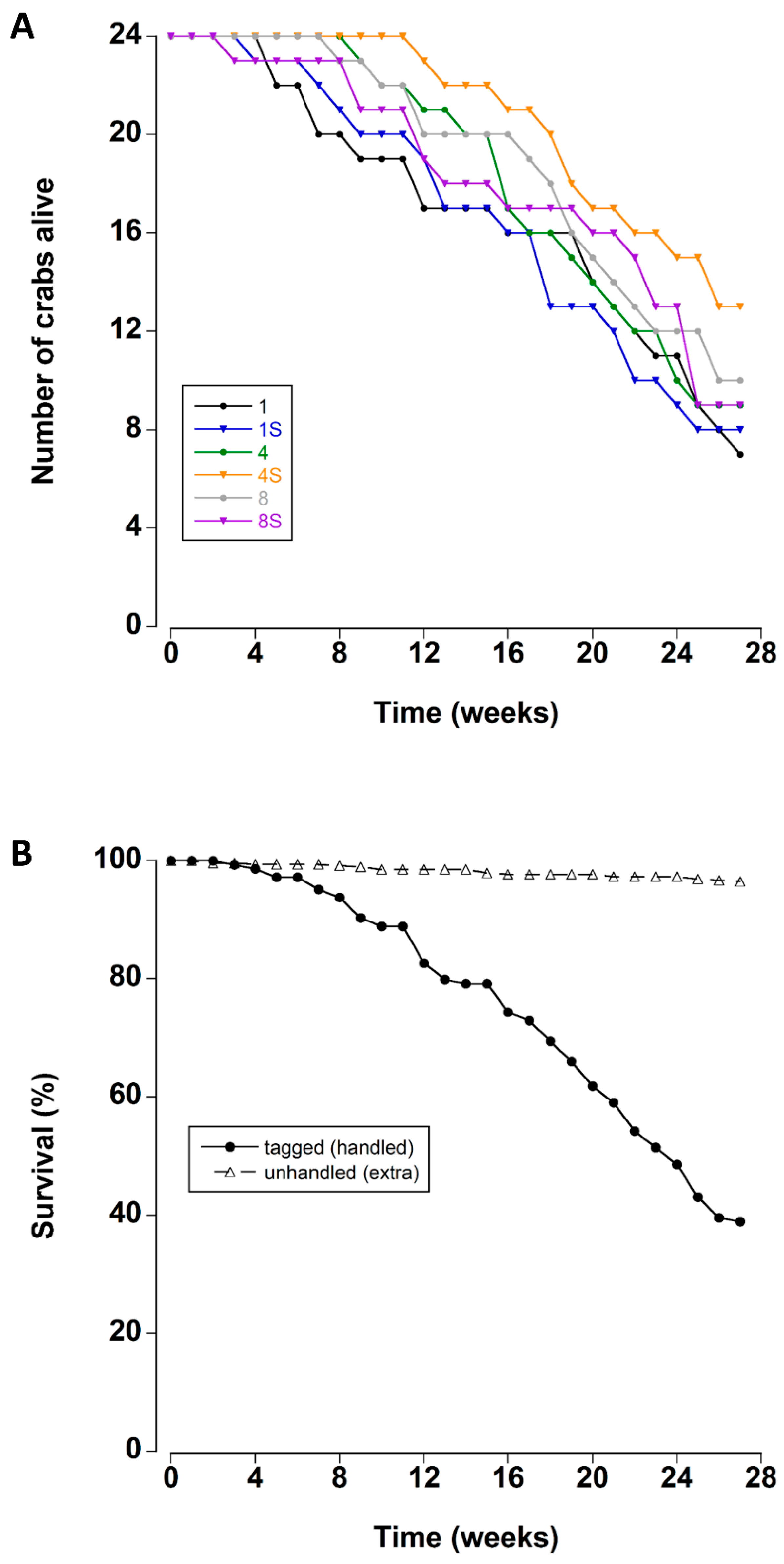
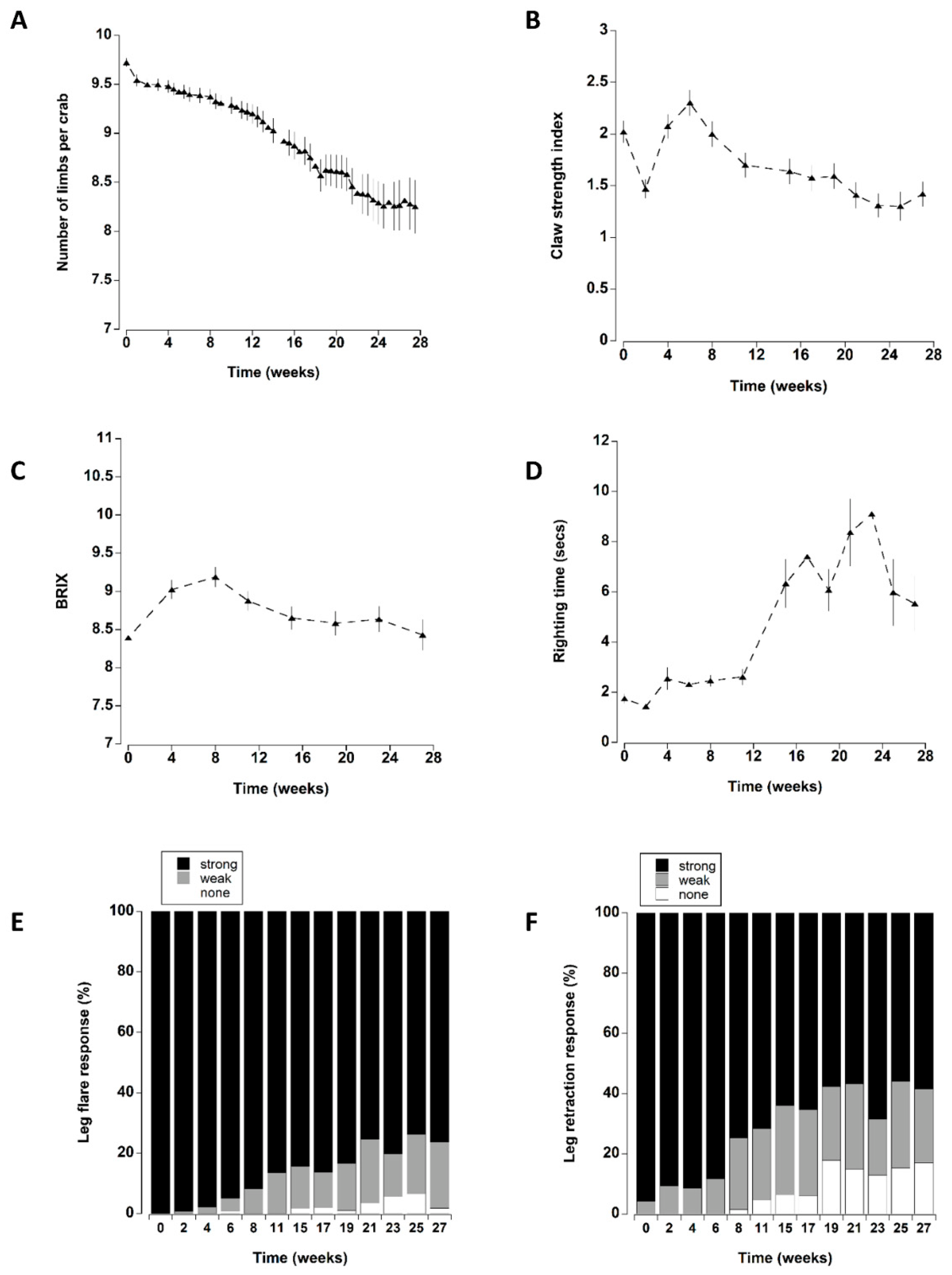
3.1. Survival
3.2. Vitality Indices
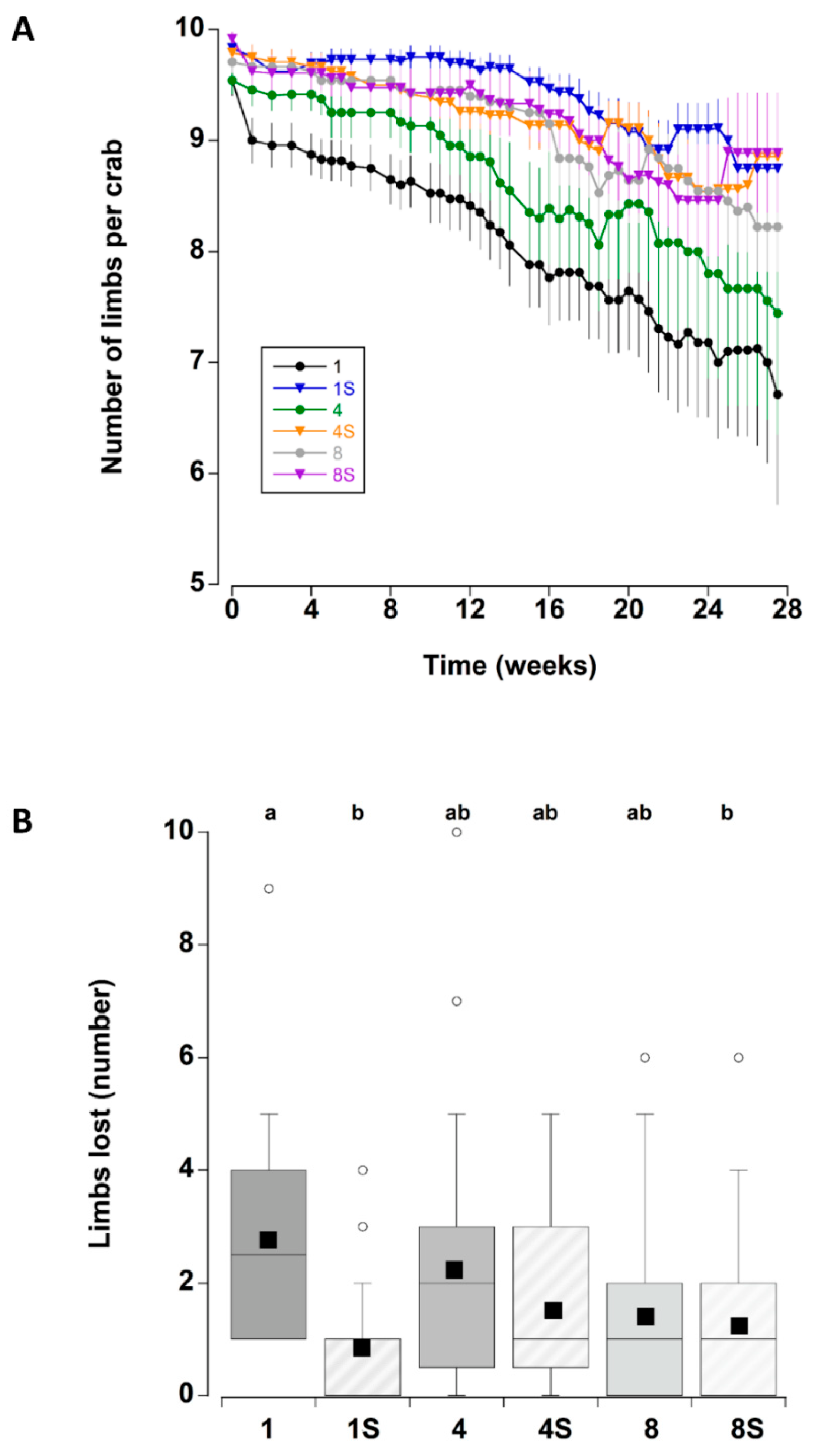
3.3. Limb Loss
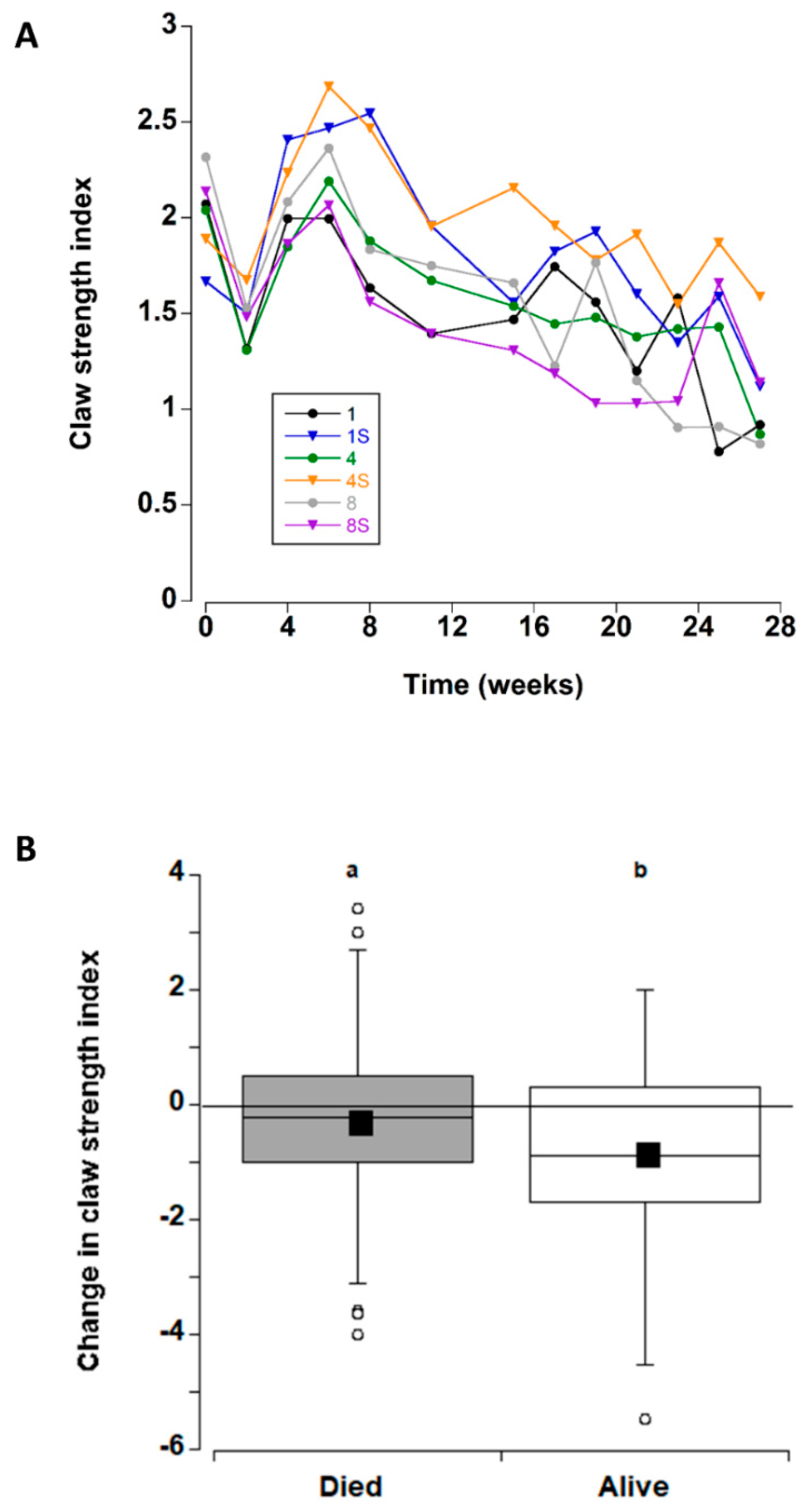
3.4. Claw Strength Index
3.5. BRIX
3.6. Righting Time
3.7. Leg Flare
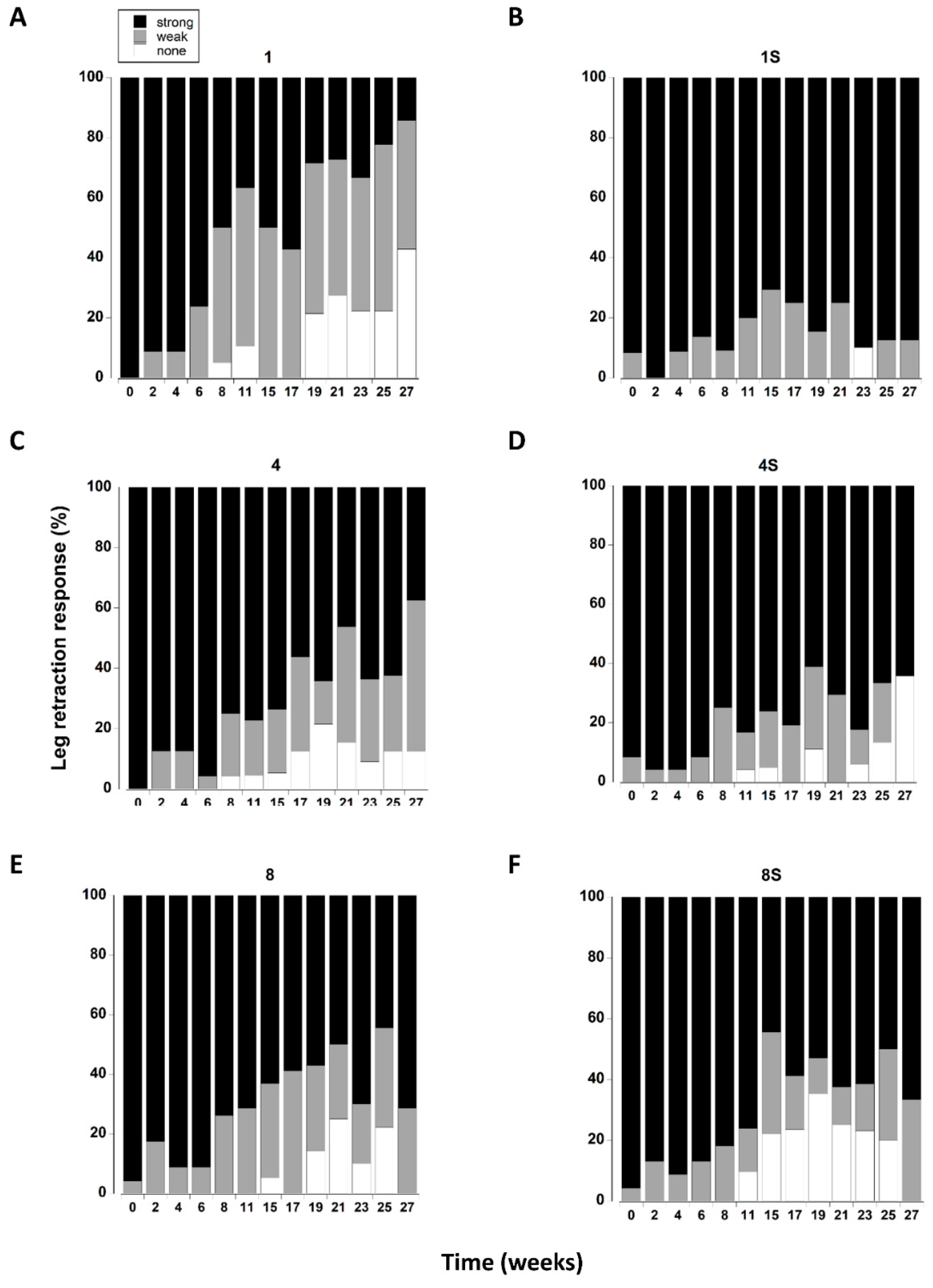
3.8. Leg Retraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trend Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sneddon, L.U.; Wolfenden, D.C.; Thomson, J.S. Stress management and welfare. In Fish Physiology; Biology of Stress in Fish; Elsevier Inc.: New York, NY, USA, 2016; Volume 35. [Google Scholar]
- Wernberg, T.; Smale, D.A.; Thomsen, M.S. A decade of climate change experiments on marine organisms: Procedures, patterns and problems. Glob. Chang. Biol. 2012, 5, 1491–1498. [Google Scholar] [CrossRef]
- Bass, A.; Wernberg, T.; Thomsen, M.; Smale, D. Another decade of marine climate change experiments: Trends, progress and knowledge gaps. Front. Mar. Sci. 2021, 1233, 71462. [Google Scholar] [CrossRef]
- Diggles, B.K. Review of some scientific issues related to crustacean welfare. ICES J. Mar. Sci. 2019, 76, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Drinkwater, E.; Robinson, E.J.H.; Hart, A.G. Keeping invertebrate research ethical in a landscape of shifting public opinion. Methods Ecol. Evol. 2019, 10, 1265–1273. [Google Scholar] [CrossRef]
- De Grave, S.; Pentcheff, N.D.; Ahyong, S.T.; Chan, T.Y.; Crandall, K.A.; Dworschak, P.C.; Wetzer, R. A classification of living and fossil genera of decapod crustaceans. Raffles Bull. Zool. 2009, 21, 1–109. [Google Scholar]
- Chang, E.S.; Thiel, M. The Natural History of the Crustacea; Physiology; Oxford University Press: New York, NY, USA, 2015; Volume 4, p. 512. [Google Scholar]
- Stein, W.; Harzsch, S. The neurobiology of ocean change–insights from decapod crustaceans. Zoology 2021, 144, 125887. [Google Scholar] [CrossRef]
- Wickens, J.F.; Lee, D.O. Crustacean Farming. Ranching and Culture, 2nd ed.; Blackwell Sciences: Oxford, UK, 2003; p. 446. [Google Scholar]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Josupeit, H.; Cai, J.; Zhou, X. The role of crustacean fisheries and aquaculture in global food security: Past, present and future. J. Invertebr. Pathol. 2012, 110, 158–165. [Google Scholar] [CrossRef]
