A Comparison of Fresh and Frozen Lamb Meat—Differences in Technological Meat Quality and Sensory Attributes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Slaughter Facilities
2.2. Sampling, Packaging and Handling of LTL Muscle
2.3. Fresh Samples
2.4. Frozen Samples
2.5. Colour
2.6. Thawing and Cooking Loss
2.7. Warner-Bratzler Shear Force (WBSF)
2.8. Sensory Evaluation
2.9. Low Field Nuclear Magnetic Resonance (LF-NMR)
2.10. Statistical Analyses
3. Results
3.1. Technological Meat Quality Attributes
3.2. Sensory Attributes
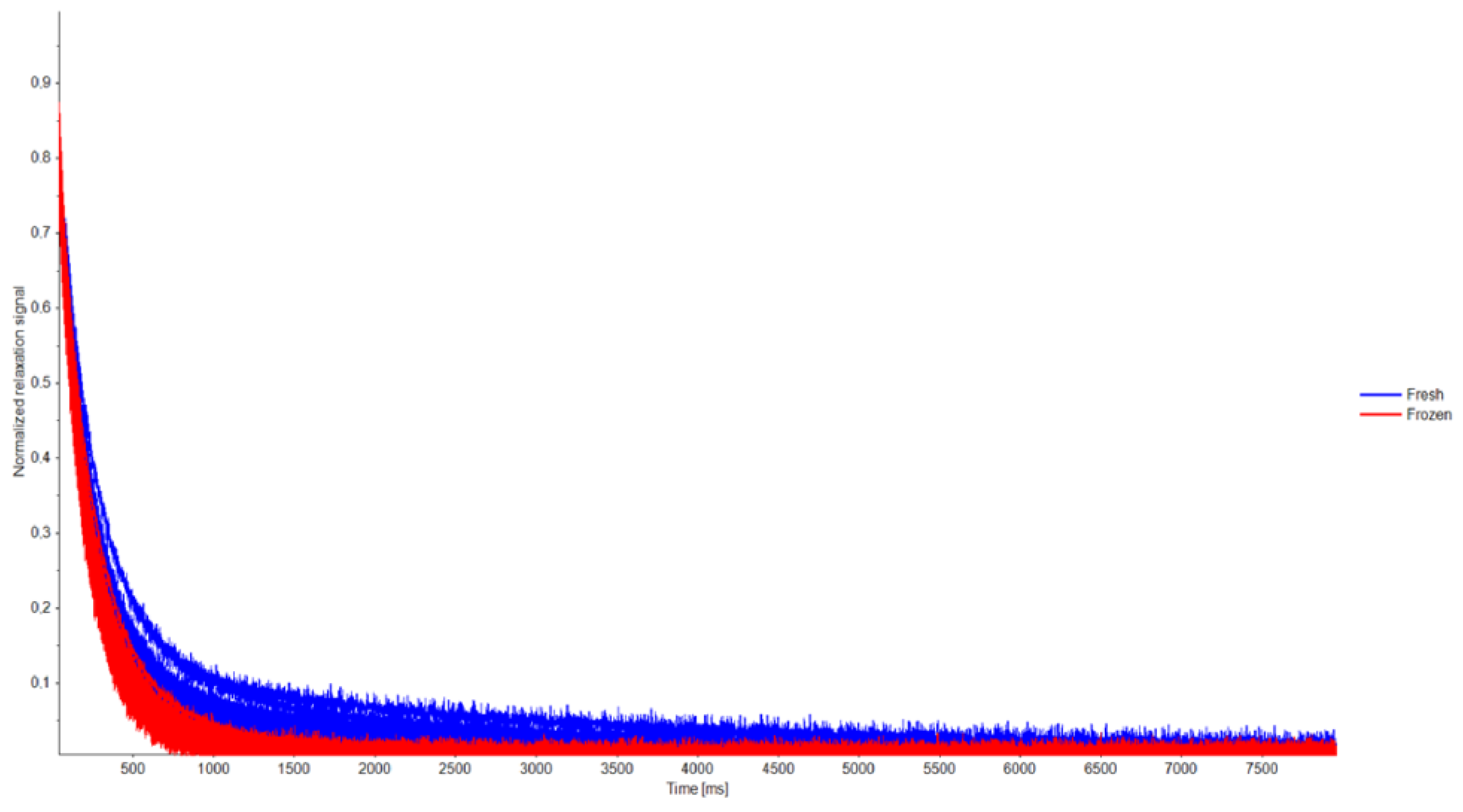
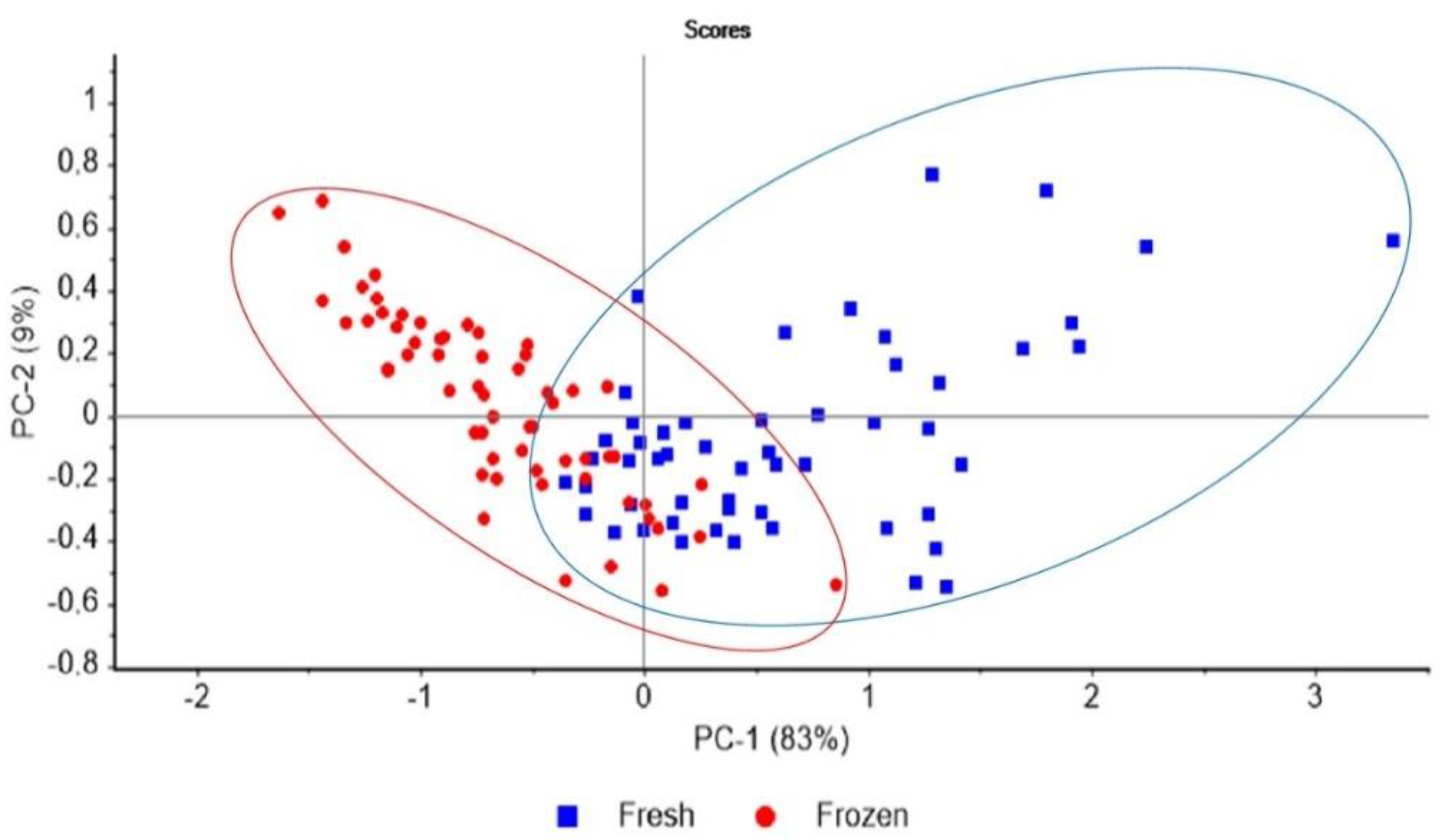
3.3. LF-NMR Analysis
4. Discussion
4.1. Fluid Losses
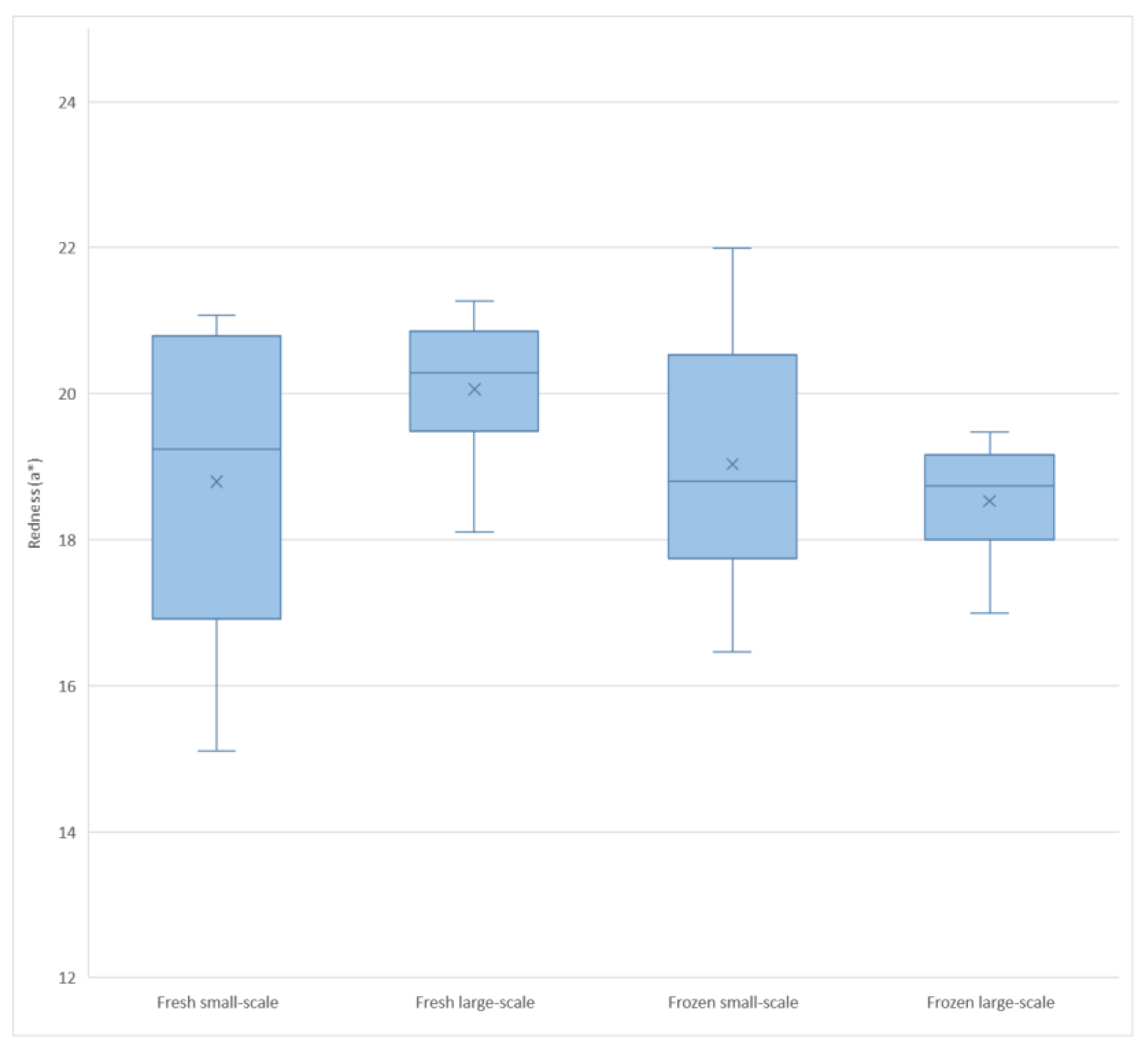
4.2. Colour
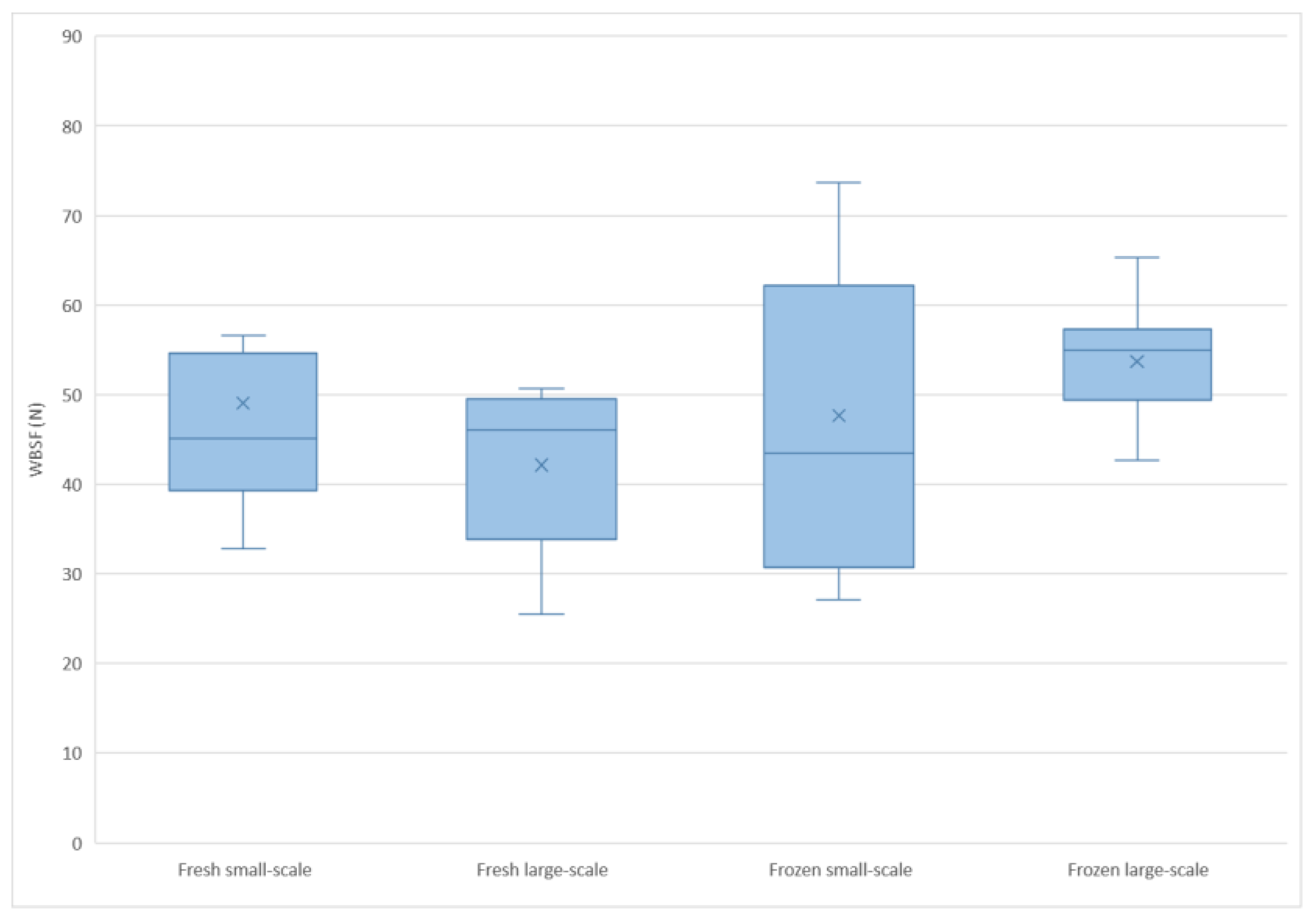
4.3. WBSF
4.4. Sensory Attributes
