Effect of Dimethylacetamide Concentration on Motility, Quality, Antioxidant Biomarkers, Anti-Freeze Gene Expression, and Fertilizing Ability of Frozen/Thawed Rooster Sperm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Management
2.2. Semen Collection and Freezing/Thawing Protocol
2.3. Sperm Motion Characteristics
2.4. Sperm Quality Characteristics
2.4.1. Viability
2.4.2. Acrosome Integrity
2.4.3. Plasma Membrane Status
2.5. Sperm Antioxidant Biomarkers
2.5.1. Semen Extraction and Protein Assay
2.5.2. Total Antioxidant Capacity
2.5.3. Superoxide Dismutase Activity
2.5.4. Glutathione Peroxidase Activity
2.5.5. Lipid Peroxidation
2.6. Anti-Freeze-Associated Gene Expression
2.7. Fertility Trial
2.8. Statistical Analysis
3. Results
3.1. Sperm Motion Characteristics
3.2. Sperm Quality Characteristics
3.3. Sperm Fertility Evaluation
3.4. Sperm Antioxidant Biomarkers
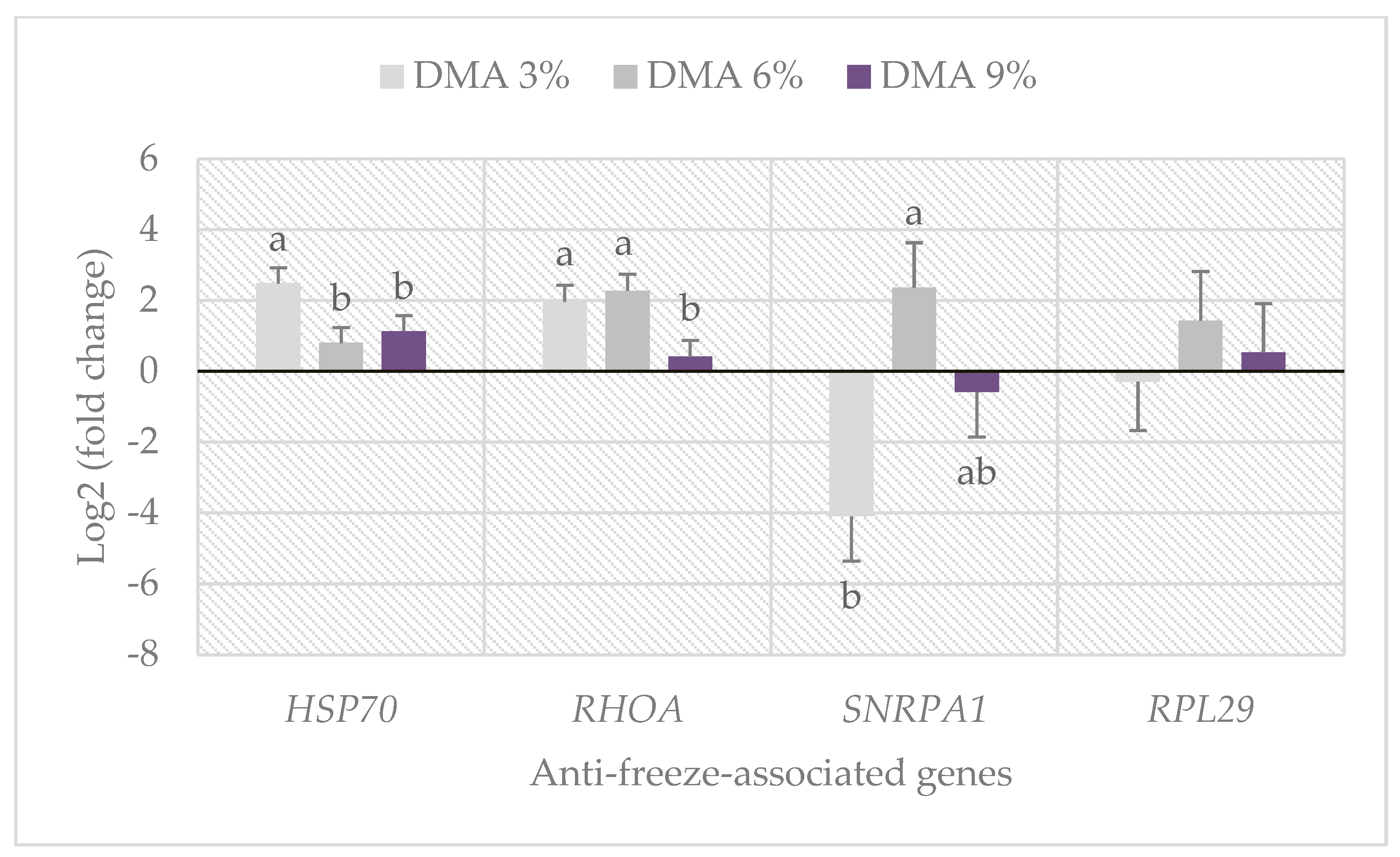
3.5. Anti-Freeze-Associated Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrabi, S.M.H.; Maxwell, W.M.C. A Review on Reproductive Biotechnologies for Conservation of Endangered Mammalian Species. Anim. Reprod. Sci. 2007, 99, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E. Current Status in Avian Semen Cryopreservation. Worlds Poult. Sci. J. 2007, 63, 213–222. [Google Scholar] [CrossRef]
- Long, J.A. Avian Semen Cryopreservation: What Are the Biological Challenges? Poult. Sci. 2006, 85, 232–236. [Google Scholar] [CrossRef]
- Partyka, A.; Nizański, W.; Łukaszewicz, E. Evaluation of Fresh and Frozen-Thawed Fowl Semen by Flow Cytometry. Theriogenology 2010, 74, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E.; Brillard, J.P. Specific Features of in Vivo and in Vitro Sperm Storage in Birds. Animal 2007, 1, 1472–1481. [Google Scholar] [CrossRef]
- Tselutin, K.; Seigneurin, F.; Blesbois, E. Comparison of Cryoprotectants and Methods of Cryopreservation of Fowl Spermatozoa. Poult. Sci. 1999, 78, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tatsumi, T.; Tsutsui, M.; Niinomi, T.; Imai, T.; Naito, M.; Tajima, A.; Nishi, Y. A Method for Cryopreserving Semen from Yakido Roosters Using N-Methylacetamide as a Cryoprotective Agent. Jap. Poult. Sci. 2010, 47, 297–301. [Google Scholar] [CrossRef]
- Osuga, T. Efficiencies of the Cryoprotectants N-Methylacetamide, N-Methylformamide and Dimethyl Sulfoxide, and the Cell Protectants Trehalose and Hydroxyethyl Starch in Cryopreservation of Swine Sperm. Nanomed. Nanotechnol. 2018, 3, 000139. [Google Scholar] [CrossRef]
- Blesbois, E.; Seigneurin, F.; Grasseau, I.; Limouzin, C.; Besnard, J.; Gourichon, D.; Coquerelle, G.; Rault, P.; Tixier-Boichard, M. Semen Cryopreservation for Ex Situ Management of Genetic Diversity in Chicken: Creation of the French Avian Cryobank. Poult. Sci. 2007, 86, 555–564. [Google Scholar] [CrossRef]