- Penn, J.W.; Caputi, N.; de Lestang, S.; Johnston, D.; Kangas, M.; Bopp, J. Crustacean Fisheries. In Encyclopedia of Ocean Sciences, 3rd ed.; Elsevier Inc.: New York, NY, USA, 2018. [Google Scholar]
- Fotedar, S.; Evans, L. Health management during handling and live transport of crustaceans: A review. J. Invertebr. Pathol. 2011, 106, 143–152. [Google Scholar] [CrossRef]
- Stoner, A.W. Assessing stress and predicting mortality in economically significant crustaceans. Rev. Fish. Sci. 2012, 20, 111–135. [Google Scholar] [CrossRef]
- Jacklin, M.; Combes, J. The Good Practice Guide to Handling and Storing Live Crustacea. Seafish Report; Sea Fish Industry Authority Publication: Lowestoft, UK, 2007; p. 151. [Google Scholar]
- Taylor, H.H.; Paterson, B.D.; Wong, R.J.; Wells, R.M.G. Physiology and live transport of lobsters: Report from a workshop. Mar. Freshw. Res. 1997, 48, 817–822. [Google Scholar] [CrossRef]
- McGaw, I.J.; Nancollas, S.J. Experimental set-up influences the cardiovascular responses of decapod crustaceans to environmental change. Can. J. Zool. 2018, 96, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, T.J.; Kwan, G.T.; Gallup, J.; Tresguerres, M. Acute fluoxetine exposure alters crab anxiety-like behaviour, but not aggressiveness. Sci. Rep. 2016, 6, 19850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiciotto, F.; Vazzana, M.; Celi, M.; Maccarrone, V.; Ceraulo, M.; Buffa, G.; de Vincenzi, G.; Mazzola, S.; Buscaino, G. Behavioural and biochemical stress responses of Palinurus elephas after exposure to boat noise pollution in tank. Mar. Pollut. Bull. 2014, 84, 104–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercier, L.; Palacios, E.; Campa-Cordova, A.I.; Tovar-Ramirez, D.; Hernandez-Herrera, R.; Racotta, I.S. Metabolic and immune responses in Pacific whiteleg shrimp Litopenaeus vannamei exposed to a repeated handling stress. Aquaculture 2006, 258, 633–640. [Google Scholar] [CrossRef]
