Evidence of Hearing Loss and Unrelated Toxoplasmosis in a Free-Ranging Harbour Porpoise (Phocoena phocoena)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Stranding and Necropsy
2.2. Inner Ear Analysis
2.2.1. Right Inner Ear: Immunofluorescence (IF)
2.2.2. Left Inner Ear: Scanning Electron Microscopy (SEM)
2.3. Life History
2.4. Molecular Studies
2.5. Toxicology
2.6. Image Processing
3. Results
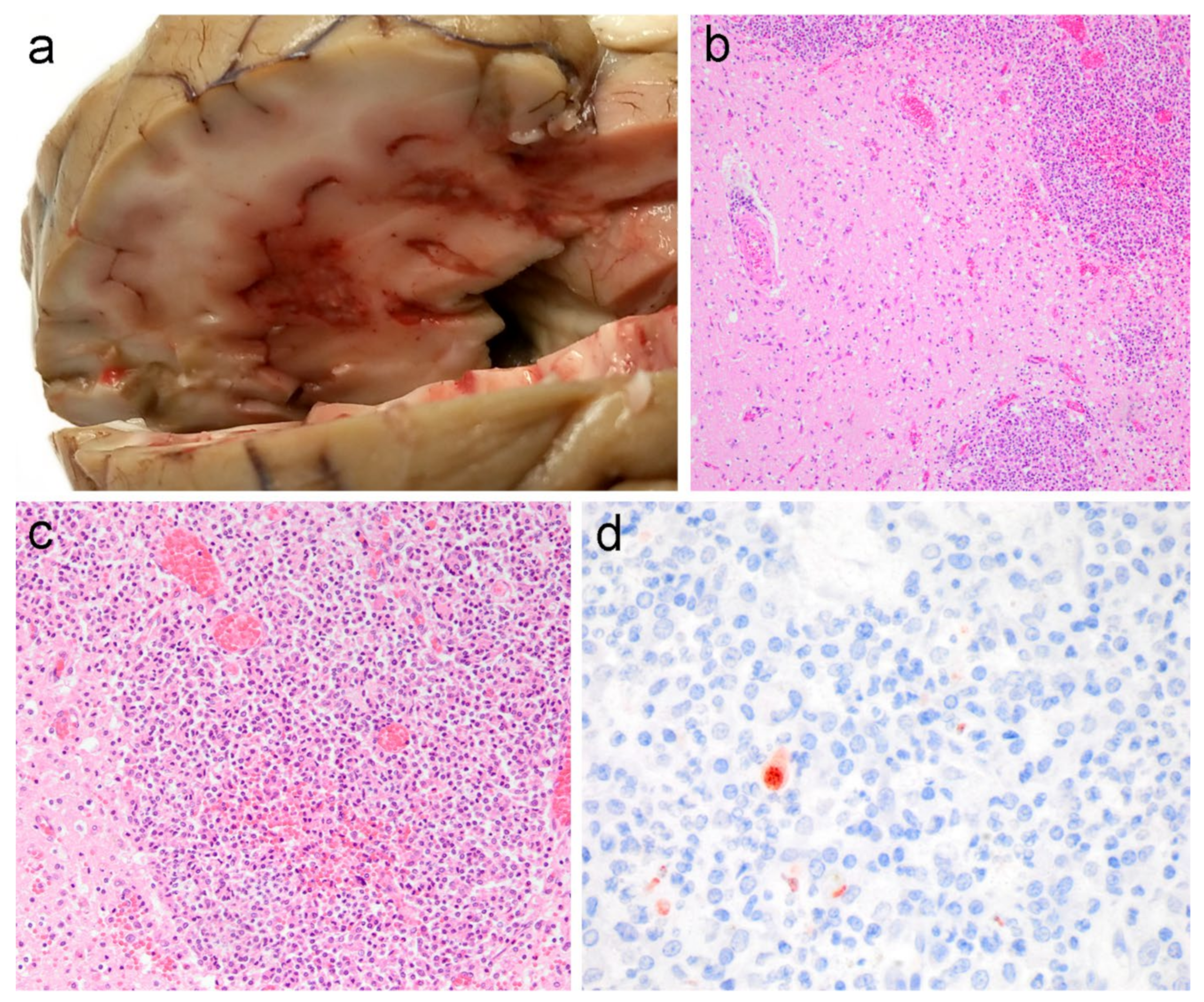
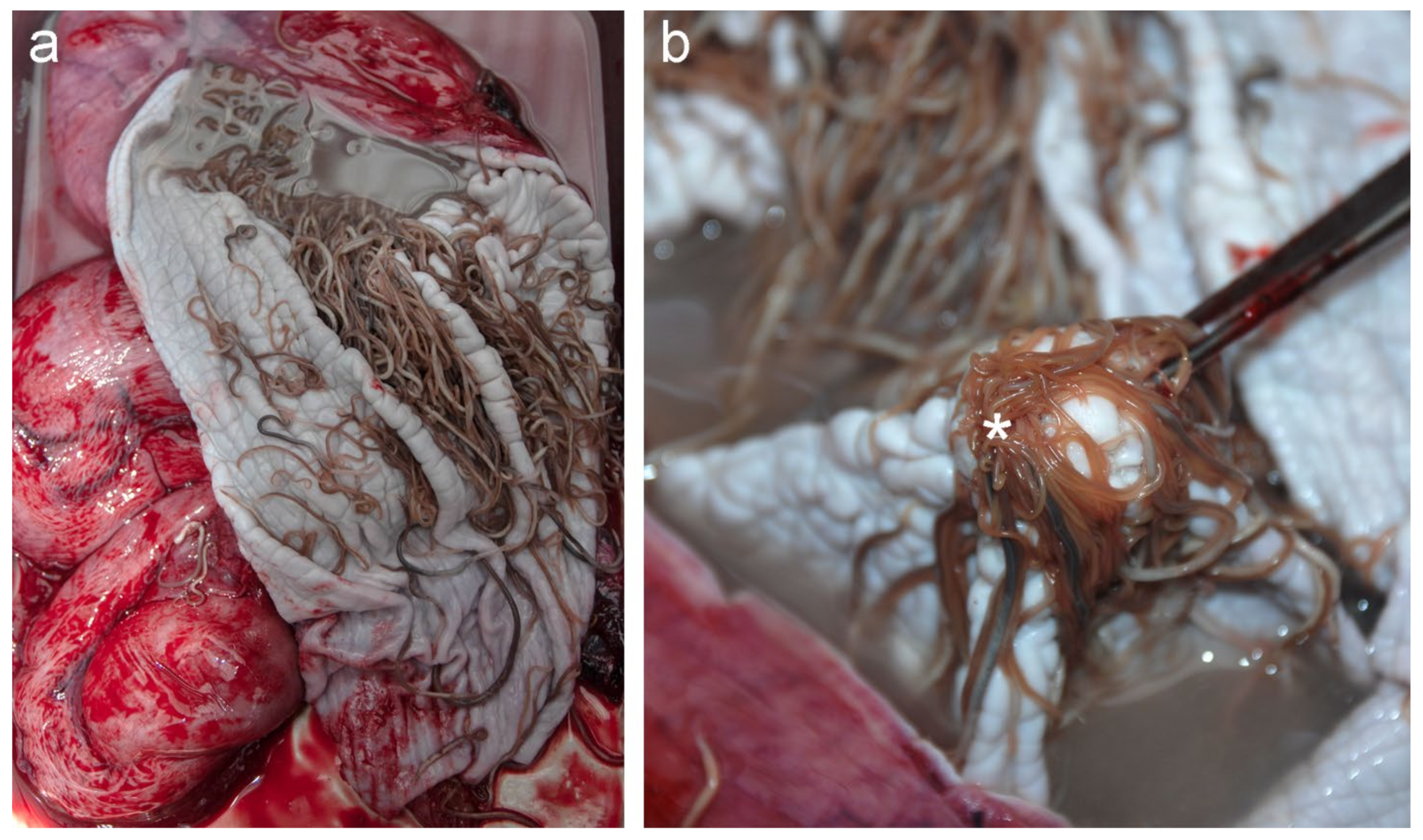
3.1. Pathological Findings
3.2. Immunohistochemistry and Molecular Studies
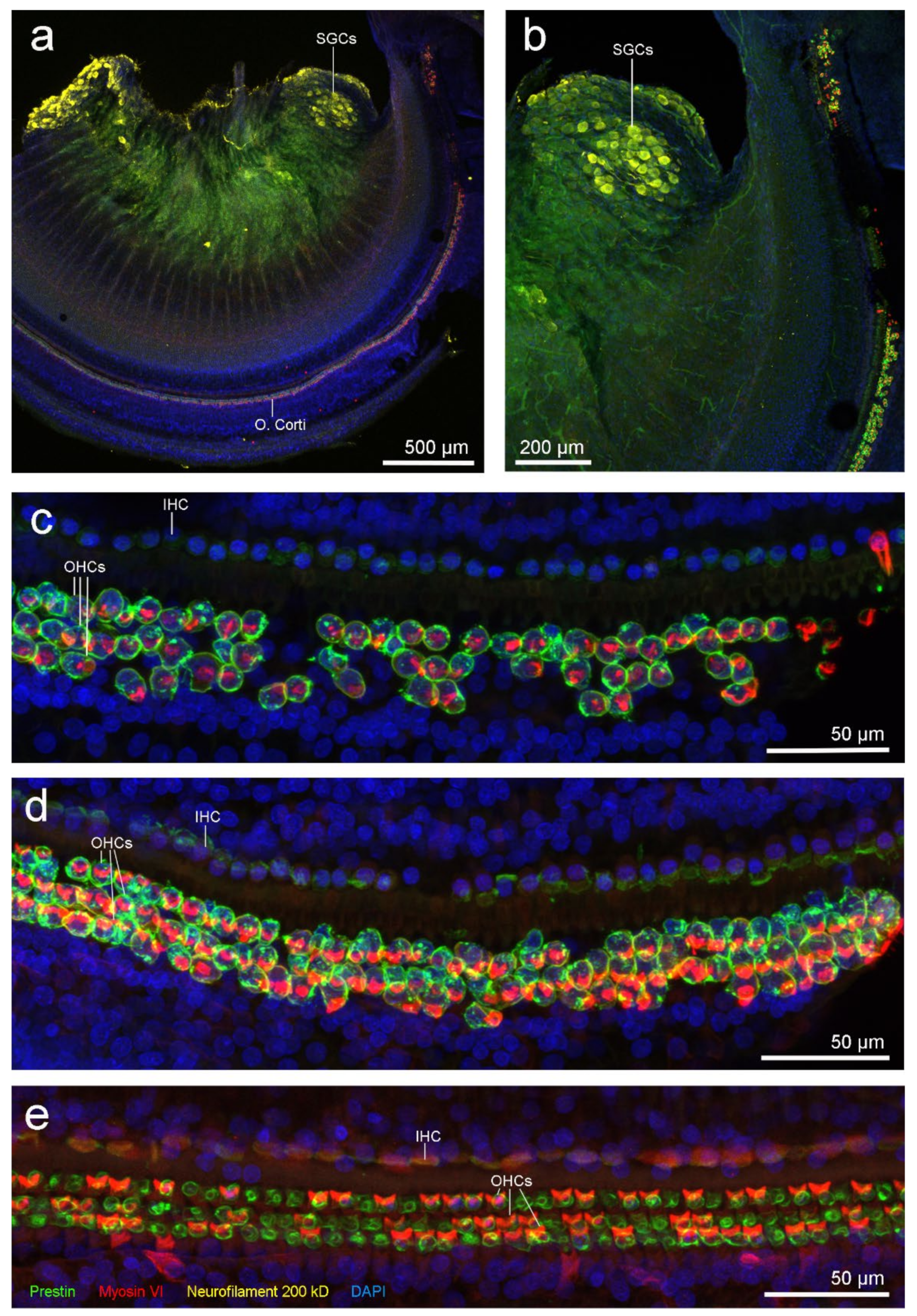
3.3. Inner Ear Analyses
3.3.1. Right Inner Ear: IF
3.3.2. Left Inner Ear: SEM
3.4. Life History
3.5. Toxicology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammond, P.S.; Berggren, P.; Benke, H.; Borchers, D.L.; Collet, A.; Heide-Jørgensen, M.P.; Heimlich, S.; Hiby, A.R.; Leopold, M.F.; Øien, N. Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. J. Appl. Ecol. 2002, 39, 361–376. [Google Scholar] [CrossRef]
- IJsseldijk, L.L.; ten Doeschate, M.T.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galatius, A.; Haelters, J.; Jepson, P.D.; Keijl, G.O.; Kinze, C.C.; et al. Spatiotemporal mortality and demographic trends in a small cetacean: Strandings to inform conservation management. Biol. Conserv. 2020, 249, 108733. [Google Scholar] [CrossRef]
- Green, M.; Caddell, R.; Eisfeld, S.; Dolman, S.; Simmonds, M. Looking Forward to ‘Strict Protection’: A Critical Review of the Current Legal Regime for Cetaceans in UK Waters; A WDCS Science Report; WDCS: Wiltshire, UK, 2012; 54p. [Google Scholar]
- Camphuysen, C.J.; Siemensma, M.L. Conservation Plan for the Harbour Porpoise Phocoena phocoena in the Netherlands: Towards a Favourable Conservation Status; NIOZ Report 2011-07; Royal Netherlands Institute for Sea Research: Texel, The Netherlands, 2011. [Google Scholar]
- Erbe, C.; Dunlop, R.; Dolman, S. Effects of Noise on Marine Mammals. In Effects of Anthropogenic Noise on Animals; Slabbekoorn, H., Dooling, R.J., Popper, A.N., Fay, R.R., Eds.; Springer Handbook of Auditory Research: New York, NY, USA, 2018; pp. 277–308. [Google Scholar]
- Simmonds, M.P.; Dolman, S.J.; Jasny, M.; Parsons, E.C.M.; Weilgart, L.; Wright, A.J.; Leaper, R. Marine noise pollution—Increasing recognition but need for more practical action. J. Ocean Technol. 2014, 9, 71–90. [Google Scholar]
- Morell, M. Ultrastructural Analysis of Odontocete Cochlea. Ph.D. Thesis, Universitat Politècnica de Catalunya, Vilanova i la Geltrú, Spain, 2012. Available online: http://www.tdx.cat/handle/10803/125113 (accessed on 4 May 2012).
