Improving Trapping Efficiency for Control of American Mink (Neovison vison) in Patagonia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Trapping
2.3. Efficiency Rates and Estimation of the Proportion of the Population Removed
2.4. Data Analysis
3. Results
3.1. Trapping
3.2. Efficiency Rates and Estimation of the Proportion of the Population Removed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaksic, F.M.; Iriarte, J.A.; Jiménez, J.E.; Martínez, D.R. Invaders without frontiers: Cross-border invasions of exotic mammals. Biol. Invasions 2002, 4, 157–173. [Google Scholar] [CrossRef]
- Bonesi, L.; Palazon, S. The American mink in Europe: Status, impacts, and control. Biol. Conserv. 2007, 134, 470–483. [Google Scholar] [CrossRef]
- Sandoval, R.J.; Schlatter, R. Estudio Ecológico del Visón Asilvestrado (Mustela vison, Schreber) en la XI Región. Bachelor’s Thesis, Universidad Austral de Chile, Valdivia, Chile, 1994; 74p. [Google Scholar]
- Previtali, A.; Cassini, M.H.; Macdonald, D.W. Habitat use and diet of the American mink (Mustela vison) in Argentinian Patagonia. J. Zool. 1998, 246, 482–486. [Google Scholar] [CrossRef]
- Anderson, C.B.; Rozzi, R.; Torres-Mura, J.C.; McGehee, S.M.; Sherriffs, M.F.; Schüttler, E.; Rosemond, A.D. Exotic vertebrate fauna in the remote and pristine sub-Antarctic Cape Horn Archipelago, Chile. Biodivers. Conserv. 2006, 15, 3295–3313. [Google Scholar] [CrossRef]
- Medina, G. A comparison of the diet and distribution of southern river otter (Lutra provocax) and mink (Mustela vison) in Southern Chile. J. Zool. 2009, 242, 291–297. [Google Scholar] [CrossRef]
- Rozzi, R.; Sherriffs, M.F. El visón (Mustela vison Schreber, Carnivora: Mustelidae), un nuevo mamífero exótico para la Isla Navarino. An. Del Inst. De La Patagon. 2003, 31, 97–104. [Google Scholar]
- Bonacic, C.; Ohrens, O.; Hernández, F. Estudio Poblacional y Orientado al Control de la Especie Invasora Neovison vison (Schreber, 1777) en el Sur de Chile; Laboratorio Fauna Australis, Pontificia Universidad Católica de Chile: Santiago, Chile, 2010. [Google Scholar]
- Mora, M.; Medina-Vogel, G.; Sepúlveda, M.A.; Noll, D.; Álvarez-Varas, R.; Vianna, J.A. Genetic structure of introduced American mink (Neovison vison) in Patagonia: Colonisation insights and implications for control and management strategies. Wildl. Res. 2018, 45, 344–356. [Google Scholar] [CrossRef]
- Lizarralde, M.S.; Escobar, J.M. Mamíferos exóticos en la Tierra del Fuego. Rev. Cienc. Hoy 2000, 10, 52–63. [Google Scholar]
- Schüttler, E.; Cárcamo, J.; Rozzi, R. Diet of the American mink Mustela vison and its potential impact on the native fauna of navarino Island, cape horn biosphere reserve, Chile. Rev. Chil. De Hist. Nat. 2008, 81, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, J.T.; Fasola, L.; MacDonald, D.W.; Rozzi, R.; Bonacic, C. Invasive american mink mustela vison in wetlands of the cape horn biosphere reserve, southern Chile: What are they eating? Oryx 2009, 43, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Schüttler, E.; Klenke, R.; McGehee, S.; Rozzi, R.; Jax, K. Vulnerability of ground-nesting waterbirds to predation by invasive American mink in the Cape Horn Biosphere Reserve, Chile. Biol. Conserv. 2009, 142, 1450–1460. [Google Scholar] [CrossRef]
- Peris, S.J.; Sanguinetti, J.; Pescador, M. Have Patagonian waterfowl been affected by the introduction of the American mink Mustela vison? Oryx 2009, 43, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, A.E.J.; Raya Rey, A.; Fasola, L.; Sáenz Samaniego, R.A.; Schiavini, A. Trophic ecology of a top predator colonizing the southern extreme of South America: Feeding habits of invasive American mink (Neovison vison) in Tierra del Fuego. Mamm. Biol. 2013, 78, 104–110. [Google Scholar] [CrossRef]
- Valenzuela, A.E.J.; Raya Rey, A.; Fasola, L.; Schiavini, A. Understanding the inter-specific dynamics of two co-existing predators in the Tierra del Fuego Archipelago: The native southern river otter and the exotic American mink. Biol. Invasions 2013, 15, 645–656. [Google Scholar] [CrossRef]
- Jiménez, J.E.; Crego, R.D.; Soto, G.E.; Román, I.; Rozzi, R.; Vergara, P.M. Potential impact of the alien American mink (Neovison vison) on Magellanic woodpeckers (Campephilus magellanicus) in Navarino Island, Southern Chile. Biol. Invasions 2014, 16, 961–966. [Google Scholar] [CrossRef]