- Montero, D.; Izquierdo, M.S.; Tort, L.; Robaina, L.; Vergara, J.M. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol. Biochem. 1999, 20, 53–60. [Google Scholar] [CrossRef]
- Tidua, S.; Briffa, M. Review of behavioural impacts of aquatic noise on crustaceans. Proc. Meet. Acoust. 2016, 27, 010028. [Google Scholar]
- Matveev, E.; McGaw, I.J. Effects of laboratory holding time and diet type on labile traits in the crab Cancer irroratus Say, 1817 (Decapoda: Brachyura: Cancridae). J. Crustac. Biol. 2022, 42, ruab076. [Google Scholar] [CrossRef]
- Childress, J.J.; Seibel, B.A. Life at stable low oxygen levels: Adaptations of animals to oceanic oxygen minimum layers. J. Exp. Biol. 1998, 201, 1223–1232. [Google Scholar] [CrossRef]
- Haukenes, A.H.; El Mejjati, S.Y.; Buck, C.L. Effects of emersion temperature on the oxygen consumption rates of male tanner crabs, Chionoecetes bairdi. J. Crustac. Biol. 2009, 29, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.H.; Nancollas, S.J.; Rivers, M.L.; Spicer, J.I.; McGaw, I.J. Effects of handling during experimental procedures on stress indices in the green shore crab, Carcinus maenas (L). Mar. Freshw. Behav. Physiol. 2021, 54, 65–86. [Google Scholar] [CrossRef]
- Chang, E.S.; Chang, S.A.; Keller, R.A.; Reddy, S.; Snyder, M.J.; Spees, J.L. Quantification of stress in lobsters: Crustacean hyperglycemic hormone, stress proteins, and gene expression. Am. Zool. 1999, 39, 487–495. [Google Scholar] [CrossRef]
- Webster, S.G. Endocrinology of metabolism and water balance: Crustacean hyperglycemic hormone. In The Natural History of the Crustacea; Chang, E.S., Thiel, M., Eds.; Oxford University Press: Oxford, UK, 2015; Volume 4, pp. 36–67. [Google Scholar]
- Whiteley, N.M.; Taylor, E.W. Oxygen and acid-base disturbances in the haemolymph of the lobster Homarus gammarus during commercial transport and storage. J. Crustac. Biol. 1992, 12, 19–30. [Google Scholar] [CrossRef]
- Crear, B.J.; Forteath, G.N.R. Recovery of the western rock lobster, Panulirus cygnus, from emersion and handling stress: The effect of oxygen concentration during re-immersion. J. Shellfish Res. 2001, 20, 921–929. [Google Scholar]