- Morell, M.; Lenoir, M.; Shadwick, R.E.; Jauniaux, T.; Dabin, W.; Begeman, L.; Ferreira, M.; Maestre, I.; Degollada, E.; Hernandez-Milian, G.; et al. Ultrastructure of the odontocete organ of Corti: Scanning and transmission electron microscopy. J. Comp. Neurol. 2015, 523, 431–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morell, M.; Vogl, W.; Ijsseldijk, L.L.; Piscitelli-Doshkov, M.; Tong, L.; Ostertag, S.; Ferreira, M.; Fraija-Fernandez, N.; Colegrove, K.; Puel, J.L.; et al. Echolocating whales and bats express the motor protein prestin in the inner ear: A potential marker for hearing loss. Front. Vet. Sci. 2020, 7, 429. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.; IJsseldijk, L.L.; Piscitelli-Doshkov, M.; Ostertag, S.; Estrade, V.; Haulena, M.; Doshkov, P.; Bourien, J.; Raverty, S.A.; Siebert, U.; et al. Cochlear apical morphology in toothed whales: Using the pairing hair cell—Deiters’ cell as a marker to detect lesions. Anat. Rec. 2021, 1–21. [Google Scholar] [CrossRef]
- Bredberg, G.; Ades, H.W.; Engstrom, H. Scanning electron microscopy of normal and pathologically altered organ of Corti. Acta Otolaryngol. 1972, 73, 3–48. [Google Scholar] [CrossRef]
- Raphael, Y.; Altschuler, R.A. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil. Cytoskeleton 1991, 18, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Morell, M.; Brownlow, A.; McGovern, B.; Raverty, S.A.; Shadwick, R.E.; André, M. Implementation of a method to visualize noise-induced hearing loss in mass stranded cetaceans. Sci. Rep. 2017, 7, 41848. [Google Scholar] [CrossRef] [Green Version]
- IJsseldijk, L.L.; Scheidat, M.; Siemensma, M.L.; Couperus, B.; Leopold, M.F.; Morell, M.; Gröne, A.; Kik, M.J.L. Challenges in the assessment of bycatch: Postmortem findings in harbor porpoises (Phocoena phocoena) retrieved from gillnets. Vet. Pathol. 2021, 58, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Leopold, M.F.; Begeman, L.; van Bleijswijk, J.D.; IJsseldijk, L.L.; Witte, H.J.; Gröne, A. Exposing the grey seal as a major predator of harbour porpoises. Proc. R. Soc. B 2015, 282, 20142429. [Google Scholar] [CrossRef] [Green Version]
- Maio, E.; Begeman, L.; Bisselink, Y.; van Tulden, P.; Wiersma, L.; Hiemstra, S.; Ruuls, R.; Gröne, A.; Roest, H.I.J.; Willemsen, P.T.J.; et al. Identification and typing of Brucella spp. in stranded harbour porpoises (Phocoena phocoena) on the Dutch coast. Vet. Microbiol. 2014, 173, 118–124. [Google Scholar] [CrossRef] [PubMed]
- van Beurden, S.J.; IJsseldijk, L.L.; Ordonez, S.R.; Förster, C.; de Vrieze, G.; Gröne, A.; Verheije, M.H.; Kik, M. Identification of a novel gammaherpesvirus associated with (muco) cutaneous lesions in harbour porpoises (Phocoena phocoena). Arch. Virol. 2015, 160, 3115–3120. [Google Scholar] [CrossRef]
- Foster, G.; Whatmore, A.M.; Dagleish, M.P.; Malnick, H.; Gilbert, M.J.; Begeman, L.; Macgregor, S.K.; Davison, N.J.; Roest, H.J.; Jepson, P.; et al. Forensic microbiology reveals that Neisseria animaloris infections in harbour porpoises follow traumatic injuries by grey seals. Sci. Rep. 2019, 9, 14338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapetanou, A.; IJsseldijk, L.L.; Willems, D.S.; Broens, E.M.; Everaarts, E.; Buil, J.B.; Verweij, P.E.; Kik, M.J.L.; Gröne, A. Mycotic infections in free-ranging harbor porpoises (Phocoena phocoena). Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Herder, V.; van de Velde, N.; Kristensen, J.H.; Van Elk, C.; Peters, M.; Kilwinski, J.; Schares, G.; Siebert, U.; Wohlsein, P. Fatal disseminated Toxoplasma gondii infection in a captive harbour porpoise (Phocoena phocoena). J. Comp. Pathol. 2015, 153, 357–362. [Google Scholar] [CrossRef]
- van de Velde, N.; Devleesschauwer, B.; Leopold, M.; Begeman, L.; IJsseldijk, L.L.; Hiemstra, S.; Jzer, J.; Brownlow, A.; Davison, N.; Haelters, J.; et al. Toxoplasma gondii in stranded marine mammals from the North Sea and Eastern Atlantic Ocean: Findings and diagnostic difficulties. Vet. Parasitol. 2016, 230, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P.; Murata, F.H.; Cerqueira-Cézar, C.K.; Kwok, O.C.; Grigg, M.E. Recent epidemiologic and clinical importance of Toxoplasma gondii infections in marine mammals: 2009–2020. Vet. Parasitol. 2020, 288, 109296. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 313. [Google Scholar]
- Cabezón, O.; Resendes, A.R.; Domingo, M.; Raga, J.A.; Agustí, C.; Alegre, F.; Mons, J.L.; Dubey, J.P.; Almería, S. Seroprevalence of Toxoplasma gondii antibodies in wild dolphins from the Spanish Mediterranean coast. J. Parasitol. 2004, 90, 643–644. [Google Scholar] [CrossRef]
- Forman, D.; West, N.; Francis, J.; Guy, E. The sero-prevalence of Toxoplasma gondii in British marine mammals. Meml. Inst. Oswaldo Cruz 2009, 104, 296–298. [Google Scholar] [CrossRef] [Green Version]
- Bossart, G.D. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IJsseldijk, L.L.; Brownlow, A.C.; Mazzariol, S. (Eds.) Best Practice on Cetacean Post Mortem Investigation and Tissue Sampling. Joint ACCOBAMS/ASCOBANS Document. 2019, p. 1. Available online: https://osf.io/zh4ra/ (accessed on 1 October 2020).