- Abouelezz, F.M.K.; Sayed, M.A.M.; Santiago-Moreno, J. Fertility Disturbances of Dimethylacetamide and Glycerol in Rooster Sperm Diluents: Discrimination among Effects Produced Pre and Post Freezing-Thawing Process. Anim. Reprod. Sci. 2017, 184, 228–234. [Google Scholar] [CrossRef]
- Murugesan, S.; Mahapatra, R. Cryopreservation of Ghagus Chicken Semen: Effect of Cryoprotectants, Diluents and Thawing Temperature. Reprod. Domest. Anim. 2020, 55, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E. Freezing Avian Semen. Avian Biol. Res. 2011, 4, 52–58. [Google Scholar] [CrossRef]
- Purdy, P.H.; Song, Y.; Silversides, F.G.; Blackburn, H.D. Evaluation of Glycerol Removal Techniques, Cryoprotectants, and Insemination Methods for Cryopreserving Rooster Sperm with Implications of Regeneration of Breed or Line or Both. Poult. Sci. 2009, 88, 2184–2191. [Google Scholar] [CrossRef]
- Mocé, E.; Grasseau, I.; Blesbois, E. Cryoprotectant and Freezing-Process Alter the Ability of Chicken Sperm to Acrosome React. Anim. Reprod. Sci. 2010, 122, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Wilmut, I. From Germ Cell Preservation to Regenerative Medicine: An Exciting Research Career in Biotechnology. Ann. Rev. Anim. Biosci. 2014, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Blesbois, E.; Grasseau, I.; Segineurin, F.; Seigneurin, F. Membrane Fluidity and the Ability of Domestic Bird Spermatozoa to Survive Cryopreservation. Reproduction 2005, 129, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, M.; Berlinguer, F.; Pasciu, V.; Succu, S.; Satta, V.; Leoni, G.G.; Zinellu, A.; Muzzeddu, M.; Carru, C.; Naitana, S. Differences in Semen Freezability and Intracellular ATP Content between the Rooster (Gallus Gallus Domesticus) and the Barbary Partridge (Alectoris Barbara). Theriogenology 2010, 74, 1010–1018. [Google Scholar] [CrossRef]
- Mehaisen, G.M.K.K.; Partyka, A.; Ligocka, Z.; Niżański, W. Cryoprotective Effect of Melatonin Supplementation on Post-Thawed Rooster Sperm Quality. Anim. Reprod. Sci. 2020, 212, 1–7. [Google Scholar] [CrossRef]
- Laura, Y.; Harimurti, S. Ismaya Effect of Different Levels of Dimethylacetamide (DMA) on Sperm Quality of Bangkok Rooster Chicken and Sperm Survivability in Reproductive Tract of Hen. Pak. J. Nutr. 2017, 16, 144–147. [Google Scholar] [CrossRef]
- Abouelezz, F.M.K.; Castaño, C.; Toledano-Díaz, A.; Esteso, M.C.; López-Sebastián, A.; Campo, J.L.; Santiago-Moreno, J. Effect of the Interaction between Cryoprotectant Concentration and Cryopreservation Method on Frozen/Thawed Chicken Sperm Variables. Reprod. Domest. Anim. 2015, 50, 135–141. [Google Scholar] [CrossRef]
- Blanco, J.M.; Long, J.A.; Gee, G.; Wildt, D.E.; Donoghue, A.M. Comparative Cryopreservation of Avian Spermatozoa: Benefits of Non-Permeating Osmoprotectants and ATP on Turkey and Crane Sperm Cryosurvival. Anim. Reprod. Sci. 2011, 123, 242–248. [Google Scholar] [CrossRef]
