Gastrointestinal Dynamics of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages in Broiler Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. BP Origin and encapsulation
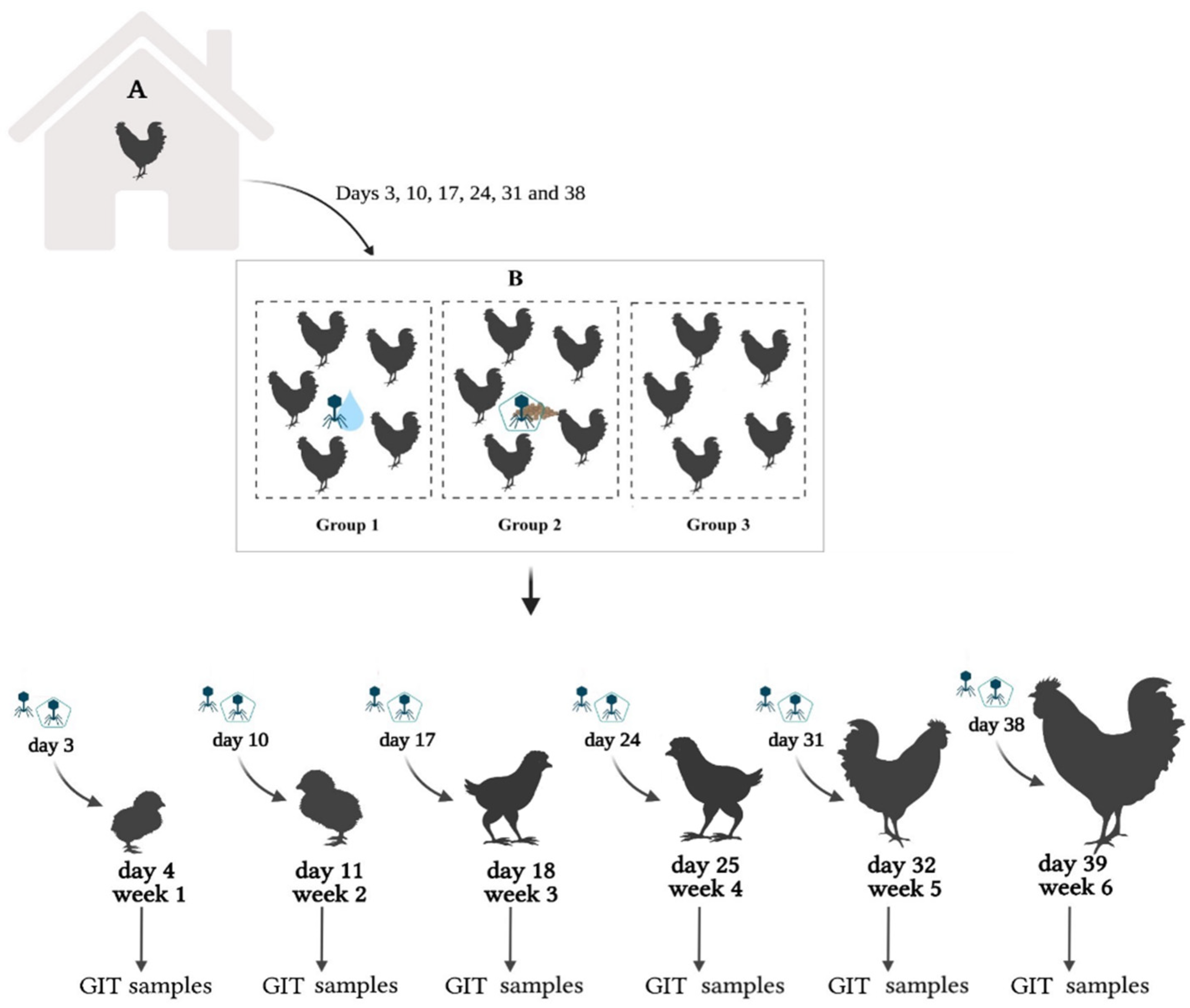
2.2. Experimental design
2.3. Processing of GIT samples
2.4. Statistical Analysis
3. Results
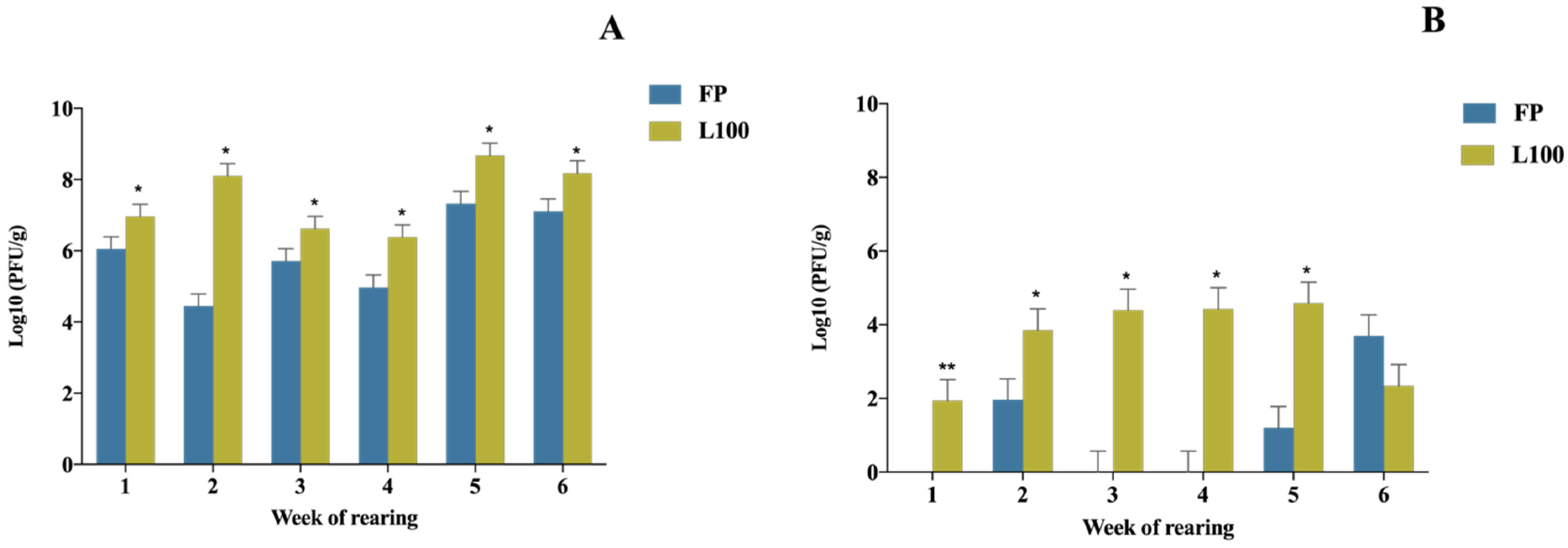
3.1. BP Gastrointestinal Dynamics in Chickens According to the Week and Form of BP Application
BP Concentration in the First Section of the GIT (Crop and PV-Gizzard)
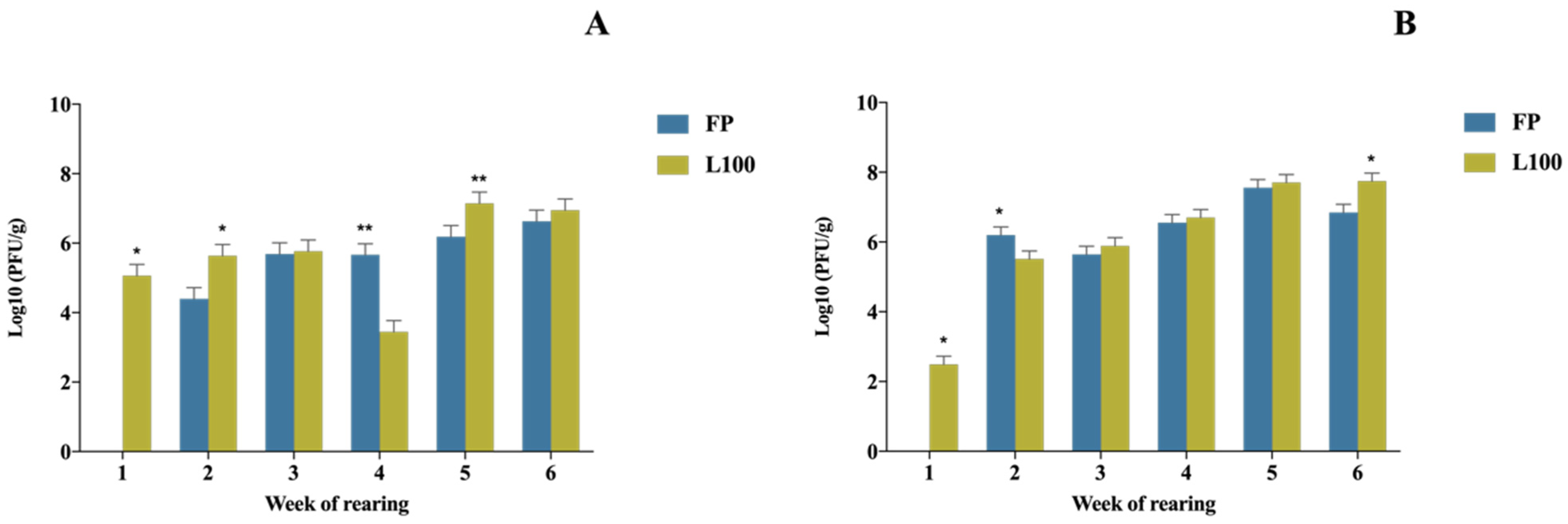
3.2. BP Concentration in the Gut and Ceca
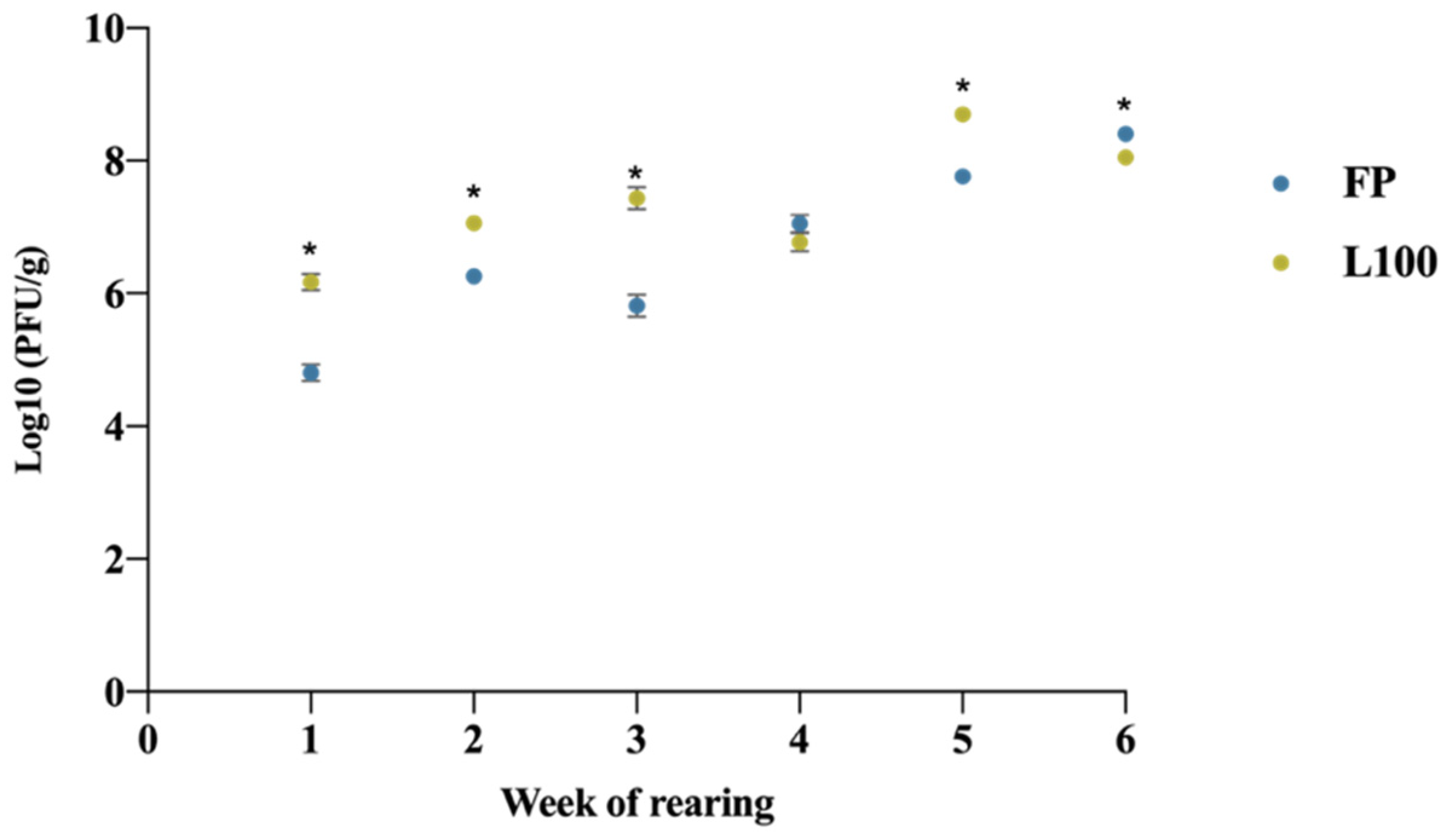
3.3. Fecal BP Excretion Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). WHO Estimates of the Global Burden of Foodborne Diseases; World Health Organization: Geneva, Switzerland, 2015; pp. 1–16. [Google Scholar]
- Dawoud, T.M.; Shi, Z.; Kwon, Y.M.; Ricke, S.C. Overview of Salmonellosis and Food-borne Salmonella: Historical and Current Perspectives. In Producing Safe Eggs Microbial Ecology of Salmonella, 2nd ed.; Ricke, S.C., Gast, R.K., Eds.; Eselvier: London, United Kingdom, 2017; Volume 1, pp. 113–138. [Google Scholar]
- European Parlament. Regulation (EC) No 2160/2003 of the European Parlament and of the Council of 17 November 2003 on the Control of Salmonella and Other Specified Food-Borne Zoonotic Agents; European Union: Maastricht, The Netherlands, 2003; Volume L325, pp. 1–15. [Google Scholar]