- Key, M. Immunohistochemical Staining Methods. In Immunohistochemical Staining Methods, 5th ed.; Kumar, G.L., Rudbeck, L., Eds.; Dako Corporation: Carpinteria, CA, USA, 2009; pp. 57–60. [Google Scholar]
- Burrells, A.; Taroda, A.; Opsteegh, M.; Schares, G.; Benavides, J.; Dam-Deisz, C.; Bartley, P.M.; Chianini, F.; Villena, I.; van der Giessen, J.; et al. Detection and dissemination of Toxoplasma gondii in experimentally infected calves, a single test does not tell the whole story. Parasites Vectors 2018, 11, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morell, M.; André, M. Cetacean Ear Extraction and Fixation Protocol. 2009. Available online: http://www.zoology.ubc.ca/files/Ear_extraction_and_fixation_protocol_UBC.pdf (accessed on 7 October 2013).
- Morell, M.; Raverty, S.A.; Mulsow, J.; Haulena, M.; Barret-Lennard, L.; Nordstrom, C.; Venail, F.; Shadwick, R.E. Combining cochlear analysis and auditory evoked potentials in a beluga whale with high-frequency hearing loss. Front. Vet. Sci. 2020, 7, 534917. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Pierce, G.J.; Law, R.J.; Bersuder, P.; Jepson, P.D.; Learmonth, J.A.; Addink, M.; Dabin, W.; Santos, M.B.; Deaville, R.; et al. Assessing the effect of persistent organic pollutants on reproductive activity in common dolphins and harbour porpoises. J. Northwest Atl. Fish Sci. 2010, 42, 153–173. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.; Barber, J.L.; Learmonth, J.A.; Read, F.L.; Deaville, R.; Perkins, M.W.; Brownlow, A.; Davison, N.; Penrose, R.; Pierce, G.J.; et al. Reproductive failure in UK harbour porpoises Phocoena phocoena: Legacy of pollutant exposure? PLoS ONE 2015, 10, e0131085. [Google Scholar] [CrossRef] [Green Version]
- van den Heuvel-Greve, M.; van den Brink, A.M.; Kotterman, M.; Kwadijk, C.; Geelhoed, S.C.V.; Murphy, S.; van den Broek, J.; Heesterbeek, H.; Gröne, A.; IJsseldijk, L.L. Polluted porpoises: Generational transfer of contaminants in harbour porpoises from the southern North Sea. Sci. Total Environ. 2021, 796, 1. [Google Scholar] [CrossRef]
- Hohn, A.A.; Lockyer, C. Protocol for Obtaining Age Estimates from Harbour Porpoise Teeth. In Biology of Phocoenids. Appendix 3, Report of the Harbour Porpoise Age Determination Workshop; International Whaling Commission: Cambridge, UK, 1995. [Google Scholar]
- Pereira, G.R.; Vogel, F.S.; Bohrer, R.C.; da Nóbrega, J.E.J.; Ilha, G.F.; da Rosa, P.R.; Glanzner, W.G.; Camillo, G.; Braunig, P.; de Oliveira, J.F.; et al. Neospora caninum DNA detection by TaqMan real-time PCR assay in experimentally infected pregnant heifers. Vet. Parasitol. 2014, 199, 129–135. [Google Scholar] [CrossRef]
- Elmore, S.A.; Jones, J.L.; Conrad, P.A.; Patton, S.; Lindsay, D.S.; Dubey, J.P. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010, 26, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Van Devanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.; Dietz, H.H.; Andersen, T.H.; Hammer, A.; Kuiken, T.; Osterhaus, A.; van de Bildt, M. Another phocine distemper outbreak in Europe. Science 2002, 297, 209. [Google Scholar] [CrossRef] [Green Version]
- Saunders, J.C.; Cohen, Y.E.; Szymko, Y.M. The structural and functional consequences of acoustic injury in the cochlea and peripheral auditory system: A five year update. J. Acoust. Soc. Am. 1991, 90, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear. Res. 2019, 377, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.A.; Mackey, C.A.; MacDonald, K.S.; Hackett, T.A.; Ramachandran, R. Changes in audiometric threshold and frequency selectivity correlate with cochlear histopathology in macaque monkeys with permanent noise-induced hearing loss. Hear. Res. 2020, 398, 108082. [Google Scholar] [CrossRef] [PubMed]
- Abrashkin, K.A.; Izumikawa, M.; Miyazawa, T.; Wang, C.; Crumling, M.A.; Swiderski, D.L.; Beyer, L.A.; Gong, T.L.; Raphael, Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear. Res. 2006, 218, 20–29. [Google Scholar] [CrossRef]