4.5. LF-NMR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chemineau, P.; Guillaume, D.; Migaud, M.; Thiéry, J.; Pellicer-Rubio, M.; Malpaux, B. Seasonality of Reproduction in Mammals: Intimate Regulatory Mechanisms and Practical Implications. Reprod. Domest. Anim. 2008, 43, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brunet, A.; Santiago-Moreno, J.; Toledano-Diaz, A.; López-Sebastián, A. Reproductive Seasonality and Its Control in Spanish Sheep and Goats. Trop. Subtrop. Agroecosyst. 2012, 15, S47–S70. [Google Scholar]
- Wheeler, T.L.; Miller, R.K.; Savell, J.W.; Cross, H.R. Palatability of Chilled and Frozen Beef Steaks. J. Food Sci. 1990, 55, 301–304. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Janz, J.A.M. Influence of freezing and thawing on the hydration characteristics, quality, and consumer acceptance of whole muscle beef injected with solutions of salt and phosphate. Meat Sci. 2009, 81, 523–532. [Google Scholar] [CrossRef]
- Campañone, L.A.; Roche, L.A.; Salvadori, V.O.; Mascheroni, R.H. Structural Studies on Unpackaged Foods during Their Freezing and Storage. J. Food Sci. 2006, 71, E218–E226. [Google Scholar] [CrossRef]
- Smith, G.C.; Spaeth, C.W.; Carpenter, Z.L.; King, G.T.; Hoke, K.E. The Effects of Freezing, Frozen Storage Conditions and Degree of Doneness on Lamb Palatability Characteristics. J. Food Sci. 1968, 33, 19–24. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- De Paula Paseto Fernandes, R.; de Alvarenga Freire, M.T.; da Costa Carrer, C.; Trindade, M.A. Evaluation of Physicochemical, Microbiological and Sensory Stability of Frozen Stored Vacuum-Packed Lamb Meat. J. Integr. Agric. 2013, 12, 1946–1952. [Google Scholar] [CrossRef]
- Vieira, C.; Diaz, M.T.; Martínez, B.; García-Cachán, M.D. Effect of frozen storage conditions (temperature and length of storage) on microbiological and sensory quality of rustic crossbred beef at different states of ageing. Meat Sci. 2009, 83, 398–404. [Google Scholar] [CrossRef]
