The Quality of Five Natural, Historical Italian Cheeses Produced in Different Months: Gross Composition, Fat-Soluble Vitamins, Fatty Acids, Total Phenols, Antioxidant Capacity, and Health Index
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Design and the Collection of Cheese Samples from Farms
2.2. Breeding Systems and Diets
2.3. Chemical Composition of Cheeses
2.4. Total Retinol, α-Tocopherol, and Cholesterol
2.5. Fatty Acid Profile
2.6. Total Phenol Content and the Antioxidant Capacity
2.6.1. Cheese Extraction
2.6.2. Total Phenolic Content
2.6.3. The Ferric Reducing Antioxidant Power (FRAP) Assay
2.6.4. The ABTS Radical Scavenging Activity Assay
2.7. The General Health Index of Cheese (GHIC)
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Fatty Acid Profile, Total Retinol, α-Tocopherol, and Cholesterol Content
3.3. Total Phenol Content and Antioxidant Capacity
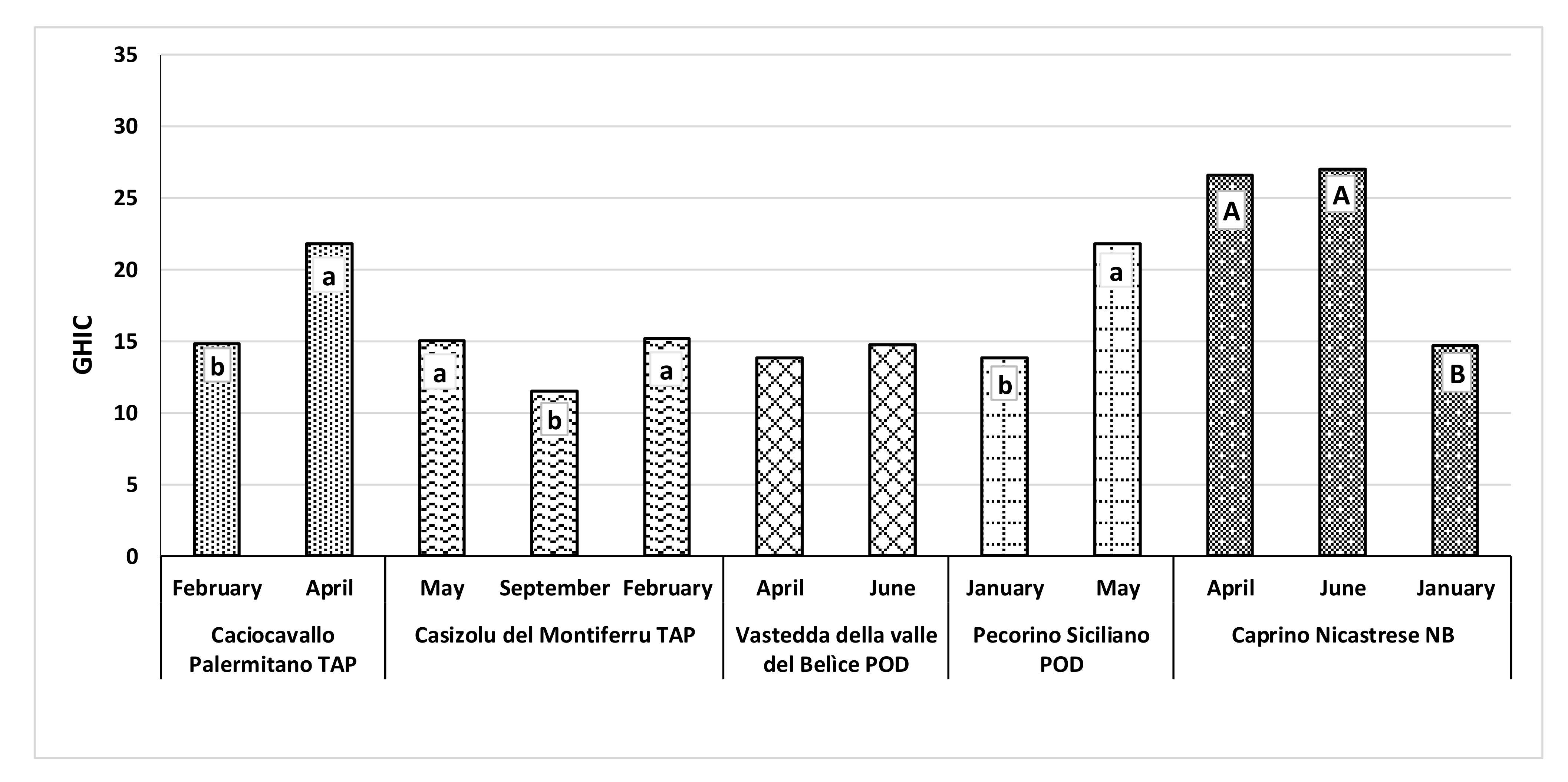
3.4. General Health Index of Cheese (GHIC)
4. Discussion
4.1. Chemical Composition
4.2. Fatty Acid Profile, Total Retinol, α-Tocopherol, and Cholesterol Content
4.3. Total Phenol Content and Antioxidant Capacity
4.4. General Health Index of Cheese (GHIC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scintu, M.F.; Piredda, G. Typicity and biodiversity of goat and sheep milk products. Small Rum. Res. 2007, 68, 221–231. [Google Scholar] [CrossRef]
- Uzun, P.; Serrapica, F.; Masucci, F.; Assunta, B.C.M.; Yildiz, H.; Grasso, F.; Di Francia, A. Diversity of traditional Caciocavallo cheeses produced in Italy. Int. J. Dairy Technol. 2020, 73, 234–243. [Google Scholar] [CrossRef]
- Bonanno, A.; Tornambè, G.; Bellina, V.; De Pasquale, C.; Mazza, F.; Maniaci, G.; Di Grigoli, A. Effect of farming system and cheesemaking technology on the physicochemical characteristics, fatty acid profile, and sensory properties of Caciocavallo Palermitano cheese. J. Dairy Sci. 2013, 96, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Cruciata, M.; Gaglio, R.; Todaro, M.; Settanni, L. Ecology of Vastedda della valle del Belìce cheeses: A review and recent findings to stabilize the traditional production. Food Rev. Int. 2019, 35, 90–103. [Google Scholar] [CrossRef]