- Zaniboni, L.; Cassinelli, C.; Mangiagalli, M.G.; Gliozzi, T.M.; Cerolini, S. Pellet Cryopreservation for Chicken Semen: Effects of Sperm Working Concentration, Cryoprotectant Concentration, and Equilibration Time during in Vitro Processing. Theriogenology 2014, 82, 251–258. [Google Scholar] [CrossRef]
- Tang, M.; Cao, J.; Yu, Z.; Liu, H.; Yang, F.; Huang, S.; He, J.; Yan, H. New Semen Freezing Method for Chicken and Drake Using Dimethylacetamide as the Cryoprotectant. Poult. Sci. 2021, 100, 101091. [Google Scholar] [CrossRef]
- Ramadan, G.S.; Moghaieb, R.E.; El-Ghamry, A.A.; El-Komy, E.M.; Stino, F.K.R. Microsatellite Marker Associated with Body Weight in Local Egyptian Broiler Line Cairo B-2. Biosci. Res. 2018, 15, 3188–3201. [Google Scholar]
- Bakst, M.R.; Dymond, J.S. Artificial Insemination in Poultry. In Success in Artificial Insemination-Quality of Semen and Diagnostics Employed; Lemma, A., Ed.; IntechOpen: London, UK, 2013; pp. 175–195. [Google Scholar] [CrossRef][Green Version]
- Łukaszewicz, E. Cryopreservation of Anser Anser L. Gander Semen. Zeszt. Nauk. Akad. Rol. We Wroc. 2002, 440, 1–111. [Google Scholar]
- Lu, J.C.; Huang, Y.F.; Lü, N.Q. Computer-Aided Sperm Analysis: Past, Present and Future. Andrologia 2014, 46, 329–338. [Google Scholar] [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Hussain, I.; Blesbois, E. Cryopreservation of Indian Red Jungle Fowl (Gallus Gallus Murghi) Semen. Anim. Reprod. Sci. 2016, 174, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of Serum Proteins by Means of the Biuret Reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the Measurement of Antioxidant Activity in Human Fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Nichi, M.; Bols, P.E.J.; Züge, R.M.; Barnabe, V.H.; Goovaerts, I.G.F.; Barnabe, R.C.; Cortada, C.N.M. Seasonal Variation in Semen Quality in Bos Indicus and Bos Taurus Bulls Raised under Tropical Conditions. Theriogenology 2006, 66, 822–828. [Google Scholar] [CrossRef]
- Kei, S. Serum Lipid Peroxide in Cerebrovascular Disorders Determined by a New Colorimetric Method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef]
- Qi, X.L.; Xing, K.; Huang, Z.; Chen, Y.; Wang, L.; Zhang, L.C.; Sheng, X.H.; Wang, X.G.; Ni, H.M.; Guo, Y. Comparative Transcriptome Analysis Digs out Genes Related to Antifreeze between Fresh and Frozen—Thawed Rooster Sperm. Poult. Sci. 2020, 99, 2841–2851. [Google Scholar] [CrossRef]
- Singh, R.P.; Shafeeque, C.M.; Sharma, S.K.; Singh, R.; Mohan, J.; Sastry, K.V.H.; Saxena, V.K.; Azeez, P.A. Chicken Sperm Transcriptome Profiling by Microarray Analysis. Genome 2016, 59, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zaniboni, L.; Madeddu, M.; Mosca, F.; Sayed, A.A.; Marelli, S.P.; di Iorio, M.; Iaffaldano, N.; Cerolini, S. Concentration Dependent Effect of Dimethylacetamide and N-Methylacetamide on the Quality and Fertility of Cryopreserved Chicken Semen. Cryobiology 2022, 106, 66–72. [Google Scholar] [CrossRef]