- Wang, G.; McGaw, I.J. Use of serum protein concentration as an indicator of quality and physiological condition in the lobster Homarus americanus (Milne-Edwards, 1837). J. Shellfish Res. 2014, 33, 805–813. [Google Scholar] [CrossRef]
- Battison, A.L. Use of the Brix value with cuticle indices to describe haemolymph biochemistry parameters in Homarus americanus H. Milne Edwards, 1837 (Decapoda: Malacostraca: Nephropidae). J. Crustac. Biol. 2018, 38, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Berry, S.E.; Simon, C.J.; Foote, A.R.; Jerry, D.R.; Wade, N.M. Evaluation of baseline haemolymph biochemistry, volume and total body energetics to determine an accurate condition index in the black tiger shrimp, Penaeus monodon. Comp. Biochem. Physiol. 2019, 228, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.W.; Ottmar, M.L. Wounding and reflex impairment may be predictors for mortality in discarded or escaped fish. Fish. Res. 2006, 82, 1–6. [Google Scholar] [CrossRef]
- Stoner, A.W.; Rose, C.S.; Munk, J.E.; Hammond, C.F.; Davis, M.W. An assessment of discard mortality for two Alaskan crab species, Tanner crab (Chionoecetes bairdi) and snow crab Chionocetes opilio), based on reflex impairment. Fish. Bull. 2008, 106, 337–347. [Google Scholar]
- Stoner, A.W. Prediction of discard mortality for Alaskan crabs after exposure to freezing temperatures, based on a reflex impairment index. Fish. Bull. 2009, 107, 351–362. [Google Scholar]
- Walters, E.A.; Crowley, C.E.; Gandy, R.L.; Behringer, D.C. A reflex action mortality predictor (RAMP) for commercially fished blue crab Callinectes sapidus in Florida. Fish. Res. 2022, 247, 106188. [Google Scholar] [CrossRef]
- Elwood, R.W. Potential Pain in Fish and Decapods: Similar Experimental Approaches. and similar results. Front. Vet. Sci. 2021, 8, 631151. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U.; Elwood, R.W.; Adamo, S.A.; Leach, M. C Defining and assessing animal pain. Anim. Behav. 2014, 97, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Birch, J. Sentience and the science-policy interface. Anim. Sentience 2022, 6, 10. [Google Scholar] [CrossRef]