- Johnsson, L.G.; Hawkins, J.E. Sensory and neural degeneration with aging, as seen in microdissections of human inner ear. Ann. Otol. Rhinol. Laryngol. 1972, 81, 179–193. [Google Scholar] [CrossRef]
- Black, R.E.; Lau, W.K.; Weinstein, R.J.; Young, L.S.; Hewitt, W.L. Ototoxicity of amikacin. Antimicrob. Agents Chemother. 1976, 9, 956–961. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.J.; Dunn, D.E. Anatomic correlates of noise induced hearing loss. Otolaryngol. Clin. N. Am. 1979, 12, 493–513. [Google Scholar] [CrossRef]
- Goldey, E.S.; Kehn, L.S.; Rehnberg, G.L.; Crofton, K.M. Developmental exposure to polychlorinated-biphenyls (Aroclor-1254) reduces circulating thyroid-hormone concentrations and causes hearing deficits in rats. Toxicol. Appl. Pharmacol. 1995, 135, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Herr, D.W.; Goldey, E.S.; Crofton, K.M. Developmental exposure to Aroclor 1254 produces low-frequency alterations in adult rat brainstem auditory evoked responses. Fundam. Appl. Toxicol. 1996, 33, 120–128. [Google Scholar] [CrossRef]
- Crofton, K.; Ding, D.; Padich, R.; Taylor, M.; Henderson, D. Hearing loss following exposure during development to polychlorinated biphenyls: A cochlear site of action. Hear. Res. 2000, 144, 196–204. [Google Scholar] [CrossRef]
- Noorbakhsh, S.; Memari, F.; Farhadi, M.; Tabatabaei, A. Sensorineural hearing loss due to Toxoplasma gondii in children: A case-control study. Clin. Otolaryngol. 2008, 33, 269–273. [Google Scholar] [CrossRef] [PubMed]
- De Castro Corrêa, C.; Maximino, L.P.; Weber, S.A.T. Hearing disorders in congenital toxoplasmosis: A literature review. Int. Arch. Otorhinolaryngol. 2018, 22, 330–333. [Google Scholar]
- Salviz, M.; Montoya, J.G.; Nadol, J.B.; Santos, F. Otopathology in congenital toxoplasmosis. Otol. Neurotol. 2013, 34, 1165–1169. [Google Scholar] [CrossRef] [Green Version]
- Cohen, B.E.; Durstenfeld, A.; Roehm, P.C. Viral causes of hearing loss: A review for hearing health professionals. Trends Hear. 2014, 18, 1–17. [Google Scholar] [CrossRef]
- Sheridan, M.D. Final report of a prospective study of children whose mothers had Rubella in early pregnancy. Br. Med. J. 1964, 2, 536–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekhar, S.S.; Connelly, P.E.; Brahmbhatt, S.S.; Shah, C.S.; Kloser, P.C.; Baredes, S. Otologic and audiologic evaluation of human immunodeficiency virus-infected patients. Am. J. Otolaryngol. 2000, 21, 1–9. [Google Scholar] [CrossRef]
- Cureoglu, S.; Schachern, P.A.; Paparella, M.M.; Lindgren, B.R. Cochlear changes in chronic otitis media. Laryngoscope 2004, 114, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Houser, D.S.; Finneran, J.J. Variation in the hearing sensitivity of a dolphin population determined through the use of evoked potential audiometry. J. Acoust. Soc. Am. 2006, 120, 4090–4099. [Google Scholar] [CrossRef]
- Houser, D.S.; Gomez-Rubio, A.; Finneran, J.J. Evoked potential audiometry of 13 Pacific bottlenose dolphins (Tursiops truncatus gilli). Mar. Mamm. Sci. 2008, 24, 28–41. [Google Scholar] [CrossRef]
- Sun, J.J.; Wang, J.B.; Wei, N.R. Histopathological observation on the inner ear barotrauma in guinea pig. J. Tongji Univ. Nat. Sci. 1987, 7, 136–142. [Google Scholar]
- Wang, J.; Puel, J.L. Toward cochlear therapies. Physiol. Rev. 2018, 98, 2477–2522. [Google Scholar] [CrossRef] [PubMed]
- Jepson, P.D.; Bennett, P.M.; Deaville, R.; Allchin, C.R.; Baker, J.R.; Law, R.J. Relationships between polychlorinated biphenyls and health status in harbor porpoises (Phocoena phocoena) stranded in the United Kingdom. Environ. Toxicol. Chem. 2005, 24, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Blankenship, A.; Jones, P.; Giesy, J. Toxicity reference values for the toxic effects of polychlorinated biphenyls to aquatic mammals. Hum. Ecol. Risk Assess. 2000, 6, 181–201. [Google Scholar] [CrossRef]