- Todaro, M.; Lo Presti, V.; Macaluso, A.; Alleri, M.; Licitra, G.; Chiofalo, V. Alkaline Phosphatase Survey in Pecorino Siciliano PDO Cheese. Foods 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.; Addis, M.; Nieddu, G.; Piredda, G.; Pirisi, A. Il Casizolu del Montiferru Formaggio artigianale a pasta filata della Sardegna. In Proceedings of the Cheese Art, Ragusa, Italy, 4–9 June 2002. [Google Scholar]
- Ministry of Agriculture and Forests. Approval of Official Methods of Analysis for Cheeses; DM 21/04/1986; Official Journal, 1986, 229, 02/10/1986 met 2; Polygraphic Institute and State Mint: Rome, Italy, 1986. [Google Scholar]
- Ministry of Agriculture and Forests. Approval of Official Methods of Analysis for Cheeses; DM 21/04/1986; Official Journal, 1986, 229, 02/10/1986 met 4; Polygraphic Institute and State Mint: Rome, Italy, 1986. [Google Scholar]
- DM 23/07/1994. Approval of Methods of Analysis for Cereals and Derivatives; Official Journal, Official Journal 186 SO114 10/08/1994 Suppl 4; Polygraphic Institute and State Mint: Rome, Italy, 1994. [Google Scholar]
- Regulation on the Provisions of Food Information to Consumers: A Comparative Analysis of Its Enforcements in Relation to Member States; 1169/11/EU; OJEU: Strasbourg, France, 2011; Volume 304, pp. 18–63.
- UNI EN 13805; Standard Reference EN 13805:2014 Foodstuffs—Determination of Trace Elements—Pressure Digestion. European Committee for Standardization, CEN: Brussels, Belgium, 2014; pp. 1–7.
- UNI EN ISO 17294-2; Water Quality: Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Part 2: Determination of Selected Elements Including Uranium Isotopes. UNI—Ente Nazionale Italiano di Unificazione Database: Milano, Italy, 2016; pp. 1–31.
- Panfili, G.; Manzi, P.; Pizzoferrato, L. High-performance Liquid Chromatographic Method for the Simultaneous Determination of Tocopherols, Carotenes, and Retinol and its Geometric Isomers in Italian Cheese. Analyst 1994, 169, 1161–1165. [Google Scholar] [CrossRef]
- Manzi, P.; Panfili, G.; Pizzoferrato, L. Normal and Reversed- Phase HPLC for more complete evaluation of tocopherols, retinols, carotenes and sterols in dairy products. Chromatographia 1996, 43, 89–93. [Google Scholar] [CrossRef]
- Jiang, J.; Bjoerck, L.; Fonden, R.; Emanuelson, M. Occurrence of con-jugated cis-9 trans-11-octadecadienoic acid in bovine milk: Effects offeed and dietary regimen. J. Dairy Sci. 1996, 79, 438–445. [Google Scholar] [CrossRef]
- ISO Methods 15884: 2002 (IDF 182: 2002); Milk Fat—Preparation of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2002.