- Elwood, R.W.; Adams, L. Electric shock causes physiological stress responses in shore crabs, consistent with prediction of pain. Biol. Lett. 2015, 11, 20150800. [Google Scholar] [CrossRef]
- De Morri, B.; Normando, S. Is history repeating itself? The case for fish and arthropod sentience and welfare. Ethics Politics 2019, 2, 491–516. [Google Scholar]
- Passantino, A.; Elwood, R.W.; Coluccio, P. Why protect decapod crustaceans used as models in biomedical research and in ecotoxicology? Ethical and legislative considerations. Animals 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Browning, H.; Schnell, A.; Burn, C.; Birch, J. Sentience in decapod crustaceans: A general framework and review of the evidence. Anim. Sentience 2022, 32, 1–35. [Google Scholar] [CrossRef]
- Birch, J.; Burn, C.; Schnell, A.; Browning, H.; Crump, A. Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans; LSE Consulting; LSE Enterprise Ltd.: London, UK; The London School of Economics and Political Science: London, UK, 2021. [Google Scholar]
- Carder, G. A preliminary investigation into the welfare of lobsters in the UK. Anim. Sentience 2017, 16, 41–59. [Google Scholar] [CrossRef]
- Klassen, G.J.; Locke, A. A biological synopsis of the European green crab, Carcinus maenas. Moncton, New Brunswick, Canada: Fisheries and Oceans Canada. Can. Manuscr. Rep. Fish. Aquat. Sci. 2007, 2818, 75. [Google Scholar]
- Leignel, V.; Stillman, J.H.; Baringou, S.; Thabet, R.; Metais, I. Overview on the European green crab Carcinus spp. (Portunidae, Decapoda), one of the most famous marine invaders and ecotoxicological models. Environ. Sci. Pollut. Res. 2014, 21, 9129–9144. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Halsey, L.G.; Bury, N.R. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017, 220, 3007–3016. [Google Scholar] [CrossRef] [Green Version]
- Siikavuopio, S.I.; James, P.; Olsen, B.R.; Evensen, T.; Mortensen, E.; Olsen, S.E. Holding wild snow crab, Chionoecetes opilio: Effects of stocking density and feeding on survival and injury. Aquac. Res. 2017, 48, 1590–1595. [Google Scholar] [CrossRef]