- Fasola, L.; Roesler, I. A familiar face with a novel behavior raises challenges for conservation: American mink in arid Patagonia and a critically endangered bird. Biol. Conserv. 2018, 218, 217–222. [Google Scholar] [CrossRef]
- Pagnoni, G.O.; Garrido, J.L.; Marín, M.R. Impacto Económico y Ambiental Del Visón, Mustela vison (Schreber, 1877) en el Norte de la Patagonia; CENPAT-CONICET, Dirección de Fauna Silvestre: Provincia de Chubut, Argentina, 1986. [Google Scholar]
- Sepúlveda, M.A.; Singer, R.S.; Silva-Rodríguez, E.A.; Eguren, A.; Stowhas, P.; Pelican, K. Invasive American mink: Linking pathogen risk between domestic and endangered carnivores. EcoHealth 2014, 11, 409–419. [Google Scholar] [CrossRef]
- Iriarte, A.; Jaksic, F.M. Los Carnívoros de Chile, 2nd ed.; Ediciones Flora & Fauna: Santiago, Chile, 2017; p. 260. [Google Scholar]
- Fasola, L.; Roesler, I. Invasive predator control program in Austral Patagonia for endangered bird conservation. Eur. J. Wildl. Res. 2016, 62, 601–608. [Google Scholar] [CrossRef]
- Birks, S.J.D. Studies on the home range of feral mink (Mustela vison). Symp. Zool. Soc. Lond. 1982, 49, 231–257. [Google Scholar]
- Dunstone, N. The Mink; T & A D Poyte Ltd.: London, UK, 1993. [Google Scholar] [CrossRef]
- MacDonald, D.W.; Harrington, L.A. The American mink: The triumph and tragedy of adaptation out of context. N. Z. J. Zool. 2003, 30, 421–441. [Google Scholar] [CrossRef]
- Zabala, J.; Zuberogoitia, I.; Martínez-Climent, J.A. Spacing pattern, intersexual competition and niche segregation in American mink. Ann. Zool. Fenn. 2007, 44, 249–258. Available online: https://www.jstor.org/stable/23736769 (accessed on 1 October 2021).
- Zabala, J.; Zuberogoitia, I.; Martínez-Climent, J.A. Winter habitat preferences of feral American mink Mustela vison in Biscay, Northern Iberian Peninsula. Acta Theriol. 2007, 52, 27–36. [Google Scholar] [CrossRef]
- Harrington, L.A.; Harrington, A.L.; Moorhouse, T.; Gelling, M.; Bonesi, L.; Macdonald, D.W. American mink control on inland rivers in southern England: An experimental test of a model strategy. Biol. Conserv. 2009, 142, 839–849. [Google Scholar] [CrossRef]
- Zabala, J.; Zuberogoitia, I.; González-Oreja, J.A. Estimating costs and outcomes of invasive American mink (Neovison vison) management in continental areas: A framework for evidence based control and eradication. Biol. Invasions 2010, 12, 2999–3012. [Google Scholar] [CrossRef]
- Bryce, R.; Oliver, M.K.; Davies, L.; Gray, H.; Urquhart, J.; Lambin, X. Turning back the tide of American mink invasion at an unprecedented scale through community participation and adaptive management. Biol. Conserv. 2011, 144, 575–583. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Richardson, S.M.; Rodgers BJ, E.; Rodgers OR, K. Effective control of non-native American mink by strategic trapping in a river catchment in mainland Britain. J. Wildl. Manag. 2013, 77, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.S.; Chauvenet AL, M.; Robertson, P.A. Removal of American mink (Neovison vison) from the Uists, Outer Hebrides, Scotland. Biol. Invasions 2015, 17, 2811–2820. [Google Scholar] [CrossRef] [Green Version]
- Macleod, I.A.; Maclennan, D.; Raynor, R.; Thompson, D.B.A.; Whitaker, S. Large scale eradication of non-native invasive American mink (Neovison vison) from the outer Hebrides of Scotland. In Island Invasives: Scaling Up to Meet the Challenge; Veitch, C.R., Clout, M.N., Martin, A.R., Russell, J.C., West, C.J., Eds.; Occasional Paper SSC No. 62; IUCN: Gland, Switzerland, 2019; pp. 261–266. [Google Scholar]
- Martin, A.R.; Lea, V.J. A mink-free GB: Perspectives on eradicating American mink Neovison vison from Great Britain and its islands. Mammal Rev. 2020, 50, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Iriarte, J.A.; Lobos, G.A.; Jaksic, F.M. Invasive vertebrate species in Chile and their control and monitoring by governmental agencies. Rev. Chil. De Hist. Nat. 2005, 78, 143–154. [Google Scholar] [CrossRef]
- Schüttler, E.; Ibarra, J.T.; Gruber, B.; Rozzi, R.; Jax, K. Abundance and habitat preferences of the southernmost population of mink: Implications for managing a recent island invasion. Biodivers. Conserv. 2010, 19, 725–743. [Google Scholar] [CrossRef]
