Selective Inner Hair Cell Loss in a Neonate Harbor Seal (Phoca vitulina)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inner Ear Analysis
2.2. Scanning Electron Microscopy (SEM)
2.3. Characterization of the Lesions
3. Results
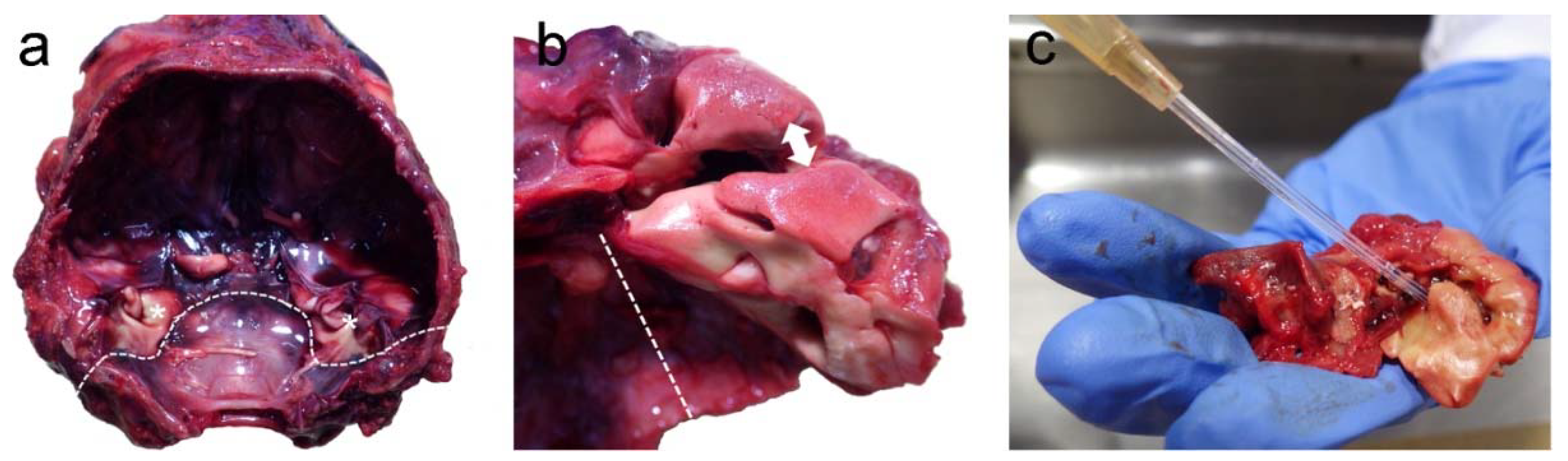
3.1. Post-Mortem Examination
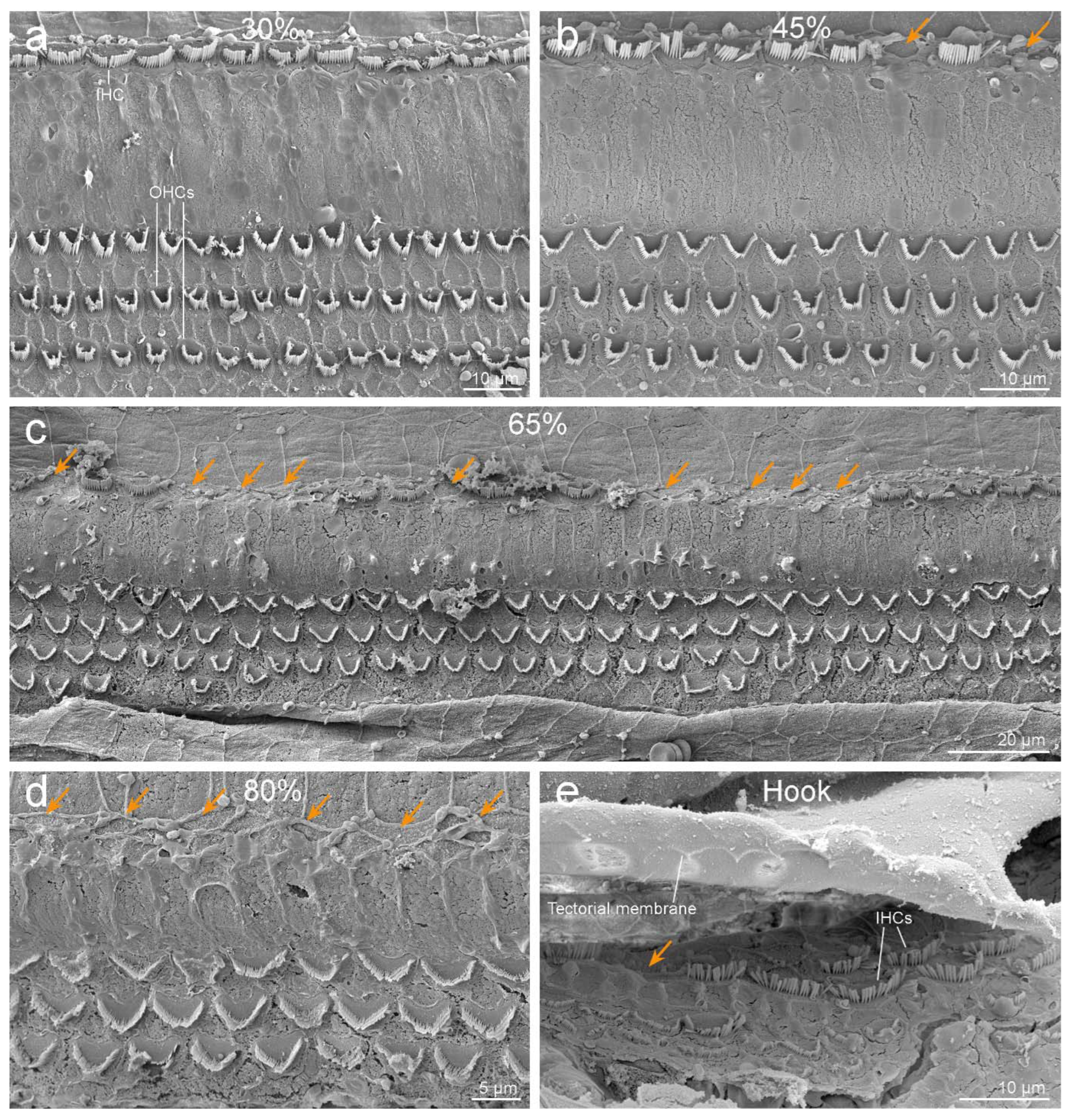
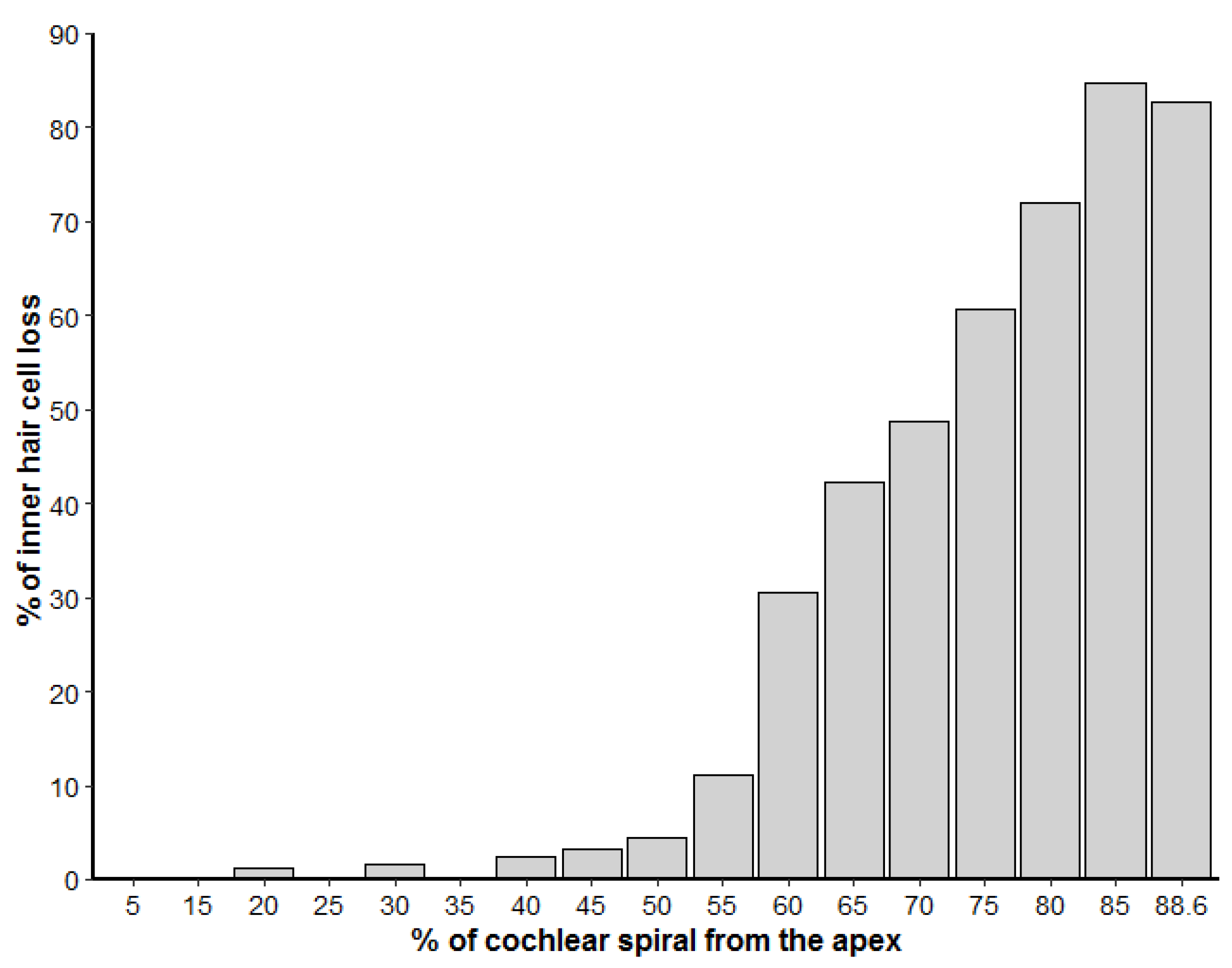
3.2. Inner Ear Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, R.; Bale, J.; White, K. Sensorineural hearing loss in children. Lancet 2005, 365, 879–890. [Google Scholar] [CrossRef]
- Belcher, R.; Virgin, F.; Duis, J.; Wootten, C. Genetic and non-genetic workup for pediatric congenital hearing loss. Front. Pediatr. 2021, 9, 536730. [Google Scholar] [CrossRef]
- Morton, N.E. Genetic epidemiology of hearing impairment. Ann. N. Y. Acad. Sci. USA 1991, 630, 16–31. [Google Scholar] [CrossRef]
- Raymond, M.; Walker, E.; Dave, I.; Dedhia, K. Genetic testing for congenital non-syndromic sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2019, 124, 68–75. [Google Scholar] [CrossRef]
- Kalatzis, V.; Petit, C. The fundamental and medical impacts of recent progress in research on hereditary hearing loss. Hum. Mol. Genet. 1998, 7, 1589–1597. [Google Scholar] [CrossRef]
- Farooq, R.; Hussain, K.; Tariq, M.; Farooq, A.; Mustafa, M. CRISPR/Cas9: Targeted genome editing for the treatment of hereditary hearing loss. J. Appl. Genet. 2020, 61, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Bredberg, G.; Ades, H.W.; Engström, H. Scanning electron microscopy of the normal and pathologically altered organ of Corti. Acta Oto-Laryngol. 1972, 73, 3–48. [Google Scholar] [CrossRef]
- Hu, B.H.; Guo, W.; Wang, P.Y.; Henderson, D.; Jiang, S.C. Intense noise-induced apoptosis in hair cells of guinea pig cochleae. Acta Oto-Laryngol. 2000, 120, 19–24. [Google Scholar] [CrossRef]
- Raphael, Y.; Altschuler, R.A. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil. Cytoskelet. 1991, 18, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.; Brownlow, A.; McGovern, B.; Raverty, S.A.; Shadwick, R.E.; André, M. Implementation of a method to visualize noise-induced hearing loss in mass stranded cetaceans. Sci. Rep. 2017, 7, 41848. [Google Scholar] [CrossRef]