- Muela, E.; Monge, P.; Sañudo, C.; Campo, M.M.; Beltrán, J.A. Sensory quality of lamb following long-term frozen storage. Meat Sci. 2016, 114, 32–37. [Google Scholar] [CrossRef]
- Muela, E.; Sañudo, C.; Campo, M.M.; Medel, I.; Beltrán, J.A. Effect of freezing method and frozen storage duration on lamb sensory quality. Meat Sci. 2012, 90, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Luyet, B. Effects of freezing on muscle tissue. In Proceedings of the 17th Reciprocal Meat Conference, Madison, Wisconsin, American Foundation for Biological Research, Washington, DC, USA, 1964. [Google Scholar]
- Petrović, L.; Grujić, R.; Petrović, M. Definition of the optimal freezing rate—2. Investigation of the physico-chemical properties of beef M. longissimus dorsi frozen at different freezing rates. Meat Sci. 1993, 33, 319–331. [Google Scholar] [CrossRef]
- Duckett, S.K.; Klein, T.A.; Leckie, R.K.; Thorngate, J.H.; Busboom, J.R.; Snowder, G.D. Effect of freezing on calpastatin activity and tenderness of callipyge lamb. J. Anim. Sci. 1998, 76, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Coombs, C.E.O.; Holman, B.W.B.; Friend, M.A.; Hopkins, D.L. Long-term red meat preservation using chilled and frozen storage combinations: A review. Meat Sci. 2017, 125, 84–94. [Google Scholar] [CrossRef]
- Stenberg, E.; Arvidsson-Segerkvist, K.; Karlsson, A.H.; Ólafsdóttir, A.; Hilmarsson, Ó.Þ.; Gudjónsdóttir, M.; Thorkelsson, G. A Comparison of Two Different Slaughter Systems for Lambs. Effects on Carcass Characteristics, Technological Meat Quality and Sensory Attributes. Animals 2021, 11, 2935. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Sensory Evaluation Practices; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- SAS 9.4, S.I.I.; SAS Version 9.4. SAS Institute Inc.: Cary, NC, USA, 2018.