- Fasola, L.; Muzio, J.; Chehébar, C.; Cassini, M.; Macdonald, D.W. Range expansion and prey use of American mink in Argentinean Patagonia: Dilemmas for conservation. Eur. J. Wildl. Res. 2011, 57, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, A.E.J.; Anderson, C.B.; Fasola, L.; Cabello, J.L. Linking invasive exotic vertebrates and their ecosystem impacts in Tierra del Fuego to test theory and determine action. Acta Oecologica 2014, 54, 110–118. [Google Scholar] [CrossRef]
- Rodriguez, V.; Poo-Muñoz, D.A.; Escobar, L.E.; Astorga, F.; Medina-Vogel, G. Carnivore-livestock conflicts in Chile: Evidence and methods for mitigation. Hum.-Wildl. Interact. 2019, 13, 50–62. [Google Scholar] [CrossRef]
- Parkes, J.P.; Panetta, F.D. Eradication of invasive species: Progress and emerging issues in the 21st century. In Invasive Species Management: A Handbook of Principles and Techniques; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Medina-Vogel, G.; Barros, M.; Monsalve, R.; Pons, D.J. Assessment of the efficiency in trapping North American mink (Neovison vison) for population control in Patagonia. Rev. Chil. De Hist. Nat. 2015, 88, 9. [Google Scholar] [CrossRef] [Green Version]
- Zalewski, A.; Piertney, S.B.; Zalewska, H.; Lambin, X. Landscape barriers reduce gene flow in an invasive carnivore: Geographical and local genetic structure of American mink in Scotland. Mol. Ecol. 2009, 18, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Zuberogoitia, I.; González-Oreja, J.A.; Zabala, J.; Rodríguez-Refojos, C. Assessing the control/eradication of an invasive species, the American mink, based on field data; how much would it cost? Biodivers. Conserv. 2010, 19, 1455–1469. [Google Scholar] [CrossRef]
- Robertson, P.A.; Adriaens, T.; Lambin, X.; Mill, A.; Roy, S.; Shuttleworth, C.M.; Sutton-Croft, M. The large-scale removal of mammalian invasive alien species in Northern Europe. Pest Manag. Sci. 2017, 73, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Sandell, M. The mating tactics and spacing patterns of solitary carnivores. In Carnivore Behavior, Ecology, and Evolution; Gittleman, J.L., Ed.; Cornell University Press: Ithaca, NY, USA, 1989; pp. 164–182. [Google Scholar] [CrossRef]
- Gerell, R. Home ranges and movements of the mink Mustela vison Shreber in Southern Sweden. Oikos 1970, 21, 160. [Google Scholar] [CrossRef]
- Melero, Y.; Palazón, S.; Revilla, E.; Martelo, J.; Gosàlbez, J. Space use and habitat preferences of the invasive American mink (Mustela vison) in a Mediterranean area. Eur. J. Wildl. Res. 2008, 54, 609–617. [Google Scholar] [CrossRef]
- Halbrook, R.S.; Petach, M. Estimated mink home ranges using various home-range estimators. Wildl. Soc. Bull. 2018, 42, 656–666. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Rushton, S.; Macdonald, D.W. Habitat preferences of feral American mink in the Upper Thames. J. Mammal. 2003, 84, 1356–1373. [Google Scholar] [CrossRef] [Green Version]
- Harrington, L.A.; Macdonald, D.W. Spatial and temporal relationships between invasive American mink and native European polecats in the southern United Kingdom. J. Mammal. 2008, 89, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Zschille, J.; Stier, N.; Roth, M.; Berger, U. Dynamics in space use of American mink (Neovison vison) in a fishpond area in Northern Germany. Eur. J. Wildl. Res. 2012, 58, 955–968. [Google Scholar] [CrossRef]
- Zuberogoitia, I.; Zabala, J.; Martínez, J.A. Evaluation of sign surveys and trappability of American mink: Management consequences. Folia Zool. 2006, 55, 257–263. [Google Scholar]
- Ireland, M.C. The Behaviour and Ecology of the American Mink Mustela vison (Schreber) in a Coastal Habitat. Ph.D. Thesis, University of Durham, Durham, UK, 1990. [Google Scholar]
- Smal, C.M. Population studies on feral American mink Mustela vison in Ireland. J. Zool. 1991, 224, 233–249. [Google Scholar] [CrossRef]
- Bonesi, L. Spatial Organisation and Feeding Ecology of the American Mink (Mustela vison) in a Coastal Habitat. Master’s Thesis, University of Durham, Durham, UK, 1996. [Google Scholar]
- Niemimaa, J. Activity patterns and home ranges of the American mink Mustela vison in the Finnish outer archipelago. Ann. Zool. Fenn. 1995, 32, 117–121. Available online: https://www.jstor.org/stable/23735570 (accessed on 1 October 2021).