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Prediction of fatty acid content in sheep milk by Mid-Infrared Spectroscopy with a selection of wavelengths by Genetic Algorithms. Food Sci. Technol. 2016, 65, 503–510. [Google Scholar] [CrossRef]
- Kramer, J.; Cruz-Hernandez, K.G.; Deng, C.; Zhou, Z.; Jahreis, J.G.; Dugan, M.E.R. Analysis of conjugated linoleic acid and trans 18:1 isomers in synthetic and animal products. Am. J. Clin. Nutr. 2004, 79, 1137S–1145S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, J.K.G.; Hernandez, M.; Cruz-Hernandez, C.; Kraft, J.; Dugan, M.E.R. Combining results of two GC separation partly achieves determination of all cis and trans 16:1 18:1, 18.2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-Ion SPE fractionation. Lipids 2008, 43, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.E.; Birch, J.; Sun-Waterhouse, D.; Everett, D.W. Effects of catechin on the phenolic content and antioxidant properties of low-fat cheese. Int. J. Food Sci. Technol. 2013, 48, 2448–2455. [Google Scholar] [CrossRef]
- ISO Method 14502-1; Determination of Substances Characteristic of Green and Black Tea—Part 1: Content of Total Polyphenols in Tea—Colorimetric Method Using Folin-Ciocalteu Reagent. 21. International Organization for Standardization: Geneva, Switzerland, 2007.
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Giorgio, D.; Di Trana, A.; Di Napoli, M.A.; Sepe, L.; Cecchini, S.; Rossi, R.; Claps, S. Comparison of cheeses from goats fed 7 forages based on a new health index. J. Dairy Sci. 2019, 102, 6790–6801. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fo, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Puchau, B.; Zulet, M.Á.; de Echávarri, A.G.; Hermsdorff, H.H.M.; Martínez, J.A. Dietary total antioxidant capacity: A novel indicator of diet quality in healthy young adults. J. Am. Coll. Nutr. 2009, 28, 648–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SYSTAT. Statistics; Version 13 Edition; SYSTAT, Inc.: Evanston, IL, USA, 2009; 750p. [Google Scholar]
- Liotta, L.; Randazzo, C.L.; Russo, N.; Zumbo, A.; Di Rosa, A.R.; Caggia, C.; Chiofalo, V. Effect of Molasses and Dried Orange Pulp as Sheep Dietary Supplementation on Physico-Chemical, Microbiological and Fatty Acid Profile of Comisana Ewe’s Milk and Cheese. Front Nutr. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Di Grigoli, A.; Francesca, N.; Gaglio, R.; Guarrasi, V.; Moschetti, M.; Scatassa, M.L.; Bonanno, A. The influence of the wooden equipment employed for cheese manufacture on the characteristics of a traditional stretched cheese during ripening. Food Microbiol. 2015, 46, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Todaro, M.; Bonanno, A.; Scatassa, M.L. The quality of Valle del Belìce sheep’s milk and cheese produced in the hot summer season in Sicily. Dairy Sci. Technol. 2014, 94, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Todaro, M.; Palmeri, M.; Settanni, L.; Scatassa, M.L.; Mazza, F.; Bonanno, A.; Di Grigoli, A. Effect of refrigerated storage on microbiological, chemical and sensory characteristics of a ewes’ raw milk stretched cheese. Food Packag. Shelf Life 2017, 11, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Gaglio, R.; Cruciata, M.; Scatassa, M.L.; Tolone, M.; Mancuso, I.; Cardamone, C.; Settanni, L. Influence of the early bacterial biofilms developed on vats made with seven wood types on PDO Vastedda della valle del Belìce cheese characteristics. Int. J. Food Microbiol. 