Dexmedetomidine Effectively Sedates Asian Elephants (Elephas maximus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Sedation Assessment
2.4. Responsiveness Assessment
2.5. Vital Sign Parameters Assessment
2.6. Clinical Observation
2.7. Data Analysis
3. Results
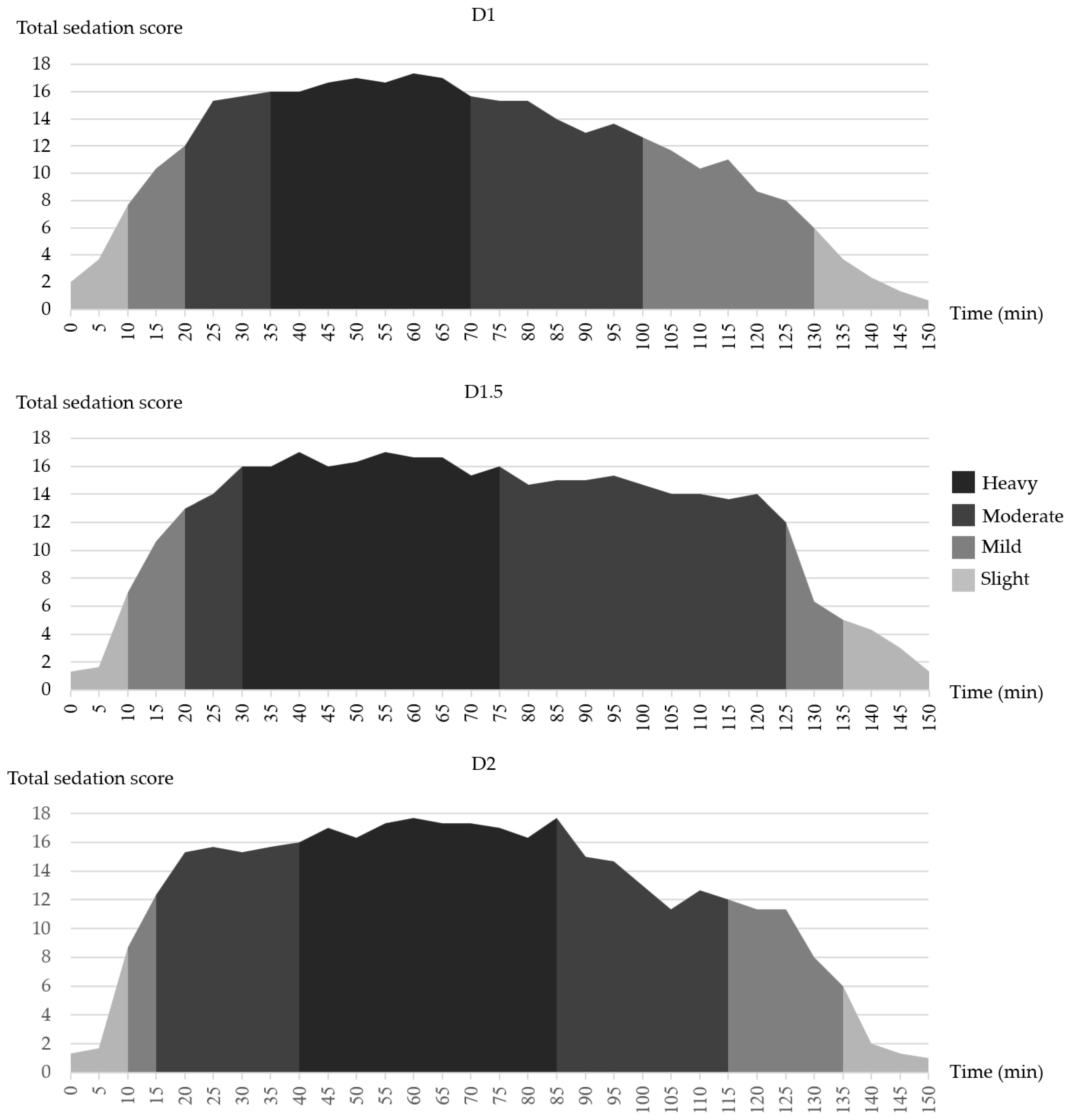
3.1. Sedation Score
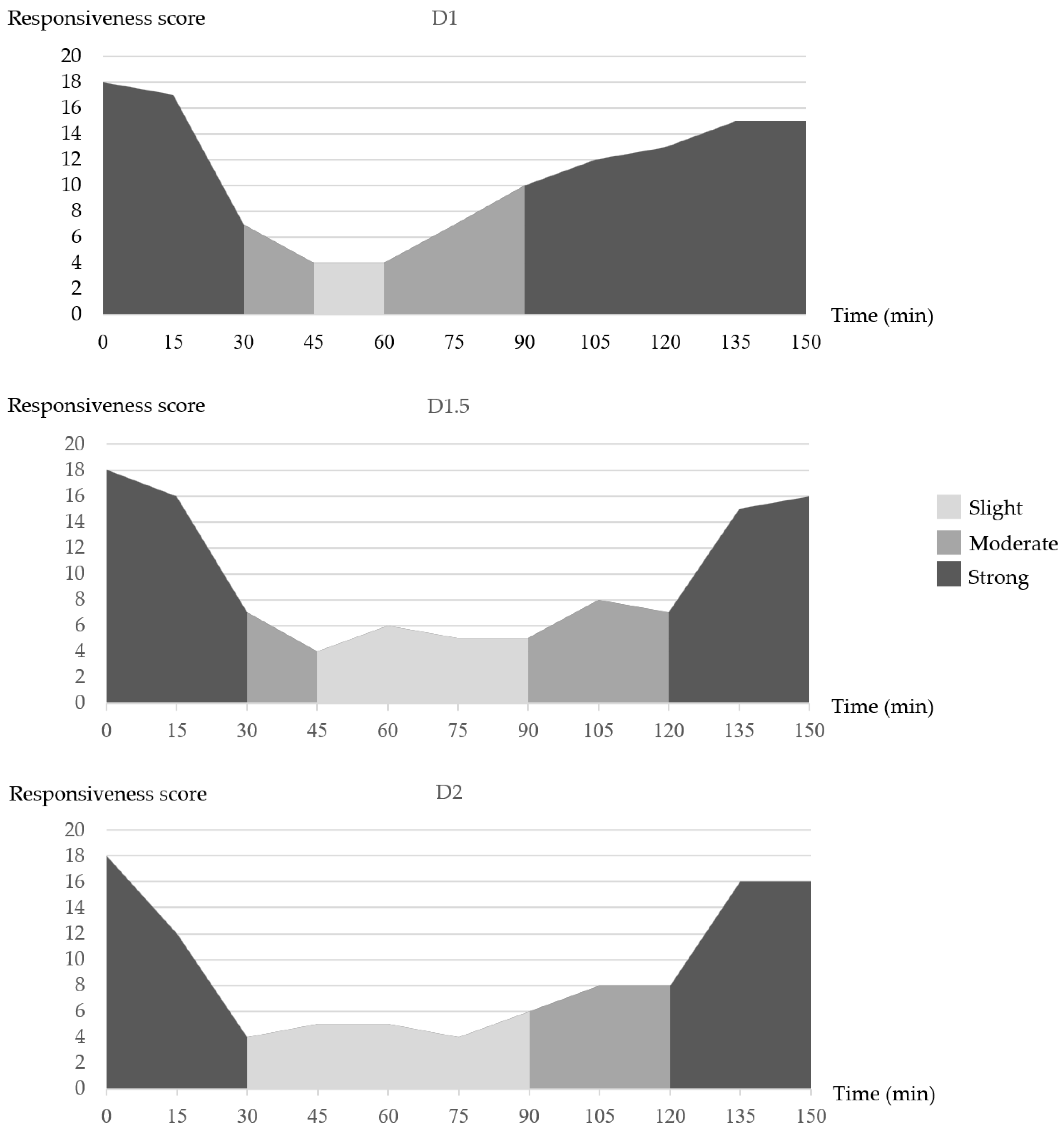
3.2. Responsiveness Score
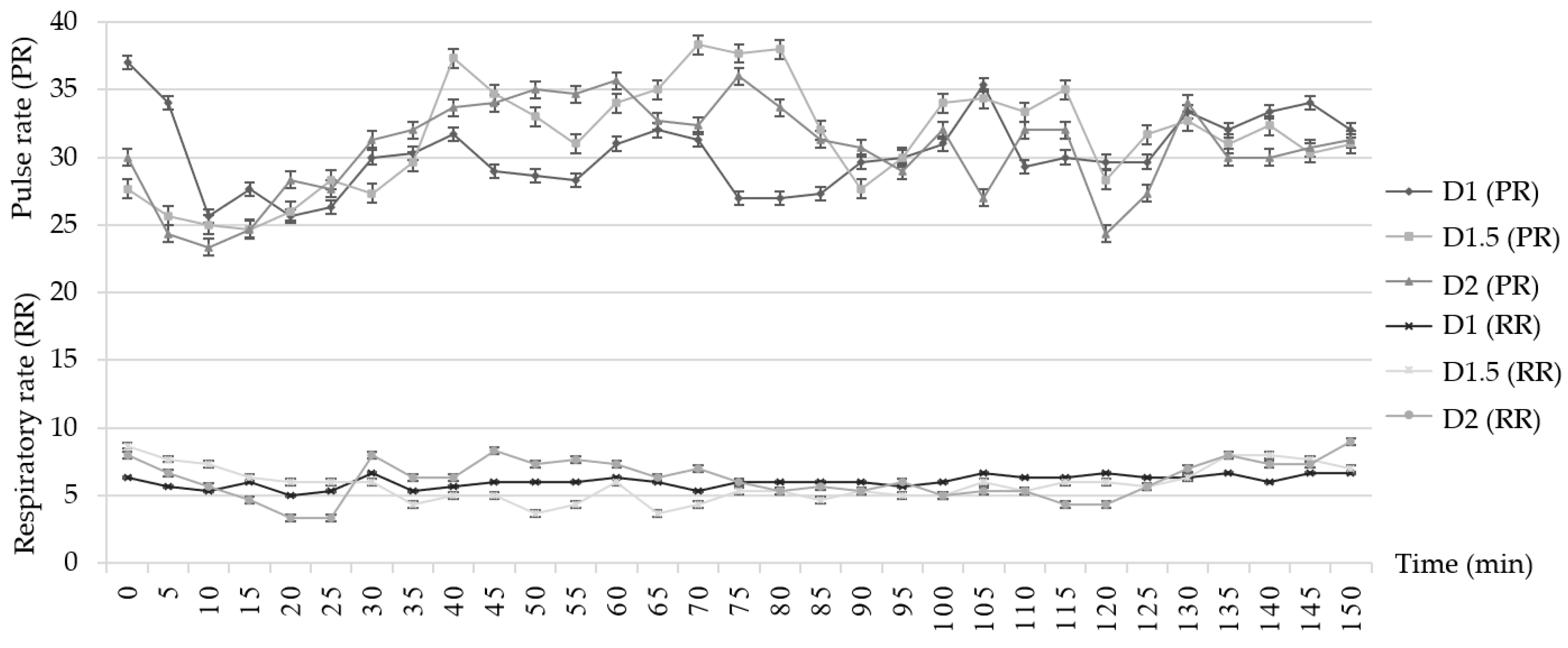
3.3. Vital Sign
3.4. Clinical Observation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansson, T.; Perera, B.V.; Edner, A.; Fahlman, Å. Standing Sedation with Xylazine and Reversal with Yohimbine in Juvenile Asian Elephants (Elephas maximus). J. Zoo Wildl. Med. 2021, 52, 437–444. [Google Scholar] [CrossRef]
- Neiffer, D.L.; Miller, M.A.; Weber, M.; Stetter, M.; Fontenot, D.K.; Robbins, P.K.; Pye, G.W. Standing sedation in African elephants (Loxodonta africana) using detomidine-butorphanol combinations. J. Zoo Wildl. Med. 2005, 36, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Buranapim, N.; Thitaram, C.; Angkawanish, T.; Sombutputorn, P.; Langkaphin, W.; Thongtip, N.; Janyamethakul, T.; Boontong, P.; Tipkantha, W. General anesthesia in Asian Elephant (Elephas maximus) using combination of medetomidine hydrochloride and etorphine hydrochloride. Vet. Integr. Sci. 2019, 17, 101–114. Available online: https://he02.tci-thaijo.org/index.php/vis/article/view/151446. (accessed on 15 September 2021).