- Buskirk, S.W.; Lindstedt, S.L. Sex biases in trapped samples of Mustelidae. J. Mammal. 1989, 70, 88–97. [Google Scholar] [CrossRef]
- Craik, J.C.A. Sex ratio in catches of American mink—How to catch the females. J. Nat. Conserv. 2008, 16, 56–60. [Google Scholar] [CrossRef]
- Davis, E.F.; Anderson, C.B.; Valenzuela AE, J.; Cabello, J.L.; Soto, N. American mink (Neovison vison) trapping in the Cape Horn Biosphere Reserve: Enhancing current trap systems to control an invasive predator. Ann. Zool. Fenn. 2012, 49, 18–22. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Short, M.J.; Leigh, R.J. Development of population control strategies for mink Mustela vison, using floating rafts as monitors and trap sites. Biol. Conserv. 2004, 120, 533–543. [Google Scholar] [CrossRef]
- Moore, N.P.; Roy, S.S.; Helyar, A. Mink (Mustela vison) eradication to protect ground-nesting birds in the western isles, Scotland, United Kingdom. N. Z. J. Zool. 2003, 30, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Haan, D.M.; Halbrook, R.S. Resting-ite selection of American minks in east-central New York. Northeast. Nat. 2014, 21, 357–368. [Google Scholar] [CrossRef]
- Santulli, G.; Palazón, S.; Melero, Y.; Gosálbez, J.; Lambin, X. Multi-season occupancy analysis reveals large scale competitive exclusion of the critically endangered European mink by the invasive non-native American mink in Spain. Biol. Conserv. 2014, 176, 21–29. [Google Scholar] [CrossRef]
- Melero, Y.; Santulli, G.; Gómez, A.; Gosàlbez, J.; Rodriguez-Refojos, C.; Palazón, S. Morphological variation of introduced species: The case of American mink (Neovison vison) in Spain. Mamm. Biol. 2012, 77, 345–350. [Google Scholar] [CrossRef]
- Zalewski, A.; Bartoszewicz, M. Phenotypic variation of an alien species in a new environment: The body size and diet of American mink over time and at local and continental scales. Biol. J. Linn. Soc. 2012, 105, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Medina-Vogel, G. Mink trapping data from Fondecyt projects 1171417, 2018–2020. Unpublished data.
- Thom, M.D.; Harrington, L.A.; Macdonald, D.W. Why are American mink sexually dimorphic? A role for niche separation. Oikos 2004, 105, 525–535. [Google Scholar] [CrossRef]
- Schlexer, F.V. Attracting animals to detection devices. In Noninvasive Survey Methods for Carnivores; MacKay, P., Zielinski, W.J., Long, R.A., Ray, J.C., Eds.; Island Press: Washington, DC, USA, 2008; pp. 263–292. [Google Scholar]
- Nordström, M. Introduced predator in the Baltic Sea: Variable effects of feral mink removal on bird and small mammal populations in the outer archipelago. Ann. Univ. Turku. 2003, AII, 158. [Google Scholar]
- Clapperton, B.K.; Minot, E.O.; Crump, D.R. Scent lures from anal sac secretions of the ferret Mustela furo L. J. Chem. Ecol. 1989, 15, 291–308. [Google Scholar] [CrossRef]
- Clapperton, B.K.; Phillipson, S.M.; Woolhouse, A.D. Field trials of slow-release synthetic lures for stoats (Mustela erminea) and ferrets (M. furo). N. Z. J. Zool. 1994, 21, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Clapperton, B.K.; McLennan, J.A.; Woolhouse, A.D. Responses of stoats to scent lures in tracking tunnels. N. Z. J. Zool. 1999, 26, 175–178. [Google Scholar] [CrossRef]
- Spurr, E.B.; O’Connor, C.E.; Morse, C.W.; Morriss, G.A.; Arnold, G.C.; Ragg, J.R.; Hamilton, W.J.; Moller, H.; Woolhouse, A.D.; Clapperton, B.K. Effect of concentration of anal gland scent lures on the capture rate of ferrets (Mustela furo) in winter and spring. N. Z. J. Zool. 2004, 31, 227–232. [Google Scholar] [CrossRef]
- Roy, S.S.; Macleod, I.; Moore, N.P. The use of scent glands to improve the efficiency of mink (Mustela vison) captures in the Outer Hebrides. N. Z. J. Zool. 2006, 33, 267–271. [Google Scholar] [CrossRef]
- Murphy, E.C.; Sjoberg, T.; Agnew, T.; Sutherland, M.; Andrews, G.; Williams, R.; Williams, J.; Ross, J.; Clapperton, B.K. In Prep. Body odours as lures for stoats Mustela erminea: Captive and field trials. Animals, Unpublished work.