2019, 291, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Francesca, N.; Reale, S.; Moschetti, G.; Vitale, F.; Settanni, L. Effect of different salting technologies on the chemical and microbiological characteristics of PDO Pecorino Siciliano cheese. Eur. Food Res. Technol. 2011, 233, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Guarcello, R.; Carpino, S.; Gaglio, R.; Pino, A.; Rapisarda, T.; Caggia, C.; Settanni, L.; Todaro, M. A large factory-scale application of selected autochthonous lactic acid bacteria for PDO Pecorino Siciliano cheese production. Food Microbiol. 2016, 59, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Pino, A.; Liotta, L.; Randazzo, C.L.; Todaro, A.; Mazzaglia, A.; De Nardo, F.; Caggia, C. Polyphasic approach to study physico-chemical, microbiological and sensorial characteristics of artisanal Nicastrese goat’s cheese. Food Microbiol. 2018, 70, 143–154. [Google Scholar] [CrossRef]
- Pino, A.; Liotta, L.; Caggia, C.; Chiofalo, V.; De Nardo, F.; Zumbo, A.; Randazzo, C.L. Effect of seasonality on physico-chemical and microbiological properties of nicastrese milk and artisanal cheese. FEMS Microbiol. Lett. 2021, 368, fnab055. [Google Scholar] [CrossRef]
- Blažić, M.; Pavić, K.; Zavadlav, S.; Marčac, N. The impact of traditional cheeses and whey on health. Croat. J. Food Sci. Technol 2017, 9, 198–203. [Google Scholar] [CrossRef]
- Chiofalo, B.; Di Rosa, A.R.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Effect of supplementation of herd diet with olive cake on the composition profile of milk and on the composition, quality and sensory profile of cheeses made therefrom. Animals 2020, 10, 977. [Google Scholar] [CrossRef]
- Czarnacka-Szymani, J.; Jezewska-Zychowicz, M. Impact of nutritional information on consumer’s acceptance of cheese with reduced sodium chloride content. Int. Dairy J. 2015, 40, 47–53. [Google Scholar] [CrossRef]
- McLean, R.; Hoek, J.; Hedderley, D. Effects of alternative label formats on choice of high-and low-sodium products in a New Zealand population sample. Public Health Nutr. 2012, 15, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Wyness, L.A.; Butriss, J.L.; Stanner, S.A. Reducing the population’s sodium intake: The UK Food Standards Agency’s salt reduction programme. Public Health Nutr. 2012, 15, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, S.; Doxey, J.; Hammond, D. Nutrition labels on pre-packaged foods: A systematic review. Public Health Nutr. 2011, 14, 1496–1506. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.A.; McGuire, M.K. Conjugated linoleic acid (CLA): A ruminant fatty acid with beneficial effects on human health. J. Anim. Sci. 2000, 77, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Jacome-Sosa, M.M.; Proctor, S.D. The role of ruminant trans fat as a potential nutraceutical in the prevention of cardiovascular disease. Food Res. Internat. 2012, 46, 460–468. [Google Scholar] [CrossRef]
- Bauman, D.E.; Baumgard, L.H.; Corl, B.A.; Griinari, J.M. Biosynthesisof conjugated linoleic acid in ruminants. Proc. Am. Soc. Anim. Sci. 1999, 77, 1–14. [Google Scholar]
- Kay, J.K.; Mackle, T.R.; Auldist, M.J.; Thomson, N.A.; Bauman, D.E. Endogenous synthesis of cis-9, trans-11 conjugated linoleic acid in dairy cows fed fresh pasture. J. Dairy Sci. 2004, 87, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Department of Health. Nutritional Aspects of Cardiovascular Disease; Report on Health and Social Subjects; HMSO, (Her Majesty’s Stationery Office): London, UK, 1994; Volume 46, pp. 37–46. [Google Scholar]