- Mosca, F.; Zaniboni, L.; Sayed, A.A.; Madeddu, M.; Iaffaldano, N.; Cerolini, S. Effect of Dimethylacetamide and N-Methylacetamide on the Quality and Fertility of Frozen/Thawed Chicken Semen. Poult. Sci. 2019, 98, 6071–6077. [Google Scholar] [CrossRef]
- Shanmugam, M.; Kumar, K.P.; Mahapatra, R.K.; Laxmi, N.A. Effect of Different Cryoprotectants on Post-Thaw Semen Parameters and Fertility in Nicobari Chicken. Indian J. Poult. Sci. 2018, 53, 208. [Google Scholar] [CrossRef]
- Dogan, S.; Mason, M.C.; Govindaraju, A.; Belser, L.; Kaya, A.; Stokes, J.; Rowe, D.; Memili, E. Interrelationships between Apoptosis and Fertility in Bull. J. Reprod. Dev. 2013, 59, 18. [Google Scholar] [CrossRef]
- Curry, M.R. Cryopreservation of Semen from Domestic Livestock. Rev. Reprod. 2000, 5, 45–52. [Google Scholar] [CrossRef]
- Mavi, G.K.; Dubey, P.P.; Cheema, R.S. Association of Antioxidant Defense System with Semen Attributes Vis a Vis Fertility in Exotic and Indigenous Chicken Breeds. Theriogenology 2020, 144, 158–163. [Google Scholar] [CrossRef]
- Ostermeier, G.C.; Goodrich, R.J.; Diamond, M.P.; Dix, D.J.; Krawetz, S.A. Toward Using Stable Spermatozoal RNAs for Prognostic Assessment of Male Factor Fertility. Fertil. Steril. 2005, 83, 1687–1694. [Google Scholar] [CrossRef]
- Alvarez-Rodríguez, M.; Alvarez, M.; Borragan, S.; Martinez-Pastor, F.; Holt, W.V.; Fazeli, A.; de Paz, P.; Anel, L. The Addition of Heat Shock Protein HSPA8 to Cryoprotective Media Improves the Survival of Brown Bear (Ursus Arctos) Spermatozoa during Chilling and after Cryopreservation. Theriogenology 2013, 79, 541–550. [Google Scholar] [CrossRef]
- Holt, W.V.; del Valle, I.; Fazeli, A. Heat Shock Protein A8 Stabilizes the Bull Sperm Plasma Membrane during Cryopreservation: Effects of Breed, Protein Concentration, and Mode of Use. Theriogenology 2015, 84, 693–701. [Google Scholar] [CrossRef]
- García-Cardeña, G.; Fan, R.; Shah, V.; Sorrentino, R.; Cirino, G.; Papapetropoulos, A.; Sessa, W.C. Dynamic Activation of Endothelial Nitric Oxide Synthase by Hsp90. Nature 1998, 392, 821–824. [Google Scholar] [CrossRef]
- Li, J.; Buchner, J. Structure, Function and Regulation of the Hsp90 Machinery. Biomed. J. 2013, 36, 106–117. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.R.; Riddick, N.; Bryden, M.; Baur, W.; Zhang, X.; Surks, H.K. ROCK Isoform Regulation of Myosin Phosphatase and Contractility in Vascular Smooth Muscle Cells. Circ. Res. 2009, 104, 531–540. [Google Scholar] [CrossRef]
- MacHacek, M.; Hodgson, L.; Welch, C.; Elliott, H.; Pertz, O.; Nalbant, P.; Abell, A.; Johnson, G.L.; Hahn, K.M.; Danuser, G. Coordination of Rho GTPase Activities during Cell Protrusion. Nature 2009, 461, 99–103. [Google Scholar] [CrossRef]