- Harrington, L.A.; Harrington, A.L.; MacDonald, D.W. The smell of new competitors: The response of American mink, Mustela vison, to the odours of otter, Lutra lutra and polecat, M. putorius. Ethology 2009, 115, 421–428. [Google Scholar] [CrossRef]
- Clapperton, B.K.; Minot, E.O.; Crump, D.R. An olfactory recognition system in the ferret Mustela furo L. (Carnivora: Mustelidae). Anim. Behav. 1988, 36, 541–553. [Google Scholar] [CrossRef]
- Available online: http://www.sag.gog.cl/noticias/autoridades-presentaron-fndr-de-control-comunitario-del-vison-en-los-rios (accessed on 11 November 2021).
- Fraser, E.J.; Macdonald, D.W.; Oliver, M.K.; Piertney, S.; Lambin, X. Using population genetic structure of an invasive mammal to target control efforts—An example of the American mink in Scotland. Biol. Conserv. 2013, 167, 35–42. [Google Scholar] [CrossRef]
- Barros, M.; Cabezón, O.; Dubey, J.P.; Almería, S.; Ribas, M.P.; Escobar, L.E.; Ramos, B.; Medina-Vogel, G. Toxoplasma gondii infection in wild mustelids and cats across an urban-rural gradient. PLoS ONE 2018, 13, e0199085. [Google Scholar] [CrossRef]
- Veblen, T.; Schlegel, F.M. Reseña ecologica de los bosques del sur de Chile. Bosque 1982, 4, 73–115. [Google Scholar] [CrossRef]
- Toledo, X.O.; Zapater, E.A. Geografía general y regional de Chile. Rev. De Geogr. Norte Gd. 1991, 18, 87–88. [Google Scholar]
- Roy, S. Strategies to improve landscape scale management of mink populations in the west coast of Scotland: Lessons learned from the Uists 2001–2006. In Island Invasives: Eradication and Management; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 114–117. [Google Scholar]
- Proulx, G.; Cattet MR, L.; Powell, R.A. Humane and efficient capture and handling methods for carnivores. In Carnivore Ecology and Conservation; Boitani, L., Powell, R.A., Eds.; Oxford University Press: London, UK, 2013; pp. 70–129. [Google Scholar] [CrossRef]
- Theuerkauf, J.; Rouys, S.; Jourdan, H.; Gula, R. Efficiency of a new reverse-bait trigger snap trap for invasive rats and a new new standardised abundance index. Ann. Zool. Fenn. 2011, 48, 308–318. [Google Scholar] [CrossRef]
- Magnusdottir, R.; von Schmalensee, M.; Stefansson, R.A.; Macdonald, D.W.; Hersteinsson, P. A foe in woe: American mink (Neovison vison) diet changes during a population decrease. Mamm. Biol. 2014, 79, 58–63. [Google Scholar] [CrossRef]
- Ahlers, A.A.; Heske, E.J.; Schooley, R.L. Prey distribution, potential landscape supplementation, and urbanization affect occupancy dynamics of American mink in streams. Landsc. Ecol. 2016, 31, 1601–1613. [Google Scholar] [CrossRef]
