Genetic Structure of the Ca Rater Mallorquí Dog Breed Inferred by Microsatellite Markers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Records
2.2. DNA Extraction
2.3. Microsatellite Amplification
2.4. Polymorphism Detection
2.5. Statistical Analysis
3. Results
3.1. Genetic Diversity within Ca Rater Mallorquí Dog Population
3.2. Genetic Diversity among Ca Rater Mallorquí and Different Balearic, Spanish and International Breeds
3.2.1. Genetic Diversity among the Ca Rater Mallorquí and Different Balearic Breeds
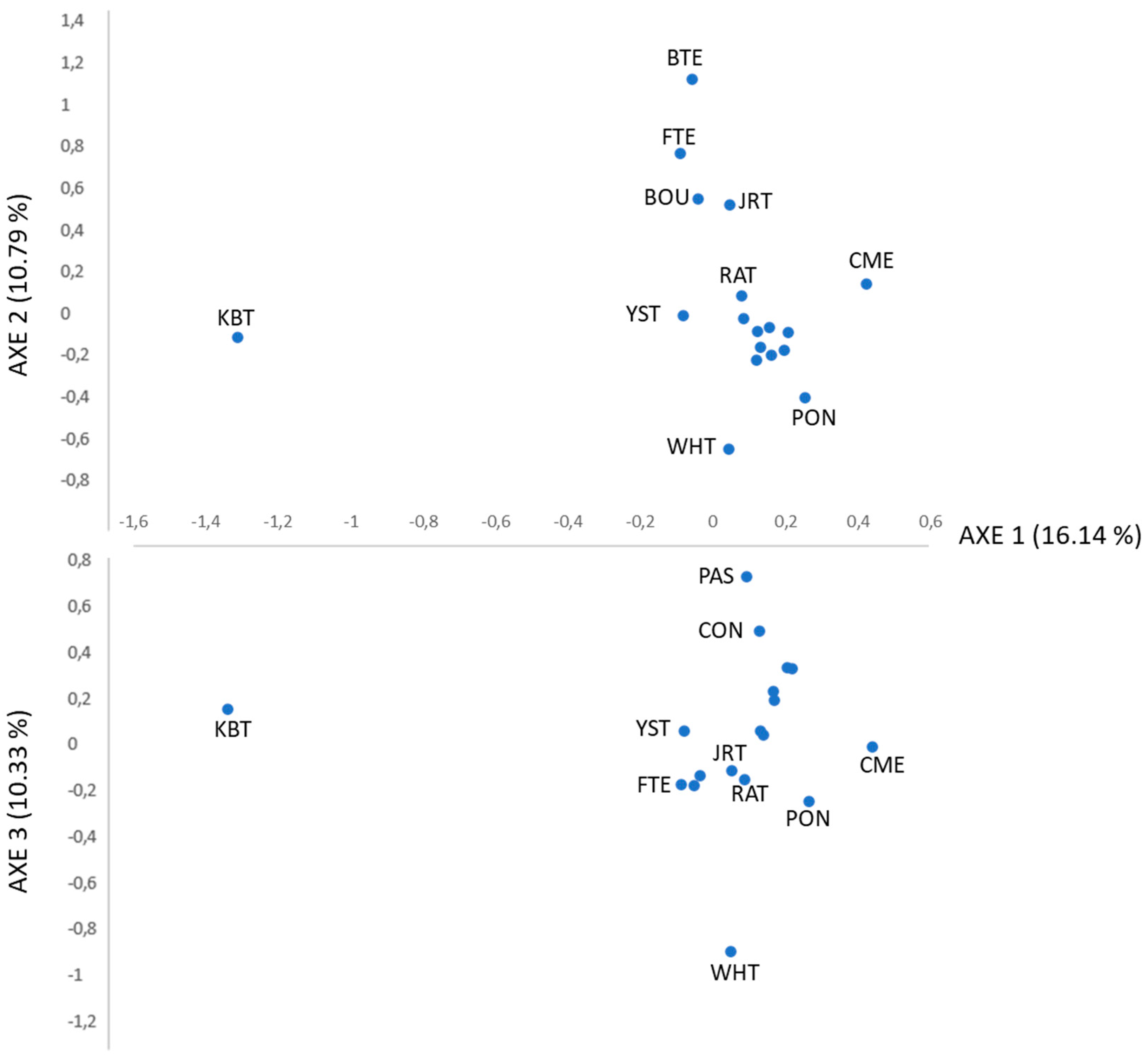
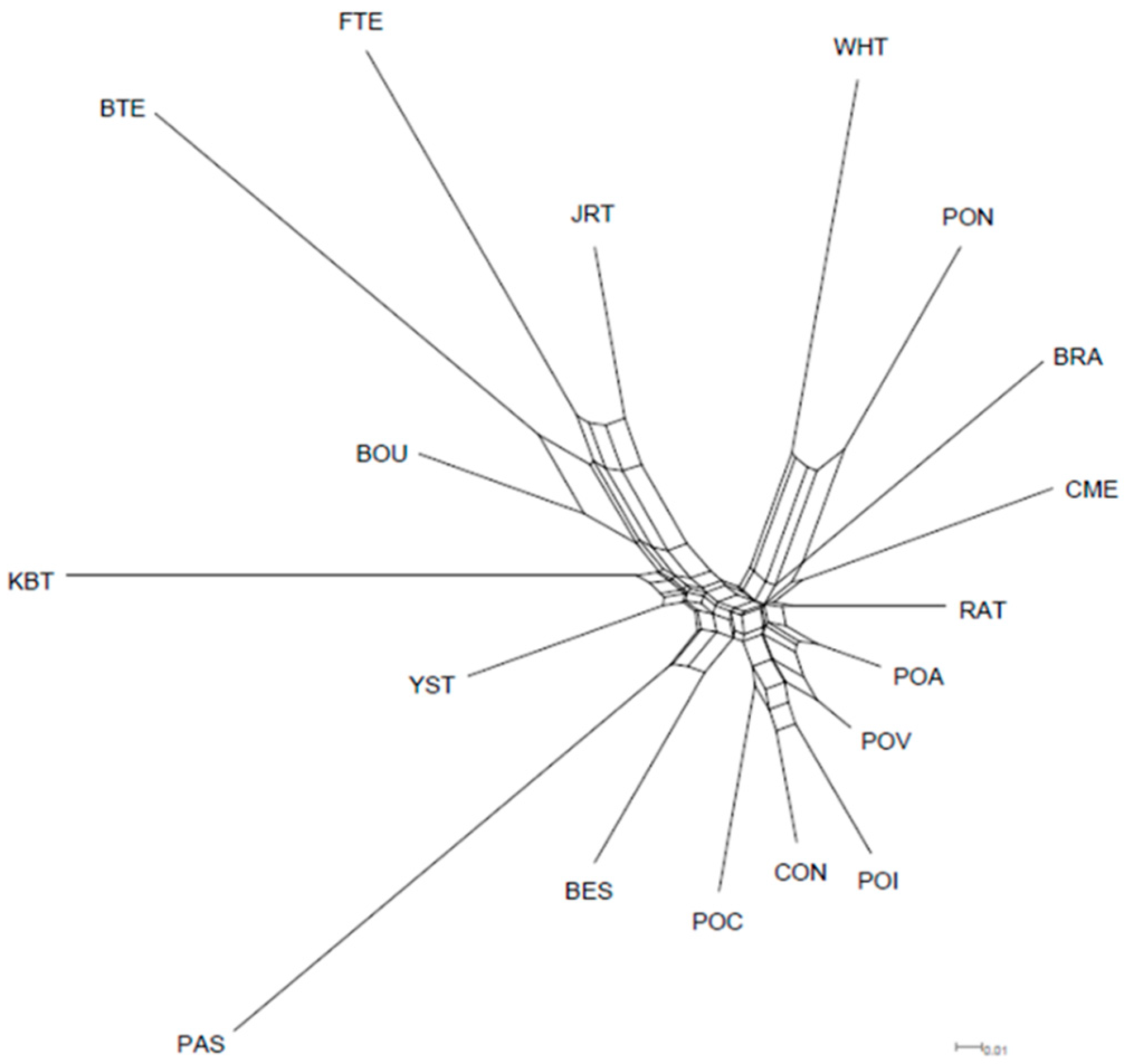
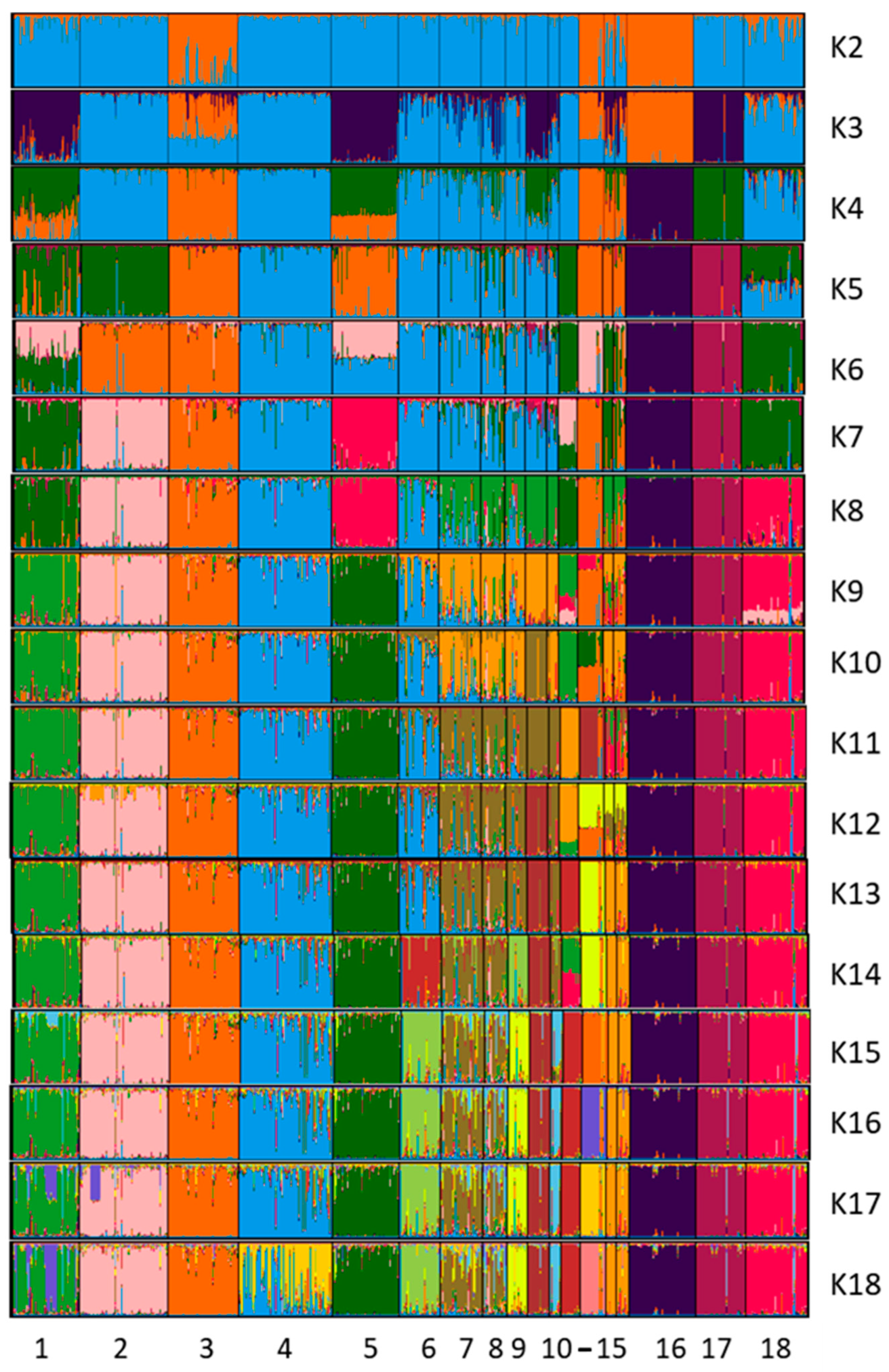
3.2.2. Genetic Diversity among Ca Rater Mallorquí and Spanish and International Breeds
4. Discussion
4.1. Moderate Level of Genetic Diversity within Ca Rater Mallorquí
4.2. Population Genetic Structure of Ca Rater Mallorquí
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- The Convention on Biological Diversity. Available online: https://www.cbd.int/convention/ (accessed on 21 August 2022).
- Global Plan of Action for Animal Genetic Resources and the Interlaken Declaration |Policy Support and Governance| Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/policy-support/tools-and-publications/resources-details/es/c/453629/ (accessed on 21 August 2022).