- Sarma, B.; Pathak, S.C.; Sarma, K.K. Medetomidine a novel immobilizing agent for the elephant (Elephas maximus). Res. Vet. Sci. 2002, 73, 315–317. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Liu, G.; Lin, J.-H.; Jin, Y.-P. Efficacy and Safety of Dexmedetomidine Premedication in Balanced Anesthesia: A Systematic Review and Meta-Analysis in Dogs. Animals 2021, 11, 3254. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, J.; Pleyers, T.; Raillard, M.; Spadavecchia, C.; Levionnois, O.L. Effect of Medetomidine, Dexmedetomidine, and Their Reversal with Atipamezole on the Nociceptive Withdrawal Reflex in Beagles. Animals 2020, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Bruniges, N.; Taylor, P.M.; Yates, D. Injectable anaesthesia for adult cat and kitten castration: Effects of medetomidine, dexmedetomidine and atipamezole on recovery. J. Feline Med. Surg. 2016, 18, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Savola, M.K.; MacIver, M.B.; Doze, V.A.; Kendig, J.J.; Maze, M. The alpha 2-adrenoceptor agonist dexmedetomidine increases the apparent potency of the volatile anesthetic isoflurane in rats in vivo and in hippocampal slice in vitro. Brain Res. 1991, 548, 23–28. [Google Scholar] [CrossRef]
- West, S.E.; Lee, J.C.; Johns, T.N.; Nunamaker, E.A. Intraperitoneal Alfaxalone and Alfaxalone-Dexmedetomidine Anesthesia in Sprague-Dawley Rats (Rattus norvegicus). J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Rioja, E.; Kerr, C.L.; Enouri, S.S.; McDonell, W.N. Sedative and cardiopulmonary effects of medetomidine hydrochloride and xylazine hydrochloride and their reversal with atipamezole hydrochloride in calves. Am. J. Vet. Res. 2008, 69, 319–329. [Google Scholar] [CrossRef]
- Samimi, A.S.; Molaei, M.M.; Azari, O.; Ebrahimpour, F. Comparative evaluation of sedative and clinical effects of dexmedetomidine and xylazine in dromedary calves (Camelus dromedarius). Vet. Anaesth. Analg. 2020, 47, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.L.; Grimsrud, K.N.; Stanley, S.D.; Steffey, E.P.; Mama, K.R. Pharmacokinetics and pharmacodynamics of intravenous dexmedetomidine in the horse. J. Vet. Pharmacol. Ther. 2015, 38, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Samimi, A.S. Evaluation of the sedative and clinical effects of xylazine, detomidine, medetomidine and dexmedetomidine in miniature donkeys. N. Z. Vet. J. 2020, 68, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, F.; Cagnardi, P.; Villa, R.; Rabbogliatti, V.; Lucatello, L.; Capolongo, F.; Gioeni, D.; Capasso, M.; Magnone, W.; Ravasio, G. Dexmedetomidine and ketamine simultaneous administration in tigers (Panthera tigris): Pharmacokinetics and clinical effects. Vet. Rec. Open 2020, 7, e000412. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.D. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 2003, 44, 885–897. [Google Scholar] [PubMed]
- Nishimura, L.T.; Auckburally, A.; Santilli, J.; Vieira, B.H.B.; Garcia, D.O.; Honsho, C.S.; de Mattos, E., Jr. Effects of dexmedetomidine combined with commonly administered opioids on clinical variables in dogs. Am. J. Vet. Res. 2018, 79, 267–275. [Google Scholar] [CrossRef]
- Vlerick, L.; Devreese, M.; Peremans, K.; Dockx, R.; Croubels, S.; Duchateau, L.; Polis, I. Pharmacokinetics, absolute bioavailability and tolerability of ketamine after intranasal administration to dexmedetomidine sedated dogs. PLoS ONE 2020, 15, e0227762. [Google Scholar] [CrossRef] [PubMed]
- Gertler, R.; Brown, H.C.; Mitchell, D.H.; Silvius, E.N. Dexmedetomidine: A novel sedative-analgesic agent. Bayl. Univ. Med. Cent. Proc. 2001, 14, 13–21. [Google Scholar] [CrossRef]
- Weerink, M.A.S.; Struys, M.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [PubMed]
- Limprasutr, V.; Sharp, P.; Jampachaisri, K.; Pacharinsak, C.; Durongphongtorn, S. Tiletamine/zolazepam and dexmedetomidine with tramadol provide effective general anesthesia in rats. Anim. Model Exp. Med. 2021, 4, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.F.; Maiello, P.; Wright, M.J., Jr.; Kracinovsky, K.B.; Newsome, J.T. Comparison of Atipamezole with Yohimbine for Antagonism of Xylazine in Mice Anesthetized with Ketamine and Xylazine. J. Am. Assoc. Lab Anim. Sci. 2017, 56, 142–147. [Google Scholar]
- Atipamezole. Available online: https://www.vetfolio.com/learn/article/pharm-profile-atipamezole (accessed on 1 April 2022).