- Melero, Y.; Palazon, S.; Revilla, E.; Gosalbez, J. Winter activity patterns in an invading Mediterranean population of American mink (Neovison vison). Folia Zool. 2011, 60, 47–53. [Google Scholar] [CrossRef]
- Bonesi, L.; Rushton, S.P.; Macdonald, D.W. Trapping for mink control and water vole survival: Identifying key criteria using a spatially explicit individual based model. Biol. Conserv. 2007, 136, 636–650. [Google Scholar] [CrossRef]
- Cloe, A.L.; Woodley, S.K.; Waters, P.; Zhou, H.; Baum, M.J. Contribution of anal scent gland and urinary odorants to mate recognition in the ferret. Physiol. Behav. 2004, 82, 871–875. [Google Scholar] [CrossRef]
- Berzins, R.; Helder, R. Olfactory communication and the importance of different odour sources in the ferret (Mustela putorius f. furo). Mamm. Biol. 2008, 73, 379–387. [Google Scholar] [CrossRef]
- King, C.M.; McDonald, R.M.; Martin, R.D.; Dennis, T. Why is eradication of invasive mustelids so difficult? Biol. Conserv. 2009, 142, 806–816. [Google Scholar] [CrossRef]
- Roy, S.; Robertson, P.A. Matching the strategy to the scenario; case studies of mink Neovison management. Mamm. Study 2017, 42, 71–80. [Google Scholar] [CrossRef]



| River Basin | No. Transects per Study Site | Habitat | Season | Trap Type | Transect Length (km) | Trapped Mink | Days | TN A | Mink/TN | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 | 13 | Male | Female | Total | |||||||||
| Toltén | 1 | Stream | Autumn | X | 4.0 | 0 | 0 | 0 | 5 | 71 | 0 | ||
| 3 | River | Autumn | X | 4.4 | 5 | 1 | 6 | 19 | 300 | 0.020 | |||
| 3 | River | Winter | X | 6.0 | 1 | 0 | 1 | 17 | 329 | 0.003 | |||
| 3 | River | Spring | X | X | 2.0 | 2 | 0 | 2 | 18 | 174 | 0.011 | ||
| 5 | River | Summer | X | 2.2 | 2 | 3 | 5 | 20 | 188 | 0.027 | |||
| Valdivia | 1 | River | Autumn | X | 7.4 | 7 | 1 | 8 | 8 | 241 | 0.033 | ||
| 4 | Stream | Winter | X | 6.8 | 5 | 1 | 6 | 21 | 590 | 0.010 | |||
| 4 | River | Winter | X | 6.0 | 11 | 3 | 14 | 26 | 498 | 0.028 | |||
| 2 | Lake | Winter | X | 6.8 | 1 | 0 | 1 | 11 | 328 | 0.003 | |||
| 5 | River | Spring | X | 8.4 | 11 | 4 | 15 | 30 | 722 | 0.021 | |||
| 9 | River | Summer | X | X | 6.8 | 21 | 8 | 29 | 56 | 865 | 0.034 | ||
| 2 | Lake | Summer | X | 6.8 | 1 | 2 | 3 | 16 | 435 | 0.007 | |||
| 1 | Stream | Summer | X | 3.0 | 4 | 5 | 9 | 6 | 90 | 0.100 | |||
| 2 | River | Summer | X | 3.4 | 6 | 2 | 8 | 14 | 227 | 0.035 | |||
| Bueno | 2 | River | Winter | X | 4.0 | 3 | 0 | 3 | 12 | 204 | 0.015 | ||