- Leadley, P.W.; Krug, C.B.; Alkemade, R.; Pereira, H.M.; Sumaila, U.R.; Walpole, M.; Marques, A.; Newbold, T.; Teh, L.S.L. Progress towards the Aichi Biodiversity Targets: An Assessment of Biodiversity Trends, Policy Scenarios and Key Actions: Global Biodiversity Outlook 4 (GBO-4) Technical Report; Secretariat of the Convention on Biological Diversity; CBD Technical Series 78: Montreal, QC, Canada, 2014. [Google Scholar]
- Compendium of Food Additive Specifications: 74th Meeting 2011; Comité Mixte FAO-OMS D’Experts des Additifs Alimentaires, Ed.; FAO JECFA monographs; FAO: Rome, Italy, 2011; ISBN 978-92-5-107004-8. [Google Scholar]
- Payeras, L.; Falconer, J. Fitxes de Les Races Autòctones de Les Illes Balears; Conselleria d’Educació, Cultura i Esport i Conselleria d’Agricultura, Comerç i Indústria.: Palma de Mallorca, Spain, 1998. [Google Scholar]
- Payeras, L.; Falconer, J. El ca Rater Mallorquí; Oficina de la caça; Consell de Mallorca, Departamente de Madi Ambient i Natura: Palma de Mallorca, Spain, 2010; ISBN 84-923765-0-3. [Google Scholar]
- Alanzor Puente, J.M.; Pons Barro, Á.L.; de la Haba Giraldo, M.R.; Delgado Bermejo, J.V.; Navas González, F.J. Does Functionality Condition the Population Structure and Genetic Diversity of Endangered Dog Breeds under Island Territorial Isolation? Animals 2020, 10, 1893. [Google Scholar] [CrossRef] [PubMed]
- Bigi, D.; Marelli, S.; Randi, E.; Polli, M. Genetic characterization of four native Italian shepherd dog breeds and analysis of their relationship to cosmopolitan dog breeds using microsatellite markers. Animal 2015, 9, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Ceh, E.; Dovc, P. Population structure and genetic differentiation of livestock guard dog breeds from the W estern B alkans. J. Anim. Breed. Genet. 2014, 131, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Vychodilova, L.; Necesankova, M.; Albrechtova, K.; Hlavac, J.; Modry, D.; Janova, E.; Vyskocil, M.; Mihalca, A.D.; Kennedy, L.J.; Horin, P. Genetic diversity and population structure of African village dogs based on microsatellite and immunity-related molecular markers. PLoS ONE 2018, 13, e0199506. [Google Scholar]
- Machová, K.; Kranjčevičová, A.; Vostrý, L.; Krupa, E. Analysis of Genetic Diversity in the Czech Spotted Dog. Animals 2020, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- San José, C.; Cárcel, M.J.; Tejedor, M.T.; Monteagudo, L.V. Microsatellite DNA markers applied to the classification of the Podenco Valenciano canine breed. Ital. J. Anim. Sci. 2018, 17, 49–52. [Google Scholar] [CrossRef]
- Pires, A.; Amorim, I.; Ginja, C.; Gomes, M.; Godinho, I.; Simoes, F.; Oom, M.; Petrucci-Fonseca, F.; Matos, J.; Bruford, M.W. Molecular structure in peripheral dog breeds: Portuguese native breeds as a case study. Anim. Genet. 2009, 40, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.; Berger, C.; Heinrich, J.; Niederstätter, H.; Hecht, W.; Hellmann, A.; Rohleder, U.; Schleenbecker, U.; Morf, N.; Freire-Aradas, A. Dog breed affiliation with a forensically validated canine STR set. Forensic Sci. Int. Genet. 2018, 37, 126–134. [Google Scholar] [CrossRef]
- Cairns, K.M.; Shannon, L.M.; Koler-Matznick, J.; Ballard, J.W.O.; Boyko, A.R. Elucidating biogeographical patterns in Australian native canids using genome wide SNPs. PLoS ONE 2018, 13, e0198754. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, S.; Ciani, E.; Sardina, M.; Sottile, G.; Pilla, F.; Portolano, B.; Consortium, B.O.I. Runs of homozygosity reveal genome-wide autozygosity in Italian sheep breeds. Anim. Genet. 2018, 49, 71–81. [Google Scholar] [CrossRef]
- Parker, H.G.; Dreger, D.L.; Rimbault, M.; Davis, B.W.; Mullen, A.B.; Carpintero-Ramirez, G.; Ostrander, E.A. Genomic analyses reveal the influence of geographic origin, migration, and hybridization on modern dog breed development. Cell Rep. 2017, 19, 697–708. [Google Scholar] [CrossRef]
- Animal Production and Health Division. Molecular Genetic Characterization of Animal Genetic Resources; Directrices de la FAO sobre producción y sanidad animal; FAO: Rome, Italy, 2011; ISBN 978-92-5-107032-1.