- Suzuki, M.; Kishimoto, M.; Hayama, S.-I.; Ohtaishi, N.; Nakane, F. A case of cleft palate in a Kuril seal (Phoca vitulina stejnegeri), from Hokkaido, Japan. J. Wildl. Dis. 1992, 28, 490–493. [Google Scholar] [CrossRef] [PubMed]
- McKnight, C.A.; Reynolds, T.L.; Haulena, M.; Delahunta, A.; Gulland, F.M.D. Congenital hemicerebral anomaly in a stranded Pacific harbor seal (Phoca vitulina richardsi). J. Wildl. Dis. 2005, 41, 654–658. [Google Scholar] [CrossRef][Green Version]
- Dennison, S.E.; Forrest, L.J.; Fleetwood, M.L.; Gulland, F.M.D. Concurrent occipital bone malformation and atlantoaxial subluxation in a neonatal harbor seal (Phoca vitulina). J. Zoo Wildl. Med. 2009, 40, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.E.; Boor, M.; Fauquier, D.; Van Bonn, W.; Greig, D.J.; Gulland, F.M.D. Foramen ovale and ductus arteriosus patency in neonatal harbor seal (Phoca vitulina) pups in rehabilitation. Aquat. Mamm. 2011, 37, 161–166. [Google Scholar] [CrossRef]
- Harris, H.S.; Facemire, P.; Greig, D.J.; Colegrove, K.M.; Ylitalo, G.M.; Yanagida, G.K.; Nutter, F.B.; Fleetwood, M.; Gulland, F.M.D. Congenital neuroglial heterotopia in a neonatal harbor seal (Phoca vitulina richardsi ) with evidence of recent exposure to polycyclic aromatic hydrocarbons. J. Wildl. Dis. 2011, 47, 246–254. [Google Scholar] [CrossRef]
- Leger, J.A.S.; Nilson, E.M. Intestinal atresia in a harbor seal (Phoca vitulina) and a review of congenital conditions of the species. Aquat. Mamm. 2014, 40, 207–212. [Google Scholar] [CrossRef]
- D’Agnese, E.R.; Lambourn, D.M.; Olson, J.K.; Huggins, J.L.; Raverty, S.; Garner, M.M.; Calambokidis, J.; Scott, A.A.; Jeffries, S.J.; Gaydos, J.K. Congenital diseases in harbor seals (Phoca vitulina richardsii) from the Salish Sea. J. Wildl. Dis. 2021, 57, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore: Baltimore, MD, USA, 2005; p. 371. [Google Scholar]