| 2 | River | Spring | X | 4.0 | 0 | 0 | 0 | 10 | 152 | 0 | |||
| 5 | River | Summer | X | X | 5.0 | 29 | 26 | 55 | 27 | 487 | 0.113 | ||
| Maullín | 3 | River | Summer | X | 2.4 | 10 | 10 | 20 | 9 | 80 | 0.250 | ||
| 2 | River | Summer | X | 3.0 | 6 | 5 | 11 | 12 | 180 | 0.061 | |||
| Total | 59 | 125 | 71 | 196 | 337 | 6161 | |||||||
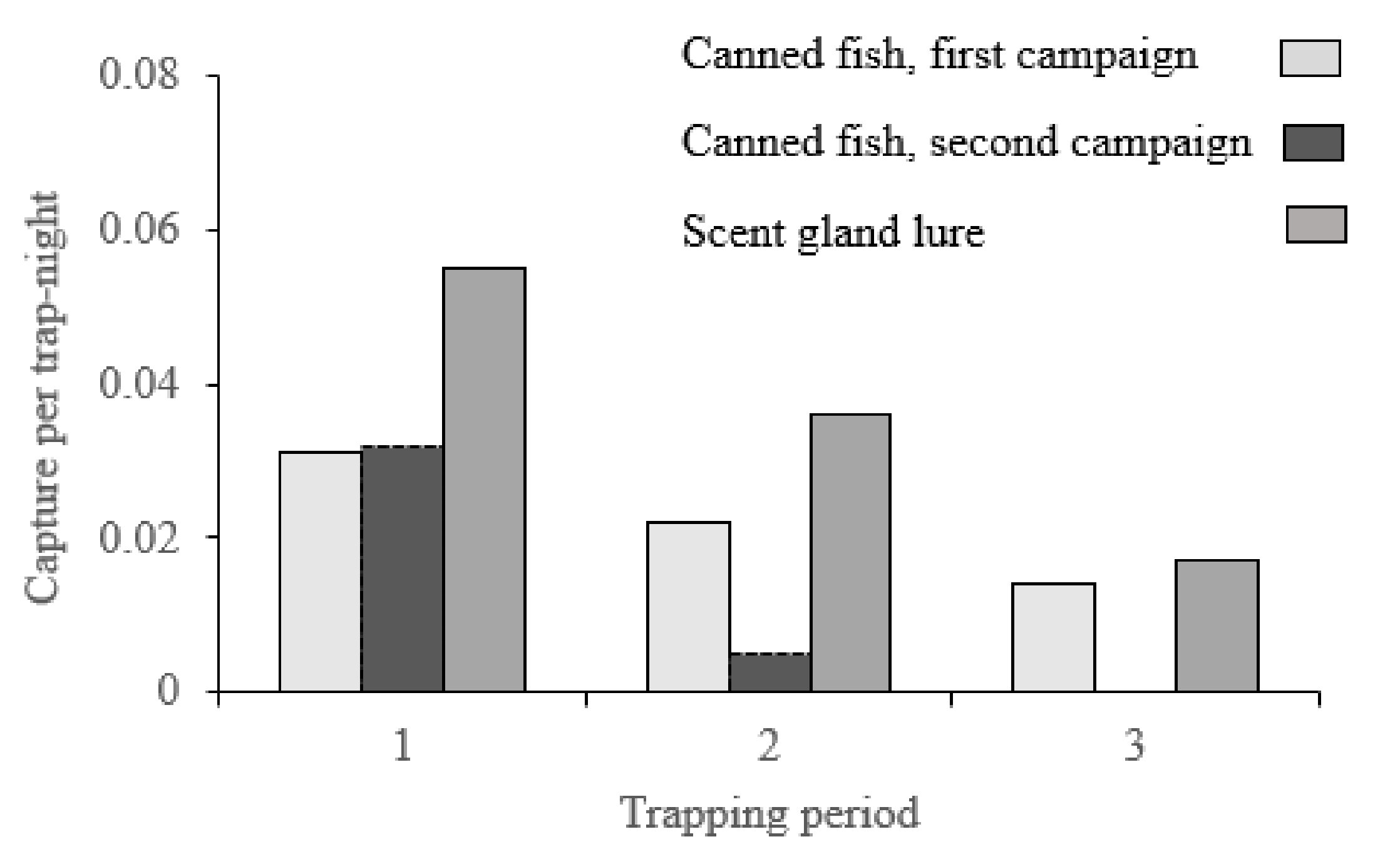
| Year of Campaign | Bait Comparison | Mean | Captures per Trap-Night | No. 3-Day Trapping Periods | |||
|---|---|---|---|---|---|---|---|
| Tukey’s Post Hoc Test p-Value | 95% Confidence Interval | ||||||
| Lower | Upper | ||||||
| 2009–2013 | Canned fish | Canned fish | 0.01 | 0.99 | −0.03 | 0.02 | 121 |
| 2018–2021 | Canned fish | Scent Lure | 0.01 | 0.00 | −0.05 | −0.02 | 26 |
| 2018–2021 | Canned fish | Scent Lure | 0.04 | 0.02 | −0.06 | −0.01 | 103 |
| Mink Trapped | Capture per Trap-Night | Tukey’s Post Hoc Test p-Value | 95% Confidence Interval | No. 3- Day Trapping Periods | ||
|---|---|---|---|---|---|---|
| Gender | Scent lure | Mean | Lower | Upper | ||
| Female | Female | 0.03 | 16 | |||
| Male | 0.07 | 0.16 | −0.09 | 0.02 | 10 | |
| Male | Female | 0.05 | 0.10 | −0.04 | 0.04 | 11 |
| Male | 0.03 | 12 | ||||
| Trap Variant A | GMV-18 | GMV-13 | |
|---|---|---|---|
| Trap-nights | 1632 | 3043 | 1486 |
| Bait type Mammal species | Canned Fish | Scent Lure | Scent Lure |
| Mink (Neovison vison) | 14 (0.0086) | 118 (0.0387 | 64 (0.0431) |
| Rat (Rattus norvegicus) | 1 (0.0006) | 25 (0.0082) | 1 (0.0007) |
| Cat (Felis silvestris catus) | 12 (0.0074) | 3 (0.0010) | 0 |
| Small dog (Canis lupus familiaris) | 1 (0.0006) | 0 | 0 |
| Skunk (Conepatus chinga) | 0 | 1 (0.0003) | 0 |
| Hare (Lepus europaeus) | 0 | 3 (0.0010) | 0 |
| Transects | Theoretical Population A | Proportion of Trapped Mink Related to Theoretical Population | Estimated Population Size | Proportion of N(1) B from Theoretical Population | Trap Efficiency C | Number of Trap-Nights Needed to Remove a % of the Estimated Discrete Mink Population | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | N(1) | k × 1000 | 70 | 80 | 90 | ||
| Nueva Tolten | 1.3 | 1.9 | 3.2 | 2.4 | 0.5 | 1.3 | 4.0 | 1.3 | 52.1 | 23.1 | 30.9 | 44.2 |
| Liquiñe | 2.3 | 3.4 | 5.6 | 0.9 | 0 | 0.4 | 2.1 | 0.4 | 19 | 63.4 | 84.7 | 121.2 |
| Liquiñe | 2.0 | 3 | 5.0 | 2.5 | 0 | 1.0 | 9.7 | 1.9 | 4.64 | 259.5 | 346.9 | 496.2 |
| Cua Cua | 1.2 | 1.8 | 3.0 | 2.5 | 0 | 1.0 | 3.1 | 1.0 | 67.6 | 17.8 | 23.8 | 34.1 |
| Liquiñe alto | 0.4 | 0.6 | 1.0 | 5.0 | 0 | 2.0 | 2.6 | 2.6 | 58.8 | 20.5 | 27.4 | 39.2 |