- Reid, D.G.; Abello, P.; Kaiser, M.J.; Warman, C.G. Carapace colour, intermoult duration and the behavioural and physiological ecology of the shore crab, Carcinus maenas. Estuar. Coast. Shelf Sci. 1997, 44, 203–211. [Google Scholar] [CrossRef]
- Lee, K.T.; Jivoff, P.; Bishop, R.P. A low cost reliable method for quantifying coloration in Carcinus maenas (Linnaeus, 1758) (Decapoda Brachyura). Crustaceana 2005, 78, 579–590. [Google Scholar]
- Klein-Breteler, W.C.M. Laboratory experiments on the influence of environmental factors on the frequency of moulting and the increase in size at moulting of juvenile shore crabs, Carcinus maenas. Neth. J. Sea Res. 1975, 9, 100–120. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Huntingford, F.A.; Taylor, A.C.; Orr, J.F. Weapon strength and competitive success in the fights of shore crabs (Carcinus maenas). J. Zool. 2000, 250, 397–403. [Google Scholar] [CrossRef]
- Taylor, G.M. Maximum force production: Why are crabs so strong? Proc. R. Soc. Lond. B 2000, 267, 1475–1480. [Google Scholar] [CrossRef]
- Taylor, G.M.; Keyghobadi, N.; Schmidt, P.S. The geography of crushing: Variation in claw performance of the invasive crab Carcinus maenas. J. Exp. Mar. Biol. Ecol. 2009, 377, 48–53. [Google Scholar] [CrossRef]
- Matveev, E. Assessment of Intra-Individual Consistency of Physiology and Behaviour in Brachyuran Crabs (Carcinus maenas and Cancer irroratus). Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2021; p. 124. [Google Scholar]
- Rebach, S.; Block, J.D. Correlates of claw strength in the rock crab, Cancer irroratus (Decapoda, Brachyura). Crustaceana 1998, 71, 468–473. [Google Scholar] [CrossRef]
- Shirley, T.C.; Stickle, W.B. Responses of Leptasterias hexactis (Echinodermata: Asteroidea) to low salinity. Mar. Biol. 1982, 69, 155–163. [Google Scholar] [CrossRef]
- Lorenzon, S.; Giulianini, P.G.; Libralato, S.; Martinis, M.; Ferrero, E.A. Stress effect of two different transport systems on the physiological profiles of the crab Cancer pagurus. Aquaculture 2008, 278, 156–163. [Google Scholar] [CrossRef]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. nparLD?: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 24 July 2022).