- Benito, J.; Monteiro, B.; Beaudry, F.; Steagall, P. Efficacy and pharmacokinetics of bupivacaine with epinephrine or dexmedetomidine after intraperitoneal administration in cats undergoing ovariohysterectomy. Can. J. Vet. Res. 2018, 82, 124–130. [Google Scholar] [PubMed]
- Gomes, L.; Czeczko, N.G.; Araújo, R.; Cartagenes, M.; Barbosa Neto, J.O.; Garcia, J.B.S. Effect of intra-articular dexmedetomidine on experimental osteoarthritis in rats. PLoS ONE 2021, 16, e0245194. [Google Scholar] [CrossRef]
- Kaye, A.D.; Chernobylsky, D.J.; Thakur, P.; Siddaiah, H.; Kaye, R.J.; Eng, L.K.; Harbell, M.W.; Lajaunie, J.; Cornett, E.M. Dexmedetomidine in Enhanced Recovery After Surgery (ERAS) Protocols for Postoperative Pain. Curr. Pain Headache Rep. 2021, 24, 21. [Google Scholar] [CrossRef]
- Li, C.; Qu, J. Efficacy of dexmedetomidine for pain management in knee arthroscopy: A systematic review and meta-analysis. Medicine 2017, 96, e7938. [Google Scholar] [CrossRef]
- Li, S.; Ouyang, B.; Zhao, X.; Wang, Y. Analgesic effect of dexmedetomidine on oxaliplatin-induced neuropathic pain in rats. Nan Fang Yi Ke Da Xue Xue Bao 2018, 38, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Wiatrowski, R. Current State of Pain Treatment: Does Dexmedetomidine Have a Role to Play? AANA. J. 2021, 89, 77–86. [Google Scholar] [PubMed]
- Zhang, W.; Yu, T.; Cui, X.; Yu, H.; Li, X. Analgesic effect of dexmedetomidine in rats after chronic constriction injury by mediating microRNA-101 expression and the E2F2-TLR4-NF-κB axis. Exp. Physiol. 2020, 105, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Pleym, H.; Spigset, O.; Kharasch, E.D.; Dale, O. Gender differences in drug effects: Implications for anesthesiologists. Acta Anaesthesiol. Scand. 2003, 47, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Zambricki, E.A.; Dalecy, L.G. Rat sex differences in anesthesia. Comp. Med. 2004, 54, 49–53. [Google Scholar] [PubMed]
- Acevedo-Arcique, C.M.; Ibancovichi, J.A.; Chavez, J.R.; Gutierrez-Blanco, E.; Moran-Muñoz, R.; Victoria-Mora, J.M.; Tendillo-Cortijo, F.; Santos-González, M.; Sanchez-Aparicio, P. Lidocaine, dexmedetomidine and their combination reduce isoflurane minimum alveolar concentration in dogs. PLoS ONE 2014, 9, e106620. [Google Scholar] [CrossRef]
- Sayce, L.J.; Powell, M.E.; Kimball, E.E.; Chen, P.; Gartling, G.J.; Rousseau, B. Continuous Rate Infusion of Ketamine Hydrochloride and Dexmedetomidine for Maintenance of Anesthesia during Laryngotracheal Surgery in New Zealand White Rabbits (Oryctolagus cuniculus). J. Am. Assoc. Lab Anim. Sci. 2020, 59, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Uilenreef, J.J.; Murrell, J.C.; McKusick, B.C.; Hellebrekers, L.J. Dexmedetomidine continuous rate infusion during isoflurane anaesthesia in canine surgical patients. Vet. Anaesth. Analg. 2008, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.S.; Carregaro, A.B.; Machado, M.; Antonow, R.R. Sedative and cardiopulmonary effects of buprenorphine and xylazine in horses. Can. J. Vet. Res. 2011, 75, 35–41. [Google Scholar] [PubMed]
- Fyffe, J.J. Effects of xylazine on humans: A review. Aust. Vet. J. 1994, 71, 294–295. [Google Scholar] [CrossRef] [PubMed]

| Elephant | Week 1 | Week 3 | Week 5 |
|---|---|---|---|
| Elephant 1 | D1 | D1.5 | D2 |