- Gaglio, R.; Franciosi, E.; Todaro, A.; Guarcello, R.; Alfeo, V.; Randazzo, C.L.; Settannia, L.; Todaro, M. Addition of selected starter/non-starter lactic acid bacterial inoculums to stabilise PDO Pecorino Siciliano cheese production. Food Res. Int. 2020, 136, 109335. [Google Scholar] [CrossRef] [PubMed]
- Narducci, V.; Finotti, E.; Galli, V.; Carcea, M. Lipids and Fatty Acids in Italian Durum Wheat (Triticum durum Desf.) Cultivars. Foods 2019, 8, 223. [Google Scholar] [CrossRef] [Green Version]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Rouel, J.; Lamberet, G. A review of nutritional and physiological factors affecting goat milk synthesis and lipolysis. J. Dairy Sci. 2003, 86, 1751–1770. [Google Scholar] [CrossRef] [Green Version]
- Serrapica, F.; Masucci, F.; Di Francia, A.; Napolitano, F.; Braghieri, A.; Esposito, G.; Romano, R. Seasonal variation of chemical composition, fatty acid profile, and sensory properties of a mountain Pecorino cheese. Foods 2020, 9, 1091. [Google Scholar] [CrossRef]
- Martin, B.; Ferlay, A.; Pradel, P.; Rock, E.; Grolier, P.; Dupont, D.; Gruffat, D.; Besle, J.M.; Ballot, N.; Chilliard, Y. Variabilité de la teneur des laits en constituants d’intérêt nutritionnel selon la nature des fourrages consommés par les vaches laitières. Rencontres Autour Rech. Rumin. 2002, 9, 347–350. [Google Scholar]
- Martin, B.; Fedele, V.; Ferlay, A.; Grolier, P.; Rock, E.; Gruffat, D.; Chilliard, Y. Effects of Grass-Based Diets on the Content of Micronutrients and Fatty Acids in Bovine and Caprine Dairy Products. In Land Use Systems in Grassland Dominated Regions, Proceedings of the 20th General Meeting of the European Grassland Federation, Luzern, Switzerland, 21–24 June 2004; Lüscher, A., Jeangros, B., Huguenin, O., Lobsiger, M., Millar, N., Suter, D., Eds.; VDF Hochschulverlag AG ETH Zentrum: Zürich, Switzerland, 2004; Volume 9, pp. 876–886. [Google Scholar]
- D’Urso, S.; Cutrignelli, M.I.; Calabrò, S.; Bovera, F.; Tudisco, R.; Piccolo, V.; Infascelli, F. Influence of pasture on fatty acid profile of goat milk. J. Anim. Phys. Anim. Nutr. 2008, 92, 405–410. [Google Scholar] [CrossRef]
- Cabiddu, A.; Delgadillo-Puga, C.; Decandia, M.; Molle, G. Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals 2019, 9, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakas, F.P.; Keskin, C.N.; Agil, F.; Zencirci, N. Profiles of vitamin B and E in wheat grass and grain of einkorn (Triticum monococcum spp. monococcum), emmer (Triticum dicoccum spp. Dicoccum Schrank.), durum (Triticum durum Desf.), and bread wheat (Triticum aestivum L.) cultivars by LC-ESI-MS/MS analysis. J. Cereal Sci. 2021, 98, 1–10. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The effect of dietary consumption of plant secondary compounds on small ruminants’ product quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- De Feo, V.; Quaranta, E.; Fedele, V.; Claps, S.; Rubino, R.; Pizza, C. Flavonoids and terpenoids in goats milk in relation to forage intake. Ital. J. Food Sci. 2006, 18, 85–92. [Google Scholar]