- Raverty, S.A.; Duignan, P.J.; Jepson, P.D.; Morell, M. Gross Necropsy and Specimen Collection Protocols (Chapter 13). In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Dierauf, L.A., Gulland, F.M., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 249–266. [Google Scholar]
- Morell, M.; Lenoir, M.; Shadwick, R.E.; Jauniaux, T.; Dabin, W.; Begeman, L.; Ferreira, M.; Maestre, I.; Degollada, E.; Hernandez-Milian, G.; et al. Ultrastructure of the Odontocete organ of Corti: Scanning and transmission electron microscopy. J. Comp. Neurol. 2015, 523, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.; Raverty, S.A.; Mulsow, J.; Haulena, M.; Barret-Lennard, L.; Nordstrom, C.; Venail, F.; Shadwick, R.E. Combining cochlear analysis and auditory evoked potentials in a beluga whale with high-frequency hearing loss. Front. Vet. Sci. 2020, 7, 534917. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.; Ijsseldijk, L.L.; Piscitelli-Doshkov, M.; Ostertag, S.; Estrade, V.; Haulena, M.; Doshkov, P.; Bourien, J.; Raverty, S.A.; Siebert, U.; et al. Cochlear apical morphology in toothed whales: Using the pairing hair cell—Deiters’ cell as a marker to detect lesions. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2021, 1–12. [Google Scholar] [CrossRef]
- Girdlestone, C.D.; Ng, J.; Kössl, M.; Caplot, A.; Shadwick, R.E.; Morell, M. Correlating cochlear morphometrics from Parnell’s mustached bat (Pteronotus parnellii) with hearing. J. Assoc. Res. Otolaryngol. 2020, 21, 425–444. [Google Scholar] [CrossRef]
- Gibson, A.K.; Raverty, S.; Lambourn, D.M.; Huggins, J.; Magargal, S.L.; Grigg, M.E. Polyparasitism is associated with in-creased disease severity in Toxoplasma gondii-infected marine sentinel species. PLoS Negl. Trop. Dis. 2011, 5, e1142. [Google Scholar] [CrossRef]
- Bohne, A.B.; Harding, G.W. Degeneration in the cochlea after noise damage: Primary versus secondary events. Am. J. Otol. 2000, 21, 505–509. [Google Scholar]