- Mancini, P.; Atturo, F.; Di Mario, A.; Portanova, G.; Ralli, M.; De Virgilio, A.; de Vincentiis, M.; Greco, A. Hearing loss in autoimmune disorders: Prevalence and therapeutic options. Autoimmun. Rev. 2018, 17, 644–652. [Google Scholar] [CrossRef]
- Davis, H.; Morgan, C.T.; Hawkins, J.E.; Galambos, R.; Smith, F.W. Temporary deafness following exposure to loud tones and noise. Acta Oto-Laryngol. 1950, Suppl. S88, 1–56. [Google Scholar] [CrossRef]
- Kastelein, R.A.; Schop, J.; Gransier, R.; Hoek, L. Frequency of greatest temporary hearing threshold shift in harbor porpoises (Phocoena phocoena) depends on the noise level. J. Acoust. Soc. Am. 2014, 136, 1410–1418. [Google Scholar] [CrossRef]
- Finneran, J.J. Noise-induced hearing loss in marine mammals: A review of temporary threshold shift studies from 1996 to 2015. J. Acoust. Soc. Am. 2015, 138, 1702–1726. [Google Scholar] [CrossRef]
- Reichmuth, C.; Sills, J.M.; Mulsow, J.; Ghoul, A. Long-term evidence of noise-induced permanent threshold shift in a harbor seal (Phoca vitulina). J. Acoust. Soc. Am. 2019, 146, 2522. [Google Scholar] [CrossRef]
- Migaki, G.; Sawa, T.R.; Dubey, J.P. Fatal disseminated toxoplasmosis in a spinner dolphin (Stenella longirostris). Vet. Pathol. 1990, 27, 463–464. [Google Scholar] [CrossRef]
- Di Guardo, G.; Proietto, U.; Di Francesco, C.E.; Marsilio, F.; Zaccaroni, A.; Scaravelli, D.; Mignone, W.; Garibaldi, F.; Kennedy, S.; Forster, F.; et al. Cerebral toxoplasmosis in striped dolphins (Stenella coeruleoalba) stranded along the Ligurian Sea coast of Italy. Vet. Pathol. 2010, 47, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Mazzariol, S.; Marcer, F.; Mignone, W.; Serracca, L.; Goria, M.; Marsili, L.; Di Guardo, G.; Casalone, C. Dolphin Morbillivirus and Toxoplasma gondii coinfection in a Mediterranean fin whale (Balaenoptera physalus). BMC Vet. Res. 2012, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- St Leger, J.; Raverty, S.; Mena, A. Cetacea. In Pathology of Wildlife and Zoo Animals; Academic Press: New York, NY, USA, 2018; pp. 533–568. [Google Scholar]
- Yan, J.; Huang, B.; Liu, G.; Wu, B.; Huang, S.; Zheng, H.; Shen, J.; Lun, Z.; Wang, Y.; Lu, F. Meta-analysis of prevention and treatment of toxoplasmic encephalitis in HIV-infected patients. Acta Trop. 2013, 127, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.P.; Tan, F.; Lindsay, D.S. Pathogenesis of Toxoplasma gondii in Humans. In Human Emerging and Re-Emerging Infections: Viral and Parasitic Infections; Singh, S.K., Ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2016; Volume I, pp. 303–317. [Google Scholar]
- Van Bressem, M.F.; Raga, J.A.; Di Guardo, G.; Jepson, P.D.; Duignan, P.J.; Siebert, U.; Barrett, T.; de Oliveira Santos, M.C.; Moreno, I.B.; Siciliano, S.; et al. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 2009, 86, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, G.; Mazzariol, S. Toxoplasma gondii: Clues from stranded dolphins. Vet. Pathol. 2013, 50, 737. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, H.; Liu, D.; Huo, X.; Gao, J.; Song, X.; Xu, X.; Huang, K.; Liu, W.; Wang, Y.; et al. Genotypes and mouse virulence of Toxoplasma gondii isolates from animals and humans in China. PLoS ONE 2013, 8, e53483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Guardo, G.; Centelleghe, C.; Mazzariol, S. Cetacean host-pathogen interaction(s): Critical knowledge gaps. Front. Immunol. 2018, 9, 2815. [Google Scholar] [CrossRef]
- Ten Doeschate, M.T.; IJsseldijk, L.L.; Hiemstra, S.; De Jong, E.A.; Strijkstra, A.; Gröne, A.; Begeman, L. Quantifying parasite presence in relation to biological parameters of harbour porpoises Phocoena phocoena stranded on the Dutch coast. Dis. Aquat. Organ. 2017, 127, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Siebert, U.; Wunschmann, A.; Weiss, R.; Frank, H.; Benke, H.; Frese, K. Post-mortem findings in harbour porpoises (Phocoena phocoena) from the German North and Baltic Seas. J. Comp. Pathol. 2001, 124, 102–114. [Google Scholar] [CrossRef]