- Pedersen, H.T.; Bro, R.; Engelsen, S.B. Towards Rapid and Unique Curve Resolution of Low-Field NMR Relaxation Data: Trilinear SLICING versus Two-Dimensional Curve Fitting. J. Magn. Reson. 2002, 157, 141–155. [Google Scholar] [CrossRef]
- Straadt, I.K.; Rasmussen, M.; Andersen, H.J.; Bertram, H.C. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss—A combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 2007, 75, 687–695. [Google Scholar] [CrossRef]
- Gudjónsdóttir, M.; Gacutan, M.D.; Mendes, A.C.; Chronakis, I.S.; Jespersen, L.; Karlsson, A.H. Effects of electrospun chitosan wrapping for dry-ageing of beef, as studied by microbiological, physicochemical and low-field nuclear magnetic resonance analysis. Food Chem. 2015, 184, 167–175. [Google Scholar] [CrossRef]
- Bertram, H.C.; Karlsson, A.H.; Rasmussen, M.; Pedersen, O.D.; Dønstrup, S.; Andersen, H.J. Origin of Multiexponential T2 Relaxation in Muscle Myowater. J. Agric. Food Chem. 2001, 49, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.-H.; Stuart, A.; Kim, Y.H.B. Effect of different aging temperatures prior to freezing on meat quality attributes of frozen/thawed lamb loins. Meat Sci. 2016, 116, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.S.B.; Francisco, C.L.; Lino, D.M.; Borba, H. Meat quality of Santa Inês lamb chilled-then-frozen storage up to 12 months. Meat Sci. 2019, 148, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Hanna, M.A.; Mandigo, R.W. Effect of Frozen Storage Conditions on Yields, Shear Strength and Color of Ground Beef Patties. J. Food Sci. 1988, 53, 696–700. [Google Scholar] [CrossRef]
- Farouk, M.M.; Wieliczko, K.J.; Merts, I. Ultra-fast freezing and low storage temperatures are not necessary to maintain the functional properties of manufacturing beef. Meat Sci. 2004, 66, 171–179. [Google Scholar] [CrossRef]
- Muela, E.; Sañudo, C.; Campo, M.M.; Medel, I.; Beltrán, J.A. Effect of freezing method and frozen storage duration on instrumental quality of lamb throughout display. Meat Sci. 2010, 84, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Moore, V.J.; Young, O.A. The effects of electrical stimulation, thawing, ageing and packaging on the colour and display life of lamb chops. Meat Sci. 1991, 30, 131–145. [Google Scholar] [CrossRef]
- Muela, E.; Monge, P.; Sañudo, C.; Campo, M.M.; Beltrán, J.A. Meat quality of lamb frozen stored up to 21months: Instrumental analyses on thawed meat during display. Meat Sci. 2015, 102, 35–40. [Google Scholar] [CrossRef]
- Farouk, M.M.; Swan, J.E. Effect of rigor temperature and frozen storage on functional properties of hot-boned manufacturing beef. Meat Sci. 1998, 49, 233–247. [Google Scholar] [CrossRef]