- Ministerio de Agricultura, Pesca y Alimentación. Orden APA/807/2004, de 24 de Marzo, por la que se Actualiza el Anexo del Real Decreto 558/2001, de 25 de Mayo, por el que se Regula el Reconocimiento Oficial de las Organizaciones o Asociaciones de Criadores de Perros de Raza Pura; Real Decreto: Madrid, Spain, 2004; Volume BOE-A-2004-5652, pp. 13430–13435.
- Fernández, M.; Gómez, M.; Delgado, J.; Adán, S.; Jiménez, M. Guía de Campo de las Razas Autóctonas Españolas; Rae 235; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2009; ISBN 978-84-491-0946-1. [Google Scholar]
- Walsh, P.S.; Varlaro, J.; Reynolds, R. A rapid chemiluminescent method for quanititation of human DNA. Nucleic Acids Res. 1992, 20, 5061–5065. [Google Scholar] [CrossRef]
- Marshall, T.C.; Slate, J.; Kruuk, L.E.B.; Pemberton, J.M. Statistical Confidence for Likelihood-Based Paternity Inference in Natural Populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.; Rousset, F. GENEPOP (Version 1.2): Population Genetics Software for Exact Tests and Ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Kim, K.S.; Sappington, T.W. Microsatellite Data Analysis for Population Genetics. In Microsatellites: Methods and Protocols; Kantartzi, S.K., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 271–295. ISBN 978-1-62703-389-3. [Google Scholar]
- Yeh, F.C.; Boyle, T.J.B. Population Genetic Analysis of Co-Dominant and Dominant Markers and Quantitative Traits. Belg. J. Bot. 1997, 129, 157. [Google Scholar]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar]
- Belkhir, K.; Borsa, P.; Chikhi, L.; Raufaste, N.; Bonhomme, F. GENETIX 4.05, Set of Population Genetics Programs, Logiciel Sous Windows TM Pour La Génétique Des Populations. Université de Montpellier II, Montpellier (France),Laboratoire Génome, Populations, Interactions, CNRS UMR 5000. 2004. Available online: https://mybiosoftware.com/genetix-4-05-set-of-population-genetics-programs.html (accessed on 26 July 2012).
- Langella, O. Populations 1.2. 28: A Population Genetic Software. 1999. Available online: http://www.pge.cnrs-gif.fr/bioinfo/populations/index.php (accessed on 28 March 2022).
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1. 4.3 Software; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2016. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.; Vonholdt, B.; Earl, D.A.; VonHoldt, B.M. Structure Harvester: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Elston, R. Linkage information content of polymorphic genetic markers. Hum. Hered. 1999, 49, 112–118. [Google Scholar] [CrossRef]
- Hartl, D.L.; Clark, A.G.; Clark, A.G. Principles of Population Genetics; Sinauer associates Sunderland: Sunderland, MA, USA, 1997; Volume 116. [Google Scholar]
- Wright, S. Evolution and the Genetics of Populations, Volume 4: Variability within and among Natural Populations; University of Chicago Press: Chicago, IL, USA, 1984; Volume 4. [Google Scholar]
- Choron, S.; Choron, H. Planet Dog: A Doglopedia; Houghton Mifflin Harcourt: Boston, MA, USA, 2005. [Google Scholar]
- Races Autòctones de Les Illes Balears-Ca Rater Mallorquí. Available online: https://www.caib.es/sites/racesautoctones/ca/ca_rater_mallorqui-4122/ (accessed on 21 August 2022).