- Vandeplas, S.; Dubois Dauphin, R.; Beckers, Y.; Thonart, P.; Théwis, A. Salmonella in chicken: Current and developing strategies to reduce contamination at farm level. J. Food Prot. 2010, 73, 774–785. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Johny, A.K. Salmonella in Poultry Meat Production. In Food Safety in Poultry Meat Production; Venkitanarayanan, K., Thakur, S., Ricke, S.C., Eds.; Springer Nature: Gewerbestrasse, Switzerland, 2019; pp. 1–25. [Google Scholar]
- Marin, C.; Lainez, M. Salmonella detection in feces during broiler rearing and after live transport to the slaughterhouse. Poult. Sci. 2009, 88, 1999–2005. [Google Scholar] [CrossRef]
- Montoro-Dasi, L.; Villagra, A.; de Toro, M.; Pérez-Gracia, M.T.; Vega, S.; Marin, C. Fast and Slow-Growing Management Systems: Characterisation of Broiler Caecal Microbiota Development throughout the Growing Period. Animals 2020, 10, 1401. [Google Scholar] [CrossRef]
- Ahmadi, M.; Amir Karimi Torshizi, M.; Rahimi, S.; Dennehy, J.J. Prophylactic bacteriophage administration more effective than post-infection administration in reducing Salmonella enterica serovar enteritidis shedding in Quail. Front. Microbiol. 2016, 7, 1253. [Google Scholar] [CrossRef] [Green Version]