- Gu, M.; Ni, H.; Sheng, X.; Pauciullo, A.; Liu, Y.; Guo, Y. RhoA Phosphorylation Mediated by Rho/RhoA-Associated Kinase Pathway Improves the Anti-Freezing Potentiality of Murine Hatched and Diapaused Blastocysts. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Wu, H.; Sun, L.; Wen, Y.; Liu, Y.; Yu, J.; Mao, F.; Wang, Y.; Tong, C.; Guo, X.; Hu, Z.; et al. Major Spliceosome Defects Cause Male Infertility and Are Associated with Nonobstructive Azoospermia in Humans. Proc. Natl. Acad. Sci. USA 2016, 113, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, A.; Socolov, R.; Socolov, D.; Iancu, I.V.; Anton, G. Methylation Pattern of Methylene Tetrahydrofolate Reductase and Small Nuclear Ribonucleoprotein Polypeptide N Promoters in Oligoasthenospermia: A Case-Control Study. Reprod. Biomed. Online 2014, 28, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Zhu, H.; Hao, H.; Zhao, X.; Qin, T.; Wang, D. Comparative Transcript Profiling of Gene Expression of Fresh and Frozen-Thawed Bull Sperm. Theriogenology 2015, 83, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, R.G.; Kirn-Safran, C.B.; Smith, M.A.; Martin-DeLeon, P.A. Ultrastructural Changes and Asthenozoospermia in Murine Spermatozoa Lacking the Ribosomal Protein L29/HIP Gene. Asian J. Androl. 2014, 16, 925. [Google Scholar] [CrossRef]
- Riesco, M.F.; Robles, V. Cryopreservation Causes Genetic and Epigenetic Changes in Zebrafish Genital Ridges. PLoS ONE 2013, 8, e67614. [Google Scholar] [CrossRef] [PubMed]
| Gene | Gene Bank Accessing Number | Primer Sequences (5′-3′) | Product Size (bp) |
|---|---|---|---|
| HSP70 | FJ217667.1 | F-TTGATAAGGGCCAGATCCAG | 105 |
| R-TGTTCAGCTCTTTGCCATTG | |||
| RHOA | NM_204704.1 | F-GAAGCAGGAGCCTGTCAAAC | 132 |
| R-GCAGCTCTAGTGGCCATTTC | |||
| SNRPA1 | NM_001005823.1 | F-CGACCTGCGGGGGTATAAAA | 176 |
| R-GTCCTTCCCCAATCCGACAA | |||
| RPL29 | NM_001171677.1 | F-GTCCCGTAAGTGGCACAGAA | 157 |
| R-CTGCTTGGCATTGTTGGCTT | |||
| GAPDH | NM_204305.1 | F-AGAACATCATCCCAGCGTCCA | 130 |
| R-CAGGTCAGGTCAACAACAGAG |
| Parameters | DMA Concentration | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 3% | 6% | 9% | Combined | Linear | Quadratic | ||
| Motile sperm (%) | 59.1 ab | 64.9 a | 55.2 b | 2.62 | 0.008 | 0.161 | 0.004 |
| PROG (%) | 33.2 ab | 38.2 a | 30.5 b | 1.98 | 0.005 | 0.207 | 0.002 |
| VAP (µm/s) | 63.9 | 63.8 | 61.1 | 3.23 | 0.619 | 0.395 | 0.642 |
| VCL (µm/s) | 126.5 | 124.2 | 120.0 | 6.43 | 0.605 | 0.331 | 0.865 |
| VSL (µm/s) | 34.9 | 35.5 | 34.6 | 1.71 | 0.853 | 0.834 | 0.607 |
| STR (%) | 54.5 | 55.2 | 56.5 | 2.19 | 0.657 | 0.376 | 0.863 |
| LIN (%) | 27.8 | 28.3 | 28.8 | 1.64 | 0.832 | 0.551 | 1.000 |
| WOB (%) | 50.3 | 51.0 | 50.7 | 1.18 | 0.854 | 0.781 | 0.632 |
| ALH (µm) | 5.5 | 5.4 | 5.3 | 0.16 | 0.530 | 0.278 | 0.812 |
| BCF (Hz) | 27.3 | 26.3 | 25.5 | 0.73 | 0.087 | 0.030 | 0.865 |