| Liquiñe alto | 0.6 | 0.9 | 1.5 | 3.3 | 0 | 1.3 | 2.1 | 1.4 | 72.4 | 16.6 | 22.2 | 31.8 |
| Santa Rita | 2.5 | 3.7 | 6.1 | 2.8 | 0.3 | 1.3 | 15.6 | 2.5 | 2.8 | 424.3 | 567.2 | 811.5 |
| Antilhue | 1.6 | 2.4 | 4.0 | 1.3 | 1.3 | 1.3 | 5.4 | 1.3 | 16.2 | 74.3 | 99.3 | 142.1 |
| Antilhue | 0.9 | 1.4 | 2.3 | 2.1 | 0.7 | 1.3 | 3.2 | 1.4 | 41.9 | 28.7 | 38.4 | 55.0 |
| Antilhue | 2.8 | 4.2 | 7.0 | 1.1 | 0 | 0.4 | 3.1 | 0.4 | 13.9 | 86.6 | 115.8 | 165.7 |
| Valdivia | 1.2 | 1.8 | 3.0 | 0.8 | 0.6 | 0.7 | 2.1 | 0.7 | 41.2 | 29.2 | 39.1 | 55.9 |
| Valdivia | 1.1 | 1.6 | 2.7 | 2.8 | 1.9 | 2.3 | 6.0 | 2.3 | 28.5 | 42.2 | 56.5 | 80.8 |
| Mantilhue | 1.5 | 2.2 | 3.7 | 3.4 | 0 | 1.4 | 5.3 | 1.4 | 61.9 | 19.5 | 26.0 | 37.2 |
| Pilmaiquén | 1.3 | 2 | 3.3 | 5.3 | 6 | 5.7 | 27.3 | 8.2 | 14.3 | 84.2 | 112.5 | 161.0 |
| Santa Rita | 1.3 | 2 | 3.3 | 0 | 1.5 | 0.9 | 4.2 | 1.3 | 12.11 | 99.4 | 132.9 | 190.1 |
| Valdivia | 0.4 | 0.6 | 1.0 | 5.0 | 0 | 2.0 | 2.8 | 2.8 | 62.5 | 19.3 | 25.8 | 36.8 |
| Valdivia | 1.3 | 1.9 | 3.2 | 7.1 | 2.1 | 4.1 | 20.1 | 6.4 | 9.9 | 121.9 | 162.9 | 233.1 |
| San Pablo | 1.5 | 2.2 | 3.7 | 2.0 | 0 | 0.8 | 3.3 | 0.9 | 35.1 | 34.3 | 45.9 | 65.6 |
| Trumao | 1.2 | 1.8 | 3.0 | 5.8 | 1.7 | 3.3 | 13.9 | 4.6 | 14.7 | 81.9 | 109.5 | 156.6 |
| Antilhue | 1 | 1.5 | 2.5 | 4 | 3.3 | 3.6 | 12.5 | 5.0 | 51.4 | 23.4 | 31.3 | 44.8 |
| Santa Rita | 1.1 | 1.7 | 2.8 | 5.3 | 0 | 2.1 | 14.4 | 5.1 | 3.5 | 344.4 | 460.4 | 658.6 |
| Maullin | 1 | 1.5 | 2.5 | 5 | 3.3 | 4.0 | 15.4 | 6.2 | 9.55 | 126.1 | 168.5 | 241.1 |
| Average | 1.3 | 2.0 | 3.3 | 3.2 | 1.1 | 1.9 | 8.1 | 2.7 | 31.5 | 92.8 | 124.0 | 177.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Vogel, G.; Muñoz, F.; Moeggenberg, M.; Calvo-Mac, C.; Barros-Lama, M.; Ulloa, N.; Pons, D.J.; Clapperton, B.K. Improving Trapping Efficiency for Control of American Mink (Neovison vison) in Patagonia. Animals 2022, 12, 142. https://doi.org/10.3390/ani12020142
Medina-Vogel G, Muñoz F, Moeggenberg M, Calvo-Mac C, Barros-Lama M, Ulloa N, Pons DJ, Clapperton BK. Improving Trapping Efficiency for Control of American Mink (Neovison vison) in Patagonia. Animals. 2022; 12(2):142. https://doi.org/10.3390/ani12020142
Chicago/Turabian StyleMedina-Vogel, Gonzalo, Francisco Muñoz, Meredith Moeggenberg, Carlos Calvo-Mac, Macarena Barros-Lama, Nickolas Ulloa, Daniel J. Pons, and B. Kay Clapperton. 2022. "Improving Trapping Efficiency for Control of American Mink (Neovison vison) in Patagonia" Animals 12, no. 2: 142. https://doi.org/10.3390/ani12020142