- R Studio Team. R Studio: Integrated Development Environment for R; R Studio: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 24 July 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Naslund, J.; Johnsson, J.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. In Fish and Fisheries; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Message, R.; Greenhough, B. “But It’s Just a Fish”: Understanding the Challenges of applying the 3Rs in laboratory aquariums in the UK. Animals 2019, 9, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, V.; Kleiber, A.; Patinote, A.; Sudan, P.; Duret, C.; Gourmelen, G.; Moreau, E.; Fournel, C.; Pineau, L.; Calvez, S.; et al. Positive welfare effects of physical enrichments from the nature, functions-and feeling based approaches in farmed rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 550, 737825. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamaguchi, E.; Fujiyama, N.; Nagayama, T. The effects of shelter quality and prior residence on marmorkrebs (marbled crayfish). J. Exp. Biol. 2019, 222, 197301. [Google Scholar] [CrossRef] [Green Version]
- Hastuti, Y.P.; Wicaksono, P.H.; Nurusallam, W.; Tridesianti, S.; Fatma, Y.S.; Nirmala, K.; Rusmana, I.; Affandi, R. Addition of shelters to control the physiological production of mud crab Scylla serrata in recirculation aquaculture system. J. Ilmu dan Teknol. Kelaut. Trop. 2020, 12, 297–308. [Google Scholar] [CrossRef]
- Orlosk, J.L.; Walker, J.M.; Morrison, A.L.; Atema, J. Conditioning the crab Carcinus maenas against instinctive light avoidance. Mar. Freshw. Behav. Physiol. 2011, 44, 375–381. [Google Scholar] [CrossRef]
- Crothers, J.H. The biology of the shore crab, Carcinus maenas (L.). 2. The life of the adult crab. Field Stud. 1968, 2, 579–614. [Google Scholar]
- McGaw, I.J. Impacts of habitat complexity on physiology: Purple shore crabs tolerate osmotic stress for shelter. Estuar. Coast. Shelf Sci. 2001, 53, 865–876. [Google Scholar] [CrossRef]
- McGaw, I.J.; Naylor, E. The effect of shelter on salinity preference behaviour of the shore crab Carcinus maenas. Mar. Behav. Physiol. 1992, 21, 145–152. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.; Kimera, F.; Sewilam, H. A review on recirculating aquaculture system: Influence of stocking density on fish and crustacean behavior, growth performance, and immunity. Ann. Anim. Sci. 2022, 22, 873–884. [Google Scholar] [CrossRef]
- Dare, P.J.; Edwards, D.B. Underwater television observations on the intertidal movements of shore crabs, Carcinus maenas, across a mudflat. J. Mar. Biol. Assoc. UK 1981, 61, 107–116. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Nutritional studies in crustaceans and the problems of applying research findings to practical farming systems. Aquac. Nutr. 2017, 1, 165–174. [Google Scholar] [CrossRef]
- Juanes, F.; Smith, L.D. The ecological consequences of limb damage and loss in. decapod crustaceans: A review and prospectus. J. Exp. Mar. Biol. Ecol. 1995, 193, 197–223. [Google Scholar] [CrossRef]
- Sekkelsten, G.I. Effect of handicap on mating success in male shore crabs Carcinus maenas. Oikos 1988, 51, 131–134. [Google Scholar] [CrossRef]
- McVean, A. The incidence of autotomy in Carcinus maenas (L.). J. Exp. Mar. Biol. Ecol. 1976, 24, 177–187. [Google Scholar] [CrossRef]
- Kennelly, S.J.; Watkins, D.; Craig, J.R. Mortality of discarded spanner crabs Ranina (Linnaeus) in a tangle-net fishery-laboratory and field experiments. J. Exp. Mar. Biol. Ecol. 1990, 140, 39–48. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Cheng, Y. Effects of limb autotomy on growth, feeding and regeneration in the juvenile Eriocheir sinensis. Aquaculture 2016, 457, 79–84. [Google Scholar] [CrossRef]
- Smith, L.D.; Palmer, A.R. Effects of manipulated diet on size and performance of brachyuran crab claws. Science 1994, 264, 710–712. [Google Scholar] [CrossRef]
- Elner, R.W. The influence of temperature, sex and chela size in the foraging strategy of the shore crab, Carcinus maenas (L.). Mar. Freshw. Behav. Physiol. 1980, 7, 15–24. [Google Scholar] [CrossRef]
- Elner, R.W. Diet of the green crab Carcinus maenas (L.) from Port Hebert, southwestern Nova Scotia. J. Shellfish Res. 1981, 1, 89–94. [Google Scholar]
- Ropes, J.W. The feeding habits of the green crab Carcinus maenas. Fish. Bull. US 1968, 67, 183–203. [Google Scholar]
- Wallace, J.C. Feeding, starvation and metabolic rate in the shore crab Carcinus maenas. Mar. Biol. 1973, 20, 277–281. [Google Scholar] [CrossRef]
- Taylor, E.W.; Butler, P.J. Aquatic and aerial respiration in the shore crab, Carcinus maenas (L.), acclimated to 15 C. J. Comp. Physiol. B 1978, 127, 315–323. [Google Scholar] [CrossRef]
- Simonik, E.; Henry, R.P. Physiological responses to emersion in the intertidal green crab, Carcinus maenas (L.). Mar. Freshw. Behav. Physiol. 2014, 47, 101–115. [Google Scholar] [CrossRef]
- Nancollas, S.J.; McGaw, I.J. Acclimation to tidal conditions alters the physiological responses of the green shore crab, Carcinus maenas to subsequent emersion. J. Exp. Biol. 2021, 224, jeb242220. [Google Scholar] [CrossRef]
- Gleeson, R.A.; Paul, L.Z. The determination of hemolymph volume in the blue crab, Callinectes sapidus, utilizing 14C-thiocyanate. Comp. Biochem. Physiol. 1977, 56, 411–413. [Google Scholar] [CrossRef]
- McMahon, B.R.; Wilkens, J.L. Periodic respiratory and circulatory performance in the red rock crab Cancer productus. J. Exp. Biol. 1977, 202, 363–374. [Google Scholar]