- Fernández, P.P.; Sanz, P.D.; Molina-García, A.D.; Otero, L.; Guignon, B.; Vaudagna, S.R. Conventional freezing plus high pressure–low temperature treatment: Physical properties, microbial quality and storage stability of beef meat. Meat Sci. 2007, 77, 616–625. [Google Scholar] [CrossRef]
- Lindahl, G.; Lundström, K.; Tornberg, E. Contribution of pigment content, myoglobin forms and internal reflectance to the colour of pork loin and ham from pure breed pigs. Meat Sci. 2001, 59, 141–151. [Google Scholar] [CrossRef]
- Khliji, S.; van de Ven, R.; Lamb, T.A.; Lanza, M.; Hopkins, D.L. Relationship between consumer ranking of lamb colour and objective measures of colour. Meat Sci. 2010, 85, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Duckett, S.K.; Klein, T.A.; Dodson, M.V.; Snowder, G.D. Tenderness of normal and callipyge lamb aged fresh or after freezing∗. Meat Sci. 1998, 49, 19–26. [Google Scholar] [CrossRef]
- Shorthose, W.R.; Powell, V.H.; Harris, P.V. Influence of Electrical Stimulation, Cooling Rates and Aging on the Shear Force Values of Chilled Lamb. J. Food Sci. 1986, 51, 889–892. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. Effect of freezing method and frozen storage duration on odor-active compounds and sensory perception of lamb. Food Res. Int. 2013, 54, 772–780. [Google Scholar] [CrossRef]
- Pouliot, E.; Gariépy, C.; Thériault, M.; Avezard, C.; Fortin, J.; Simmons, N.J.; Castonguay, F.W. Effects of low-voltage electrical stimulation and aging on lamb meat quality. Can. J. Anim. Sci. 2012, 92, 59–66. [Google Scholar] [CrossRef]
- Hagyard, C.J.; Keiller, A.H.; Cummings, T.L.; Chrystall, B.B. Frozen storage conditions and rancid flavour development in lamb. Meat Sci. 1993, 35, 305–312. [Google Scholar] [CrossRef]
- Lambelet, P.; Berrocal, R.; Ducret, F. Low resolution NMR spectroscopy: A tool to study protein denaturation: I. Application to diamagnetic whey proteins. J. Dairy Res. 1989, 56, 211–222. [Google Scholar] [CrossRef]
- Gudjónsdóttir, M.; Jónsson, Á.; Bergsson, A.B.; Arason, S.; Rustad, T. Shrimp Processing Assessed by Low Field Nuclear Magnetic Resonance, Near Infrared Spectroscopy, and Physicochemical Measurements—The Effect of Polyphosphate Content and Length of Prebrining on Shrimp Muscle. J. Food Sci. 2011, 76, E357–E367. [Google Scholar] [CrossRef]
| Parameter | Fresh, n = 18 | Frozen, n = 20 | SEM 1 | p-Value 2 |
|---|---|---|---|---|
| Lightness (L*) | 37.5 ± 1.7 | 36.7 ± 1.4 | 0.38 | 0.0654 |
| Redness (a*) | 19.5 ± 1.8 | 18.8 ± 1.4 | 0.37 | 0.0080 |
| Yellowness (b*) | 4.5 ± 1.2 | 6.0 ± 1.4 | 0.33 | 0.0006 |
| Fluid loss (%) | 15 ± 3.1 | 27 ± 3.3 | 0.70 | <0.0001 |
| WBSF (N) 3 | 46 ± 13.1 | 51 ± 13.0 | 3.03 | 0.0837 |