- Ministerio de Agricultura; Pesca y Alimentación. Real Decreto 558/2001, de 25 de Mayo, Por El Que Se Regula El Reconocimiento Oficial de Las Organizaciones o Asociaciones de Criadores de Perros de Raza Pura; Real Decreto: Madrid, Spain, 2001; Volume BOE-A-2001-11347, pp. 21156–21182. [Google Scholar]
- Méndez, S.; Dunner, S.; García, J.; de Argüello, S.; Crespo, M.; Chomón, N.; Calderón, L.; Sañudo, B.; Cañón, J. Caracterización del Perro de Agua del Cantábrico. Arch. De Zootec. 2011, 60, 405–408. [Google Scholar] [CrossRef]
- Martínez, A.; Vega, J.L.; Delgado, J.V. Caracterización genética de razas de caza: Casos aplicados a Mallorca;; Recerca i gestió dins l’àmbit cinegètic. Mon. Soc. Hist. Nat. Balears 2019, 28, 115–117. [Google Scholar]
- Hill, W.G.; Rasbash, J. Models of long term artificial selection in finite population. Genet. Res. 1986, 48, 41–50. [Google Scholar] [CrossRef]
- Parra, D.; Méndez, S.; Canon, J.; Dunner, S. Genetic differentiation in pointing dog breeds inferred from microsatellites and mitochondrial DNA sequence. Anim. Genet. 2008, 39, 1–7. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Pooch, A.S.; Liu, H. A genetic assessment of the English bulldog. Canine Genet. Epidemiol. 2016, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Smetanová, M.; Černá Bolfíková, B.; Randi, E.; Caniglia, R.; Fabbri, E.; Galaverni, M.; Kutal, M.; Hulva, P. From wolves to dogs, and back: Genetic composition of the Czechoslovakian Wolfdog. PLoS ONE 2015, 10, e0143807. [Google Scholar] [CrossRef]
- Lee, E.-W.; Choi, S.-K.; Cho, G.-J. Molecular genetic diversity of the Gyeongju Donggyeong dog in Korea. J. Vet. Med. Sci. 2014, 76, 1359–1365. [Google Scholar] [CrossRef]
- Luikart, G.; Amish, S.; Winnie, J.; Beja-Pereira, A.; Godinho, R.; Allendorf, F.; Harris, R. High connectivity among argali sheep from Afghanistan and adjacent countries: Inferences from neutral and candidate gene microsatellites. Conserv. Genet. 2011, 12, 921–931. [Google Scholar] [CrossRef]
- Wright, S. The relation of livestock breeding to theories of evolution. J. Anim. Sci. 1978, 46, 1192–1200. [Google Scholar] [CrossRef]
- Hauser, S.S.; Athrey, G.; Leberg, P.L. Waste Not, Want Not: Microsatellites Remain an Economical and Informative Technology for Conservation Genetics. Ecol. Evol. 2021, 11, 15800–15814. [Google Scholar] [CrossRef] [PubMed]
| Breed (Acronym) *, ** | N | FCI | RSCE |
|---|---|---|---|
| 1 * CA RATER (RAT) | 79 | NR | NR |
| 2 * CA DE BESTIAR (BES) | 106 | 1 | 1 |
| 3 * CA BOU (BOU) | 83 | 2 | 2 |
| 4 * CA DE CONILLS DE MENORCA (CON) | 112 | NR | NR |
| 5 * CA MÈ MALLORQUÍ (CME) | 80 | NR | NR |
| 6 *, ** PODENCO IBICENCO (POI) | 49 | 5 | 5 |
| 7 ** PODENCO VALENCIANO (POV) | 50 | NR | 5 |
| 8 ** PODENCO ANDALUZ (POA) | 29 | NR | 11 |
| 9 ** PODENCO CANARIO (POC) | 25 | 5 | 5 |
| 10 BRACO (BRA) | 26 | 7 | 7 |
| 11 GERMAN SHEPHERD (PAS) | 14 | 1 | 1 |
| 12 POINTER INGLÉS (PON) | 23 | 7 | 7 |
| 13 BULL TERRIER (BTE) | 30 | 3 | 3 |
| 14 FOX TERRIER (FTE) | 13 | 3 | 3 |
| 15 JACK RUSELL TERRIER (JRT) | 14 | 3 | 3 |