- Siebert, U.; Pawliczkac, I.; Benke, H.; von Vietinghoff, V.; Wolf, P.; Pilāts, V.; Kesselring, T.; Lehnert, K.; Prenger-Berninghoff, E.; Galatius, A.; et al. Health assessment of harbour porpoises (Phocoena phocoena) from Baltic area of Denmark, Germany, Poland and Latvia. Environ. Int. 2020, 143, 105904. [Google Scholar] [CrossRef]
- Jauniaux, T.; Petitjean, D.; Brenez, C.; Borrens, M.; Brosens, L.; Haelters, J.; Tavernier, T.; Coignoul, F. Post-mortem findings and causes of death of harbour porpoises (Phocoena phocoena) stranded from 1990 to 2000 along the coastlines of Belgium and Northern France. J. Comp. Pathol. 2002, 126, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Jepson, P.D.; Baker, J.R.; Kuiken, T.; Simpson, V.R.; Kennedy, S.; Bennett, P.M. Pulmonary pathology of harbour porpoises stranded in England and Wales between 1990 and 1996. Vet. Rec. 2000, 146, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Siebert, U.; MacLachlan, M.; Bruhn, R.; Thron, K.; Failing, K.; Müller, G.; Baumgärtner, W. Investigations of the potential influence of environmental contaminants on the thymus and spleen of harbor porpoises (Phocoena phocoena). Environ. Sci. Technol. 2005, 39, 3933–3938. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.-W.; Sonne, C.; Levin, M.; Siebert, U.; De Guise, S.; Dietz, R. Immunotoxic effects of environmental pollutants in marine mammals. Environ. Int. 2015, 86, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Jepson, P.D.; Deaville, R.; Barber, J.L.; Aguilar, A.; Borrell, A.; Murphy, S.; Barry, J.; Brownlow, A.; Barnett, J.; Berrow, S.; et al. PCB pollution continues to impact populations of orcas and other dolphins in European waters. Sci. Rep. 2016, 6, 18573. [Google Scholar] [CrossRef] [Green Version]
- Møhl, B.; Andersen, S. Echolocation: High-frequency component in click of harbor porpoise (Phocoena ph. L.). J. Acoust. Soc. Am. 1973, 54, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Villadsgaard, A.; Wahlberg, M.; Tougaard, J. Echolocation signals of wild harbour porpoises, Phocoena phocoena. J. Exp. Biol. 2007, 210, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.E.; Wahlberg, M. Echolocation by the harbor porpoise: Life in coastal waters. Front. Physiol. 2013, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Kastelein, R.A.; Helder-Hoek, L.; Van de Voorde, S. Hearing thresholds of a male and a female harbor porpoise (Phocoena phocoena). J. Acoust. Soc. Am. 2017, 142, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Ketten, D.R. Marine Mammal Auditory System Noise Impacts: Evidence and Incidence. In The Effects of Noise on Aquatic Life. Advances in Experimental Medicine and Biology; Popper, A.N., Hawkins, A., Eds.; Springer: New York, NY, USA, 2012; Volume 730, pp. 207–212. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morell, M.; IJsseldijk, L.L.; Berends, A.J.; Gröne, A.; Siebert, U.; Raverty, S.A.; Shadwick, R.E.; Kik, M.J.L. Evidence of Hearing Loss and Unrelated Toxoplasmosis in a Free-Ranging Harbour Porpoise (Phocoena phocoena). Animals 2021, 11, 3058. https://doi.org/10.3390/ani11113058
Morell M, IJsseldijk LL, Berends AJ, Gröne A, Siebert U, Raverty SA, Shadwick RE, Kik MJL. Evidence of Hearing Loss and Unrelated Toxoplasmosis in a Free-Ranging Harbour Porpoise (Phocoena phocoena). Animals. 2021; 11(11):3058. https://doi.org/10.3390/ani11113058
Chicago/Turabian StyleMorell, Maria, Lonneke L. IJsseldijk, Alinda J. Berends, Andrea Gröne, Ursula Siebert, Stephen A. Raverty, Robert E. Shadwick, and Marja J. L. Kik. 2021. "Evidence of Hearing Loss and Unrelated Toxoplasmosis in a Free-Ranging Harbour Porpoise (Phocoena phocoena)" Animals 11, no. 11: 3058. https://doi.org/10.3390/ani11113058