- Huizing, E.H.; De Groot, J.C.M.J. Human cochlear pathology in aminoglycoside ototoxicity—A review. Acta Oto-Laryngologica 1987, 104, 117–125. [Google Scholar] [CrossRef]
- Takeno, S.; Harrison, R.V.; Mount, R.J.; Wake, M.; Harada, Y. Induction of selective inner hair cell damage by carboplatin. Scanning Microsc. 1994, 8, 97–106. [Google Scholar]
- Saito, T.; Saito, H.; Saito, K.; Wakui, S.; Manabe, Y.; Tsuda, G. Ototoxicity of carboplatin in guinea pigs. Auris Nasus Larynx 1989, 16, 13–21. [Google Scholar] [CrossRef]
- Karimi-Boroujeni, M.; Zahedi-Amiri, A.; Coombs, K.M. Embryonic origins of virus-induced hearing loss: Overview of molecular etiology. Viruses 2021, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Suzutani, T.; Baba, Y.; Koyano, S.; Nozawa, N.; Ishibashi, K.; Fujieda, K.; Inoue, N.; Omori, K. Etiology of severe sensorineural hearing loss in children: Independent impact of congenital cytomegalovirus infection and GJB2 mutations. J. Infect. Dis. 2007, 195, 782–788. [Google Scholar] [CrossRef]
- Grosse, S.D.; Ross, D.S.; Dollard, S.C. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: A quantitative assessment. J. Clin. Virol. 2008, 41, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Cheeran, M.; Lokensgard, J.R.; Schleiss, M.R. Neuropathogenesis of congenital cytomegalovirus infection: Disease mechanisms and prospects for intervention. Clin. Microbiol. Rev. 2009, 22, 99–126. [Google Scholar] [CrossRef]
- Tsuprun, V.; Keskin, N.; Schleiss, M.R.; Schachern, P.; Cureoglu, S. Cytomegalovirus-induced pathology in human temporal bones with congenital and acquired infection. Am. J. Otolaryngol. 2019, 40, 102270. [Google Scholar] [CrossRef] [PubMed]
- Schachtele, S.J.; Mutnal, M.B.; Schleiss, M.R.; Lokensgard, J.R. Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J. NeuroVirology 2011, 17, 201–211. [Google Scholar] [CrossRef]
- Nomura, Y.; Kurata, T.; Saito, K. Cochlear changes after Herpes simplex virus infection. Acta Oto-Laryngol. 1985, 99, 419–427. [Google Scholar] [CrossRef]
- Cohen, B.E.; Durstenfeld, A.; Roehm, P.C. Viral causes of hearing loss: A review for hearing health professionals. Trends Hear. 2014, 18. [Google Scholar] [CrossRef]