- Svircev, A.; Roach, D.; Castle, A. Framing the future with bacteriophages in agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The use of bacteriophages in the poultry industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Borie, C.; Albala, I.; Sànchez, P.; Sánchez, M.L.; Ramírez, S.; Navarro, C.; Morales, M.A.; Retamales, J.; Robeson, J. Bacteriophage Treatment Reduces Salmonella Colonization of Infected Chickens. Avian Dis. 2008, 52, 64–67. [Google Scholar] [CrossRef]
- Gigante, A.; Atterbury, R.J. Veterinary use of bacteriophage therapy in intensively-reared livestock. Virol. J. 2019, 16, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atterbury, R.J.; Van Bergen, M.A.P.; Ortiz, F.; Lovell, M.A.; Harris, J.A.; De Boer, A.; Wagenaar, J.A.; Allen, V.M.; Barrow, P.A. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007, 73, 4543–4549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018, 8, 1539056. [Google Scholar] [CrossRef]
- Sobhy, H.; Soliman, E.A.; El-Tawab, A.A.A.; Elhofy, F.I.; Askora, A.; El-Nahas, E.M.; Wareth, G.; Ahmed, W. Isolation, Characterization, and Efficacy of Three Lytic Phages Infecting Multidrug-Resistant Salmonella Serovars from Poultry Farms in Egypt. Arch. Razi Inst. 2021, 76, 507. [Google Scholar] [CrossRef]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef]