- Garcia, V.; Rovira, S.; Boutoial, K.; Lopez, M.B. Improvements in goat milk quality: A review. Small Rumin. Res. 2014, 121, 51–57. [Google Scholar] [CrossRef]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; Di Grigoli, A.; Claps, S. Effects of Sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilario, M.C.; Puga, C.D.; Ocana, A.N.; Romo, F.P.G. Antioxidant activity, bioactive polyphenols in Mexican goats’ milk cheeses on summer grazing. J. Dairy Res. 2010, 77, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branciari, R.; Ranucci, D.; Trabalza-Marinucci, M.; Codini, M.; Orru, M.; Ortenzi, R.; Valiani, A. Evaluation of the antioxidant properties and oxidative stability of P ecorino cheese made from the raw milk of ewes fed Rosmarinus officinalis L. leaves. Int. J. Food Sci. 2015, 50, 558–565. [Google Scholar] [CrossRef]
- Chavez-Servin, J.L.; Andrade-Montemayor, H.M.; Vazquez, C.V.; Barreyro, A.A.; Garcia-Gasca, T.; Martinez, R.A.F.; Ramirez, A.M.O.; de la Torre-Carbot, K. Effects of feeding system, heat treatment and season on phenolic compounds and antioxidant capacity in goat milk, whey and cheese. Small Rumin. Res. 2018, 16, 54–58. [Google Scholar] [CrossRef]
- Jeangros, B.; Scehovic, J.; Schubiger, F.X.; Lehmann, J.; Daccord, R.; Arrigo, Y. Valeur nutritive des plantes des prairies 4. Composés phénoliques. Revue Suisse d’Agriculture 2001, 33, 147–151. [Google Scholar]
- Piluzza, G.; Bullitta, S. The dynamics of phenolic concentration in some pasture species and implications for animal husbandry. J. Sci. Food Agric. 2010, 90, 1452–1459. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Casalino, E.; D’Alessandro, A.G.; Laudadio, V. Dietary phenolic compounds: Biochemistry, metabolism and significance in animal and human health. Curr. Drug Metab. 2017, 18, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Blomhoff, R. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutrients 2010, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E. In vitro and in vivo antioxidant potential of milks, yoghurts, fermented milks and cheeses: A narrative review of evidence. Nutr. Res. Rev. 2018, 31, 52–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, A.; Rock, E.; Chamba, J.F.; Verdier-Metz, I.; Brachet, P.; Coulon, J.B. Respective effects of milk composition and the cheese-making process on cheese compositional variability in components of nutritional interest. Lait 2006, 86, 21–41. [Google Scholar] [CrossRef] [Green Version]
- Pattorn, S.; Hongsprabhas, P. Effect of coagulants on antioxidant capacity of milk protein curds and their tryptic hydrolysates. J. Food Biochem. 2013, 37, 203–211. [Google Scholar] [CrossRef]
- Revilla, I.; Gonzalez-Martin, M.I.; Vivar-Quintana, A.M.; Blanco-Lopez, M.A.; Lobos-Ortega, I.A.; Hernandez-Hierro, J.M. Antioxidant capacity of different cheeses: Affecting factors and prediction by near infrared spectroscopy. J. Dairy Sci. 2016, 99, 5074–5082. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. Antioxidant activity of Cheddar cheeses at different stages of ripening. Int. J. Dairy Technol. 2009, 62, 339–347. [Google Scholar] [CrossRef]
- Kuhnen, S.; Moacyr, J.R.; Mayer, J.K.; Navarro, B.B.; Trevisan, R.; Honorato, L.A.; Pinheiro Machado Filho, L.C. Phenolic content and ferric reducing antioxidant power of cow’s milk produced in different pasture-based production systems in southern Brazil. J. Sci. Food Agric. 2014, 94, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Coulon, J.B.; Agabriel, C.; Chilliard, Y.; Rock, E. Relationships between the conditions of goat’s milk production and the contents of some components of nutritional interest in Rocamadour cheese. Small Rum. Res. 2008, 74, 91–106. [Google Scholar] [CrossRef]
- Grażyna, C.; Hanna, C.; Adam, A.; Magdalena, B.M. Natural antioxidants in milk and dairy products. Int. J. Dairy Technol. 2017, 70, 165–178. [Google Scholar] [CrossRef]