- Hemenway, W.G.; Sando, I.; McChesney, D. Temporal bone pathology following maternal rubella. Arch. Klin. Exp. Ohr. Nas. Kehlk. Heilk. 1969, 193, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, M.; Pujol, R. Age-related structural investigation of the Bronx waltzer mutant mouse cochlea: Scanning and transmission electron microscopy. Hear. Res. 1984, 13, 123–134. [Google Scholar] [CrossRef]
- Liberman, M.C.; Tartaglini, E.; Fleming, J.C.; Neufeld, E.J. Deletion of SLC19A2, the high affinity Thiamine transporter, causes selective inner hair cell loss and an auditory neuropathy phenotype. J. Assoc. Res. Otolaryngol. 2006, 7, 211–217. [Google Scholar] [CrossRef]
- Schrott, A.; Stephan, K.; Spoendlin, H. Hearing with selective inner hair cell loss. Hear. Res. 1989, 40, 213–219. [Google Scholar] [CrossRef]
- Kelsell, D.P.; Dunlop, J.; Stevens, H.P.; Lench, N.; Liang, J.N.; Parry, G.; Mueller, R.F.; Leigh, I.M. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997, 387, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Zelante, L.; Gasparini, P.; Estivill, X.; Melchionda, S.; D’Agruma, L.; Govea, N.; Mila, M.; Monica, M.D.; Lutfi, J.; Shohat, M.; et al. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum. Mol. Genet. 1997, 6, 1605–1609. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Lin, X.; Kong, W. Down regulated connexin26 at different postnatal stage displayed different types of cellular degeneration and formation of organ of Corti. Biochem. Biophys. Res. Commun. 2014, 445, 71–77. [Google Scholar] [CrossRef] [PubMed]
- De Siati, R.D.; Rosenzweig, F.; Gersdor, G.; Gregoire, A.; Rombaux, P.; Deggouj, N. Auditory neuropathy spectrum disorders: From diagnosis to treatment: Literature review and case reports. J. Clin. Med. 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Starr, A. Auditory neuropathy–neural and synaptic mechanisms. Nat. Rev. Neurol. 2016, 12, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Shearer, A.E.; Hansen, M.R. Auditory synaptopathy, auditory neuropathy, and cochlear implantation. Laryngoscope Investig. Otolaryngol. 2019, 4, 429–440. [Google Scholar] [CrossRef]