- Ryan, E.M.; Gorman, S.P.; Donnelly, R.F.; Gilmore, B.F. Recent advances in bacteriophage therapy: How delivery routes, formulation, concentration and timing influence the success of phage therapy. J. Pharm. Pharmacol. 2011, 63, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, A.; Cloastre, L.; Bernard, J.; Laveran, H.; Ackermann, H.W.; Licois, D.; Joly, B. Characteristics and diffusion in the rabbit of a phage for Escherichia coli 0103. Attempts to use this phage for therapy. Vet. Microbiol. 1992, 30, 203–212. [Google Scholar] [CrossRef]
- Stanford, K.; McAllister, T.A.; Niu, Y.D.; Stephens, T.P.; Mazzocco, A.; Waddell, T.E.; Johnson, R.P. Oral Delivery Systems for Encapsulated Bacteriophages Targeted Escherichia coli O157: H7 in Feedlot Cattle. J. Food Prot. 2010, 73, 1304–1312. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Aríñez-Soriano, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Microencapsulation with alginate/CaCO 3: A strategy for improved phage therapy. Sci. Rep. 2017, 7, 41441. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Rebenaque, L.; Malik, D.J.; Catalá-Gregori, P.; Marin, C.; Sevilla-Navarro, S. In Vitro and In Vivo Gastrointestinal Survival of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages: Implications for Bacteriophage Therapy in Poultry. Pharmaceuticals 2021, 14, 434. [Google Scholar] [CrossRef]