| Item | Caciocavallo Palermitano TAP | Vastedda della Valle del Belìce PDO | Pecorino Siciliano PDO | Casizolu del Montiferru TAP | Caprino Nicastrese NB |
|---|---|---|---|---|---|
| Region origin | Sicily | Sicily | Sicily | Sardinia | Calabria |
| Production area | Province of Palermo | Western Sicily | Central and western Sicily | Montiferru | Nicastro-Lamezia Terme-Catanzaro |
| Milk nature and species | Raw bovine | Raw ovine | Raw ovine | Raw bovine | Raw caprine |
| Animal breed/s | Cinisara/Pezzata Rossa/Bruna/ Half-breed | Valle del Belice | Valle del Belìce/Comisana/ Pinzirita | Sardo-Modicana/Bruno-Sarda | Nicastrese |
| Curd/Paste | Stretched/hard | Stretched/soft | Pressed/hard | Stretched/hard | Semi-hard |
| Starter | No | No | No | No | No |
| Rennet | Lamb or Kid, paste | Lamb, paste | Lamb, paste | Calf, liquid | Kid, paste |
| Curd breaking | Seed of chickpea or lentil | Grain of rice | Grain of rice | Hazelnut size | Grain of rice (only in May, as grain of mais or hazelnut) |
| Cooking | For 3 h and, after about 20 h, stretched. In both cases scotta whey is used (85–90 °C) | Stretched with hot (85–90 °C) scotta whey | Under hot (70–75 °C) scotta whey for 3 h | Stretched with hot water (65–75 °C) | No |
| Salting | Brine | Brine | Dry or brine | Brine | Dry |
| Shape | Parallelepiped | Cylindrical | Cylindrical | Pear with head | Cylindrical |
| Diameter (cm) | 14 side of the square section | 15.17 | 15–20 | 13–16 | 11–13 |
| Weight (kg) | ~10 | 0.5–0.7 | 10 | 3–3.5 | 0.8–1.3 |
| Height (cm) | 38 | 3 | 15–25 | 22–23 | 5–6 |
| Ripening (d) | 60 | 20 | 210 | 180 | 20 |
| Cheese production (t/year) | 162.5–212.5 | 40 | 60 | 60–65 | 7.2–9.0 |
| Total producers (n) | 25 | 10 | 13 | 4 | 6 |
| Sampled cheeses (n) | 10 | 14 | 11 | 18 | 11 |
| Cheese Name | Ripening Days | Month of Production | Moisture | Fat | Saturated Fat | Protein | NaCl |
|---|---|---|---|---|---|---|---|
| Caciocavallo Palermitano TAP | 60 | February | 39.1 | 26.4 | 17.2 | 27.8 | 2.2 |
| April | 38.4 | 26.1 | 16.3 | 28.5 | 2.2 | ||
| SEM | 0.6 | 0.8 | 0.7 | 0.5 | 0.1 | ||
| Casizolu del Montiferru TAP | 180 | February | 36.2A | 27.8b | 18.2b | 29.9a | 2.0 |
| May | 31.4B | 29.3a | 19.5a | 27.4b | 2.1 | ||
| September | 35.5A | 27.7b | 18.2b | 29.9a | 2.1 | ||
| SEM | 0.3 | 0.4 | 0.3 | 0.6 | 0.01 | ||
| Vastedda valle del Belìce PDO | 20 | April | 46.2 | 19.7 | 13.4 | 27.7$ | 1.7 |
| June | 45.0 | 21.9 | 13.8 | 26.1& | 1.6 | ||
| SEM | 0.7 | 0.9 | 0.6 | 0.6 | 0.1 | ||
| Pecorino Siciliano PDO | 210 | January | 30.9A | 30.2 | 21.0 | 29.9b | 2.6a |
| May | 27.7B | 30.8 | 20.5 | 35.1a | 2.3b | ||
| SEM | 0.5 | 1.1 | 0.9 | 1.1 | 0.1 | ||
| Caprino Nicastrese NB | 20 | January | 42.5 | 27.0 | 17.9 | 21.0 | 2.3 |
| April | 39.7 | 26.5 | 18.1 | 25.7 | 2.1 | ||
| June | 39.8 | 26.1 | 17.4 | 26.0 | 2.1 | ||
| SEM | 2.0 | 1.0 | 0.9 | 1.6 | 0.1 |
| Cheese Name | Ripening Days | Month of Production | CLA cis9 trans 11 | MUFA | PUFA | PUFA ω6 | PUFA ω3 | Total Retinol | α-Tocopherol | Cholesterol |
|---|---|---|---|---|---|---|---|---|---|---|
| Caciocavallo Palermitano TAP | 60 | February | 0.21b | 5.8 | 0.72 | 0.50 | 0.21 | 0.25 | 0.60 | 78 |
| April | 0.41a | 5.4 | 0.79 | 0.48 | 0.28 | 0.26 | 0.72 | 86 | ||
| SEM | 0.05 | 0.3 | 0.03 | 0.03 | 0.04 | 0.04 | 0.10 | 4 | ||
| Casizolu del Montiferru TAP | 180 | February | 0.22A | 5.9 | 0.87 | 0.63 | 0.24a | 0.29a | 0.71b | 102 |
| May | 0.20B | 5.9 | 0.85 | 0.59 | 0.25a | 0.27ab | 1.18a | 106 | ||
| September | 0.15C | 6.3 | 0.82 | 0.66 | 0.16b | 0.21b | 0.70b | 97 | ||