| Parameter | Fresh, n = 18 | Frozen, n = 20 | SEM 1 | p-Value 2 | |
|---|---|---|---|---|---|
| Odour attributes 3 | Frying | 33 ± 8.7 | 36 ± 6.4 | 1.78 | 0.2465 |
| Sour | 12 ± 3.8 | 14 ± 2.9 | 0.82 | 0.1043 | |
| Fatty | 27 ± 3.2 | 32 ± 7.0 | 1.38 | 0.0229 | |
| Liver | 30 ± 4.6 | 32 ± 4.4 | 1.05 | 0.1665 | |
| Appearance attribute 3 | Colour | 31 ± 4.7 | 30 ± 6.8 | 1.44 | 0.4780 |
| Flavour attributes 3 | Frying | 24 ± 4.5 | 29 ± 6.8 | 1.33 | 0.0340 |
| Sour | 25 ± 5.1 | 31 ± 8.0 | 1.55 | 0.0204 | |
| Fatty | 16 ± 3.5 | 22 ± 4.8 | 0.98 | 0.0003 | |
| Sweet | 9 ± 1.6 | 9 ± 1.8 | 0.40 | 0.6240 | |
| Liver | 41 ± 5.2 | 45 ± 5.3 | 1.38 | 0.0222 | |
| Texture attributes 3 | Soft | 50 ± 13.9 | 53 ± 13.8 | 3.22 | 0.5586 |
| Tender | 48 ± 16.0 | 50 ± 14.8 | 3.71 | 0.6816 | |
| Juicy | 49 ± 9.6 | 35 ± 11.2 | 2.40 | 0.0001 | |
| Mushy | 16 ± 4.7 | 14 ± 3.7 | 1.00 | 0.0714 | |
| Parameters | Fresh, n = 18 | Frozen, n = 18 | SEM 1 | p-Value 2 |
|---|---|---|---|---|
| Translational relaxation parameters | ||||
| T2b (ms) | 13.6 ± 6.9 | 12.1 ± 8.8 | 1.92 | 0.5972 |
| T21 (ms) | 48.7 ± 4.3 | 41.0 ± 4.3 | 1.05 | <0.0001 |
| T22 (ms) | 224.1 ± 89.3 | 105.5 ± 20.4 | 15.8 | <0.0001 |
| Water population within muscle sample | ||||
| A2b (%) | 9.1 ± 7.5 | 9.0 ± 14.5 | 2.80 | 0.9657 |
| A21 (%) | 81.5 ± 7.2 | 78.0 ± 13.2 | 2.59 | 0.3549 |
| A22 (%) | 9.4 ± 2.1 | 13.0 ± 3.9 | 0.75 | 0.0029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenberg, E.; Arvidsson-Segerkvist, K.; Karlsson, A.H.; Ólafsdóttir, A.; Hilmarsson, Ó.Þ.; Gudjónsdóttir, M.; Thorkelsson, G. A Comparison of Fresh and Frozen Lamb Meat—Differences in Technological Meat Quality and Sensory Attributes. Animals 2022, 12, 2830. https://doi.org/10.3390/ani12202830
Stenberg E, Arvidsson-Segerkvist K, Karlsson AH, Ólafsdóttir A, Hilmarsson ÓÞ, Gudjónsdóttir M, Thorkelsson G. A Comparison of Fresh and Frozen Lamb Meat—Differences in Technological Meat Quality and Sensory Attributes. Animals. 2022; 12(20):2830. https://doi.org/10.3390/ani12202830
Chicago/Turabian StyleStenberg, Elin, Katarina Arvidsson-Segerkvist, Anders H. Karlsson, Aðalheiður Ólafsdóttir, Óli Þór Hilmarsson, María Gudjónsdóttir, and Guðjón Thorkelsson. 2022. "A Comparison of Fresh and Frozen Lamb Meat—Differences in Technological Meat Quality and Sensory Attributes" Animals 12, no. 20: 2830. https://doi.org/10.3390/ani12202830
APA StyleStenberg, E., Arvidsson-Segerkvist, K., Karlsson, A. H., Ólafsdóttir, A., Hilmarsson, Ó. Þ., Gudjónsdóttir, M., & Thorkelsson, G. (2022). A Comparison of Fresh and Frozen Lamb Meat—Differences in Technological Meat Quality and Sensory Attributes. Animals, 12(20), 2830. https://doi.org/10.3390/ani12202830