| Elephant 2 | D1.5 | D2 | D1 |
| Elephant 3 | D2 | D1 | D1.5 |
| Criterions | 0–5 Slight Sedation | 6–11 Mild | 12–15 Moderate | 16–18 Heavy |
|---|---|---|---|---|
| Awareness of surroundings | Yes | Moderately aware | Less aware | Not present |
| Standing posture and balance | Moving, rocking back and forth | Stop moving, moderately rocking back and forth | Standstill, less movement, less rocking | Completely motionless |
| Ear movement | Constant flipping (> 10 times/min) | Moderately flipping (5–10 times/min) | Little flipping (< 5 times/min) | Not present |
| Tail movement | Constant tail movement (> 10 times/min) | Moderate tail movement (5–10 times/min) | Little tail movement (< 5 times/min) | Not present |
| Trunk movement | Constant trunk movement | Moderate trunk movement | Little trunk movement, Relaxed, lying on the ground, occasional motion | Relaxed, lying on the ground, motionless |
| Genital protruding | Intact (no prolapse) | Mild prolapse | Protruding from prepuce (incomplete prolapse) | Protruding from prepuce (complete prolapse) |
| Response | 0–6 Slight | 7–11 Moderate | 12–18 Strong |
|---|---|---|---|
| Palpebral reflex | Intermittent or slow response, sluggish | Moderate reflex | Present and strong |
| Eye | Slightly open, able to lift eyelid, third eyelid intact | Moderately open | Wide open |
| Ear tone (Gently pull the ears) | Little response/ flipping ears | Moderate response/ flipping ears | Strong response/ flipping ears |
| Jaw tone (Gently pull lower lip to open the mouth) | Slight resistance | Moderate resistance | Strong resistance |
| Tail tone (Lifting tail to observe resistance) | Slight resistance | Moderate resistance | Strong resistance |
| Pain reflex (Pinch forelimb skin with a hemostat forceps) | Slight resistance | Moderate resistance | Strong resistance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buranapim, N.; Kulnanan, P.; Chingpathomkul, K.; Angkawanish, T.; Chansitthiwet, S.; Langkaphin, W.; Sombutputorn, P.; Monchaivanakit, N.; Kasemjai, K.; Namwongprom, K.; et al. Dexmedetomidine Effectively Sedates Asian Elephants (Elephas maximus). Animals 2022, 12, 2787. https://doi.org/10.3390/ani12202787
Buranapim N, Kulnanan P, Chingpathomkul K, Angkawanish T, Chansitthiwet S, Langkaphin W, Sombutputorn P, Monchaivanakit N, Kasemjai K, Namwongprom K, et al. Dexmedetomidine Effectively Sedates Asian Elephants (Elephas maximus). Animals. 2022; 12(20):2787. https://doi.org/10.3390/ani12202787
Chicago/Turabian StyleBuranapim, Nithidol, Pawinee Kulnanan, Kullapassorn Chingpathomkul, Taweepoke Angkawanish, Saran Chansitthiwet, Warangkhana Langkaphin, Petthisak Sombutputorn, Natcha Monchaivanakit, Kankawee Kasemjai, Kittikul Namwongprom, and et al. 2022. "Dexmedetomidine Effectively Sedates Asian Elephants (Elephas maximus)" Animals 12, no. 20: 2787. https://doi.org/10.3390/ani12202787
APA StyleBuranapim, N., Kulnanan, P., Chingpathomkul, K., Angkawanish, T., Chansitthiwet, S., Langkaphin, W., Sombutputorn, P., Monchaivanakit, N., Kasemjai, K., Namwongprom, K., Boonprasert, K., Bansiddhi, P., Thitaram, N., Sharp, P., Pacharinsak, C., & Thitaram, C. (2022). Dexmedetomidine Effectively Sedates Asian Elephants (Elephas maximus). Animals, 12(20), 2787. https://doi.org/10.3390/ani12202787