- Söderhäll, I. Crustacean hematopoiesis. Dev. Comp. Immunol. 2016, 58, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Baltar, F.; Lindh, M.V.; Parparov, A.; Berman, T.; Pinhassi, J. Prokaryotic community structure and respiration during long term incubations. Microsc. Open 2012, 1, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.N.; Nguyen, P.N.; Le, D.V.; Nguyen, D.V.; Bossier, P. Effects of stocking density of gray mullet Mugil cephalus on water quality, growth performance, nutrient conversion rate, and microbial community structure in the white shrimp Litopenaeus vannamei integrated system. Aquaculture 2018, 496, 123–133. [Google Scholar] [CrossRef]
- Reuter, R.E.; Geddes, M.C.; Evans, L.H.; Bryars, S.R. Tail disease in southern rock lobsters (Jasus edwardsii). In Proceedings on the International Symposium of Lobster Health and Management; Curtin University: Perth, Australia, 1999; pp. 88–95. [Google Scholar]
- Evans, L.H. Lobster health and disease: Basic concepts. In Proceedings on the International Symposium of Lobster Health and Management; Curtin University: Perth, Western Australia, 1999; pp. 3–8. [Google Scholar]
- Jussila, J.; Jago, J.; Tsvetnenko, E.; Evans, L.H. Effects of handling or injury disturbance on total hemocyte counts in western rock lobster (Panulirus cygnus George). In Proceedings on the International Symposium of Lobster Health and Management; Curtin University: Perth, Australia, 1999; pp. 52–63. [Google Scholar]
- McEwen, B.; Seeman, T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Pascual, C.; Sánchez, A.; Vargas-Albores, F.; Le Moullac, G.; Rosas, C. Hemolymph metabolic variables and immune response in Litopenaeus setiferus adult males: The effect of acclimation. Aquaculture 2001, 198, 13–28. [Google Scholar] [CrossRef]



| Reflex | Test | Strong Response | Weak Response | No Response | No Longer Used for Testing If: |
|---|---|---|---|---|---|
| Leg flare | Crabs gently lifted out of water by the carapace, dorsum up | All walking legs spread wide and high. Back legs can be extended higher than horizontal | Partial response, some legs remain below horizontal | No attempt to flare legs, all appendages remain below horizontal | Loss of both right and left 4th walking legs, 3 or more limbs on one side |
| Leg retraction | Crabs gently lifted out of water, dorsum up. Attempt to manually retract the first walking leg anteriorly | Crabs resist to the motion–leg stays in its current position | Crabs show less resistance, but leg always returns to original position after manipulation | No resistance from crab, and leg droops down. | Loss of both left and right first walking legs, or loss of more than three limbs on one side |
| Treatment | Mean ± SE (weeks) | Median (weeks) |
|---|---|---|
| 1 crab | 19.6 ± 1.7 | 22 |
| 1 crab + shelter | 19.4 ± 1.6 | 22 |
| 4 crabs | 21.2 ± 1.3 | 24 |
| 4 crabs + shelter | 23.6 ± 1.0 | - |
| 8 crabs | 21.8 ± 1.3 | 26 |
| 8 crabs + shelter | 21.0 ± 1.5 | 25 |
| Vitality Index | F Statistic | DF | p Value |
|---|---|---|---|
| Limb Loss | |||
| Density | 0.8 | 2 | 0.437 |
| Shelter | 10.4 | 1 | 0.001 |
| Time | 30.4 | 47 | <0.000 |
| Density × Shelter | 3.0 | 2 | 0.047 |
| Density × Time | 0.8 | 94 | 0.534 |
| Shelter × Time | 0.8 | 47 | 0.487 |
| Density × Shelter × Time | 0.8 | 94 | 0.564 |
| Claw Strength | |||
| Density | 1.0 | 2 | 0.374 |
| Shelter | 1.3 | 1 | 0.289 |
| Time | 11.3 | 10 | <0.000 |
| Density × Shelter | 1.3 | 2 | 0.269 |
| Density × Time | 1.3 | 20 | 0.237 |
| Shelter × Time | 0.7 | 10 | 0.647 |
| Density × Shelter × Time | 0.9 | 20 | 0.538 |
| BRIX | |||
| Density | 0.3 | 2 | 0.756 |
| Shelter | 0.0 | 1 | 0.908 |
| Time | 7.5 | 6 | <0.000 |
| Density × Shelter | 1.4 | 2 | 0.248 |
| Density × Time | 1.1 | 12 | 0.376 |
| Shelter × Time | 0.6 | 6 | 0.601 |
| Density × Shelter × Time | 1.6 | 12 | 0.164 |
| Righting Time | |||
| Density | 0.7 | 2 | 0.51 |
| Shelter | 1.5 | 1 | 0.222 |
| Time | 70.6 | 12 | <0.000 |
| Density × Shelter | 0.9 | 2 | 0.414 |
| Density × Time | 0.7 | 24 | 0.808 |
| Shelter × Time | 1.1 | 12 | 0.395 |
| Density × Shelter × Time | 0.5 | 24 | 0.902 |
| Leg Flare | |||
| Density | 0.4 | 2 | 0.666 |
| Shelter | 0.0 | 1 | 0.94 |
| Time | 8.0 | 12 | <0.000 |
| Density × Shelter | 2.5 | 2 | 0.088 |
| Density × Time | 1.7 | 24 | 0.083 |
| Shelter × Time | 1.1 | 12 | 0.355 |
| Density × Shelter × Time | 1.5 | 24 | 0.121 |
| Leg Retraction | |||
| Density | 0.6 | 2 | 0.525 |
| Shelter | 8.9 | 1 | 0.003 |
| Time | 15.4 | 12 | <0.000 |
| Density × Shelter | 5.2 | 2 | 0.006 |
| Density × Time | 0.9 | 24 | 0.579 |
| Shelter × Time | 2.0 | 12 | 0.053 |
| Density × Shelter × Time | 1.3 | 24 | 0.192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, C.H.; Wyeth, R.C.; Spicer, J.I.; McGaw, I.J. Effect of Animal Stocking Density and Habitat Enrichment on Survival and Vitality of Wild Green Shore Crabs, Carcinus maenas, Maintained in the Laboratory. Animals 2022, 12, 2970. https://doi.org/10.3390/ani12212970
Wilson CH, Wyeth RC, Spicer JI, McGaw IJ. Effect of Animal Stocking Density and Habitat Enrichment on Survival and Vitality of Wild Green Shore Crabs, Carcinus maenas, Maintained in the Laboratory. Animals. 2022; 12(21):2970. https://doi.org/10.3390/ani12212970
Chicago/Turabian StyleWilson, Charlotte H., Russell C. Wyeth, John I. Spicer, and Iain J. McGaw. 2022. "Effect of Animal Stocking Density and Habitat Enrichment on Survival and Vitality of Wild Green Shore Crabs, Carcinus maenas, Maintained in the Laboratory" Animals 12, no. 21: 2970. https://doi.org/10.3390/ani12212970
APA StyleWilson, C. H., Wyeth, R. C., Spicer, J. I., & McGaw, I. J. (2022). Effect of Animal Stocking Density and Habitat Enrichment on Survival and Vitality of Wild Green Shore Crabs, Carcinus maenas, Maintained in the Laboratory. Animals, 12(21), 2970. https://doi.org/10.3390/ani12212970