| Parameters | DMA Concentration | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 3% | 6% | 9% | Combined | Linear | Quadratic | ||
| Viability (%) | 66.7 b | 85.6 a | 74.8 ab | 5.00 | 0.006 | 0.123 | 0.004 |
| Intact acrosome (%) | 85.9 | 89.4 | 87.4 | 2.61 | 0.438 | 0.574 | 0.253 |
| Intact plasma membrane (%) | 83.2 | 83.2 | 86.1 | 2.56 | 0.447 | 0.277 | 0.522 |
| Parameters | DMA Concentration | p-Value | ||
|---|---|---|---|---|
| 3% | 6% | 9% | ||
| No. of incubated eggs | 67 | 83 | 86 | |
| Fertile eggs% (n) 1 | 28.4 (19) | 18.1 (15) | 23.3 (20) | 0.327 |
| Early embryo death% (n) 2 | 10.5 (2) | 13.3 (2) | 25.0 (5) | 0.441 |
| Late embryo death% (n) 2 | 5.3 (1) | 0.0 (0) | 5.0 (1) | 0.670 |
| Pipped eggs% (n) 2 | 42.1 (8) | 53.3 (8) | 45.0 (9) | 0.800 |
| Hatched eggs% (n) 2 | 42.1 (8) | 33.3 (5) | 25.0 (5) | 0.527 |
| Parameters | DMA Concentration | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 3% | 6% | 9% | Combined | Linear | Quadratic | ||
| TAC (µM/mg) * | 0.02 | 0.02 | 0.01 | 0.004 | 0.182 | 0.080 | 0.591 |
| GPX (mU/mg) * | 4.9 a | 1.1 b | 1.5 b | 0.383 | <0.001 | <0.001 | <0.001 |
| SOD (U/mg) * | 22.0 a | 15.3 b | 13.6 b | 1.056 | <0.001 | <0.001 | 0.015 |
| LPO (nM/mg) * | 0.04 b | 0.23 a | 0.22 a | 0.046 | 0.001 | 0.001 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehaisen, G.M.K.; Elomda, A.M.; Hamad, S.K.; Ghaly, M.M.; Sun, Y.; Li, Y.; Zong, Y.; Chen, J.; Partyka, A.; Nazmi, A.; et al. Effect of Dimethylacetamide Concentration on Motility, Quality, Antioxidant Biomarkers, Anti-Freeze Gene Expression, and Fertilizing Ability of Frozen/Thawed Rooster Sperm. Animals 2022, 12, 2739. https://doi.org/10.3390/ani12202739
Mehaisen GMK, Elomda AM, Hamad SK, Ghaly MM, Sun Y, Li Y, Zong Y, Chen J, Partyka A, Nazmi A, et al. Effect of Dimethylacetamide Concentration on Motility, Quality, Antioxidant Biomarkers, Anti-Freeze Gene Expression, and Fertilizing Ability of Frozen/Thawed Rooster Sperm. Animals. 2022; 12(20):2739. https://doi.org/10.3390/ani12202739
Chicago/Turabian StyleMehaisen, Gamal M. K., Ahmed M. Elomda, Shaimaa K. Hamad, Mona M. Ghaly, Yanyan Sun, Yunlei Li, Yunhe Zong, Jilan Chen, Agnieszka Partyka, Ali Nazmi, and et al. 2022. "Effect of Dimethylacetamide Concentration on Motility, Quality, Antioxidant Biomarkers, Anti-Freeze Gene Expression, and Fertilizing Ability of Frozen/Thawed Rooster Sperm" Animals 12, no. 20: 2739. https://doi.org/10.3390/ani12202739
APA StyleMehaisen, G. M. K., Elomda, A. M., Hamad, S. K., Ghaly, M. M., Sun, Y., Li, Y., Zong, Y., Chen, J., Partyka, A., Nazmi, A., Abbas, A. O., & Stino, F. K. R. (2022). Effect of Dimethylacetamide Concentration on Motility, Quality, Antioxidant Biomarkers, Anti-Freeze Gene Expression, and Fertilizing Ability of Frozen/Thawed Rooster Sperm. Animals, 12(20), 2739. https://doi.org/10.3390/ani12202739