- Foerst, A.; Beutner, D.; Lang-Roth, R.; Huttenbrink, K.B.; von Wedel, H.; Walger, M. Prevalence of auditory neuropathy/synaptopathy in a population of children with profound hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Amatuzzi, M.; Liberman, M.C.; Northrop, C. Selective inner hair cell loss in prematurity: A temporal bone study of infants from a neonatal intensive care unit. J. Assoc. Res. Otolaryngol. 2011, 12, 595–604. [Google Scholar] [CrossRef]
- Soons, J.A.M.; Ricci, A.J.; Steele, C.R.; Puria, S. Cytoarchitecture of the mouse organ of Corti from base to apex, determined using in situ two-photon imaging. J. Assoc. Res. Otolaryngol. 2014, 16, 47–66. [Google Scholar] [CrossRef]
- Lobarinas, E.; Salvi, R.; Ding, D. Selective inner hair cell dysfunction in chinchillas impairs hearing-in-noise in the absence of outer hair cell loss. J. Assoc. Res. Otolaryngol. 2015, 17, 89–101. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morell, M.; Rojas, L.; Haulena, M.; Busse, B.; Siebert, U.; Shadwick, R.E.; Raverty, S.A. Selective Inner Hair Cell Loss in a Neonate Harbor Seal (Phoca vitulina). Animals 2022, 12, 180. https://doi.org/10.3390/ani12020180
Morell M, Rojas L, Haulena M, Busse B, Siebert U, Shadwick RE, Raverty SA. Selective Inner Hair Cell Loss in a Neonate Harbor Seal (Phoca vitulina). Animals. 2022; 12(2):180. https://doi.org/10.3390/ani12020180
Chicago/Turabian StyleMorell, Maria, Laura Rojas, Martin Haulena, Björn Busse, Ursula Siebert, Robert E. Shadwick, and Stephen A. Raverty. 2022. "Selective Inner Hair Cell Loss in a Neonate Harbor Seal (Phoca vitulina)" Animals 12, no. 2: 180. https://doi.org/10.3390/ani12020180
APA StyleMorell, M., Rojas, L., Haulena, M., Busse, B., Siebert, U., Shadwick, R. E., & Raverty, S. A. (2022). Selective Inner Hair Cell Loss in a Neonate Harbor Seal (Phoca vitulina). Animals, 12(2), 180. https://doi.org/10.3390/ani12020180