| SEM | 0.01 | 0.1 | 0.03 | 0.05 | 0.02 | 0.02 | 0.12 | 3 | ||
| Vastedda valle del Belìce PDO | 20 | April | 0.26 | 2.4B | 0.86 | 0.33B | 0.50 | 0.26 | 0.58B | 74 |
| June | 0.25 | 4.1A | 1.03 | 0.55A | 0.46 | 0.26 | 1.11A | 68 | ||
| SEM | 0.02 | 0.2 | 0.07 | 0.04 | 0.04 | 0.01 | 0.07 | 4 | ||
| Pecorino Siciliano PDO | 210 | January | 0.22b | 4.9 | 0.96b | 0.61 | 0.33b | 0.25 | 0.36& | 86 |
| May | 0.41a | 4.3 | 1.29a | 0.61 | 0.65a | 0.27 | 0.58$ | 75 | ||
| SEM | 0.05 | 0.3 | 0.10 | 0.06 | 0.07 | 0.03 | 0.07 | 6 | ||
| Caprino Nicastrese NB | 20 | January | 0.17 | 5.5 | 0.63b | 0.42£ | 0.19b | 0.41 | 1.50 | 104 |
| April | 0.18 | 4.5 | 1.09a | 0.63$ | 0.45a | 0.29 | 0.72 | 95 | ||
| June | 0.20 | 5.0 | 0.93a | 0.51& | 0.39a | 0.32 | 0.85 | 103 | ||
| SEM | 0.03 | 0.5 | 0.06 | 0.05 | 0.04 | 0.04 | 0.27 | 6 |
| Cheese Name | Ripening Days | Month of Production | TPC (g GAE/kg Cheese) | FRAP (mmol FeSO4/kg Cheese) | TEAC (mmol Trolox/kg Cheese) |
|---|---|---|---|---|---|
| Caciocavallo Palermitano TAP | 60 | February | 3.52b | 1.84 | 52.37 |
| April | 4.65a | 2.00 | 46.83 | ||
| SEM | 0.27 | 0.08 | 3.61 | ||
| Casizolu del Montiferru TAP | 180 | February | 2.98 | 2.08 | 10.34b |
| May | 3.25 | 1.97 | 18.89a | ||
| September | 3.65 | 1.69 | 12.81ab | ||
| SEM | 0.29 | 0.19 | 2.09 | ||
| Vastedda valle del Belìce PDO | 20 | April | 4.62 | 2.19$ | 69.47a |
| June | 4.95 | 1.74& | 47.91b | ||
| SEM | 0.20 | 0.17 | 3.08 | ||
| Pecorino Siciliano PDO | 210 | January | 4.63 | 2.93 | 52.95a |
| May | 4.55 | 2.64 | 25.42b | ||
| SEM | 0.16 | 0.24 | 1.82 | ||
| Caprino Nicastrese NB | 20 | January | 2.30B | 2.32 | 6.89B |
| April | 3.58Ab | 1.76 | 49.11A | ||
| June | 4.46Aa | 1.77 | 38.33A | ||
| SEM | 0.18 | 0.31 | 3.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Trana, A.; Di Rosa, A.R.; Addis, M.; Fiori, M.; Di Grigoli, A.; Morittu, V.M.; Spina, A.A.; Claps, S.; Chiofalo, V.; Licitra, G.; et al. The Quality of Five Natural, Historical Italian Cheeses Produced in Different Months: Gross Composition, Fat-Soluble Vitamins, Fatty Acids, Total Phenols, Antioxidant Capacity, and Health Index. Animals 2022, 12, 199. https://doi.org/10.3390/ani12020199
Di Trana A, Di Rosa AR, Addis M, Fiori M, Di Grigoli A, Morittu VM, Spina AA, Claps S, Chiofalo V, Licitra G, et al. The Quality of Five Natural, Historical Italian Cheeses Produced in Different Months: Gross Composition, Fat-Soluble Vitamins, Fatty Acids, Total Phenols, Antioxidant Capacity, and Health Index. Animals. 2022; 12(2):199. https://doi.org/10.3390/ani12020199
Chicago/Turabian StyleDi Trana, Adriana, Ambra Rita Di Rosa, Margherita Addis, Myriam Fiori, Antonino Di Grigoli, Valeria Maria Morittu, Anna Antonella Spina, Salvatore Claps, Vincenzo Chiofalo, Giuseppe Licitra, and et al. 2022. "The Quality of Five Natural, Historical Italian Cheeses Produced in Different Months: Gross Composition, Fat-Soluble Vitamins, Fatty Acids, Total Phenols, Antioxidant Capacity, and Health Index" Animals 12, no. 2: 199. https://doi.org/10.3390/ani12020199
APA StyleDi Trana, A., Di Rosa, A. R., Addis, M., Fiori, M., Di Grigoli, A., Morittu, V. M., Spina, A. A., Claps, S., Chiofalo, V., Licitra, G., & Todaro, M. (2022). The Quality of Five Natural, Historical Italian Cheeses Produced in Different Months: Gross Composition, Fat-Soluble Vitamins, Fatty Acids, Total Phenols, Antioxidant Capacity, and Health Index. Animals, 12(2), 199. https://doi.org/10.3390/ani12020199