| 16 KERRY BLUE TERRIER (KBT) | 80 | 3 | 3 |
| 17 WEST HIGHLAND WHITE TERRIER (WHT) | 60 | 3 | 3 |
| 18 YORKSHIRE TERRIER (YTE) | 71 | 3 | 3 |
| Breed | N | NMA | Ae | Rt | He | Ho | FIS | CI |
|---|---|---|---|---|---|---|---|---|
| RAT | 79 | 7.25 | 4.85 | 3.72 | 0.713 | 0.700 | 0.00953 | (−0.02321–0.03284) |
| BES | 106 | 6.95 | 4.67 | 3.88 | 0.712 | 0.706 | 0.12109 | (0.07375–0.15422) |
| BOU | 83 | 6.35 | 4.05 | 3.19 | 0.644 | 0.566 | 0.00804 | (−0.13240–0.04131) |
| CON | 112 | 7.20 | 4.7 | 3.73 | 0.712 | 0.686 | 0.26097 | (0.09149–0.34834) |
| CME | 80 | 6.70 | 4.23 | 3.27 | 0.675 | 0.675 | −0.00095 | (−0.03597–0.01973) |
| POI | 49 | 5.75 | 4.26 | 3.44 | 0.699 | 0.718 | 0.19955 | (−0.01783–0.25776) |
| POV | 50 | 7.65 | 5.38 | 4.45 | 0.759 | 0.717 | 0.03798 | (0.00756–0.05897) |
| POA | 29 | 7.10 | 5.34 | 4.24 | 0.743 | 0.710 | 0.08302 | (−0.05333–0.11452) |
| POC | 25 | 5.90 | 4.6 | 3.46 | 0.674 | 0.690 | −0.02857 | (−0.08105–0.00964) |
| PON | 26 | 5.30 | 4.17 | 2.96 | 0.612 | 0.614 | −0.02982 | (−0.11950–0.00741) |
| BRA | 14 | 5.55 | 4.89 | 3.59 | 0.728 | 0.723 | 0.04539 | (−0.02694–0.08134) |
| PAS | 23 | 4.05 | 3.43 | 2.59 | 0.589 | 0.607 | −0.02354 | (−0.10593–0.01139) |
| BTE | 30 | 3.70 | 2.82 | 1.89 | 0.428 | 0.318 | −0.02687 | (−0.08048–0.00434) |
| FTE | 13 | 4.15 | 3.82 | 2.74 | 0.600 | 0.485 | −0.00416 | (−0.07732–0.02652) |
| JRT | 14 | 5.05 | 4.48 | 3.3 | 0.665 | 0.612 | 0.05560 | (0.01131–0.07960) |
| KBT | 80 | 3.80 | 2.95 | 2.09 | 0.490 | 0.504 | 0.01938 | (−0.02246–0.04994) |
| WHT | 60 | 4.70 | 2.94 | 2.14 | 0.465 | 0.431 | 0.07309 | (−0.00767–0.13856) |
| YST | 71 | 6.90 | 4.71 | 3.77 | 0.713 | 0.688 | 0.03520 | (−0.00873–0.06431) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, L.S.A.; Vergara, A.M.C.; Herrera, P.Z.; Puente, J.M.A.; Barro, Á.L.P.; Dunner, S.; Marques, C.S.J.; Bermejo, J.V.D.; Martínez, A.M. Genetic Structure of the Ca Rater Mallorquí Dog Breed Inferred by Microsatellite Markers. Animals 2022, 12, 2733. https://doi.org/10.3390/ani12202733
García LSA, Vergara AMC, Herrera PZ, Puente JMA, Barro ÁLP, Dunner S, Marques CSJ, Bermejo JVD, Martínez AM. Genetic Structure of the Ca Rater Mallorquí Dog Breed Inferred by Microsatellite Markers. Animals. 2022; 12(20):2733. https://doi.org/10.3390/ani12202733
Chicago/Turabian StyleGarcía, Lourdes Sofía Aguilera, Amado Manuel Canales Vergara, Pedro Zurita Herrera, José Manuel Alanzor Puente, Águeda Laura Pons Barro, Susana Dunner, Carlos San José Marques, Juan Vicente Delgado Bermejo, and Amparo Martínez Martínez. 2022. "Genetic Structure of the Ca Rater Mallorquí Dog Breed Inferred by Microsatellite Markers" Animals 12, no. 20: 2733. https://doi.org/10.3390/ani12202733
APA StyleGarcía, L. S. A., Vergara, A. M. C., Herrera, P. Z., Puente, J. M. A., Barro, Á. L. P., Dunner, S., Marques, C. S. J., Bermejo, J. V. D., & Martínez, A. M. (2022). Genetic Structure of the Ca Rater Mallorquí Dog Breed Inferred by Microsatellite Markers. Animals, 12(20), 2733. https://doi.org/10.3390/ani12202733