- Widjaja, M.; Gan, J.; Talpaneni, J.S.R.; Tjandrawinata, R.R. Determination of eudragit® L100 in an enteric-coated tablet formulation using size-exclusion chromatography with charged-aerosol detection. Sci. Pharm. 2018, 86, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministerio de la Presidencia. Royal Degree 53/2013, 1st of Febrary, por el que se Establecen las Normas Básicas Aplicables Para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia; Boletín Oficial del Estado: Madrid, Spain, 2013; pp. 11370–11421.
- Sevilla-Navarro, S.; Catalá-Gregori, P.; Marin, C. Salmonella Bacteriophage Diversity According to Most Prevalent Salmonella Serovars in Layer and Broiler Poultry Farms from Eastern Spain. Animals 2020, 10, 1456. [Google Scholar] [CrossRef]
- Ministerio de Agricultura Pesca y Alimentación (MAPA). Programa Nacional de Control de Determinados Serotipos de Salmonella en la Especie Gallus Gallus; Ministerio de Agricultura Pesca y Alimentación (MAPA): Madrid, Spain, 2020.
- Malik, D.J. Bacteriophage encapsulation using spray drying for phage therapy. Curr. Issues Mol. Biol. 2021, 40, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kislalioglu, M.S.; Khan, M.A.; Blount, C.; Goettsch, R.W.; Bolton, S. Physical characterization and dissolution properties of ibuprofen: Eudragit coprecipitates. J. Pharm. Sci. 1991, 80, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.; Malik, D.J. Microencapsulation of Bacteriophages Using Membrane Emulsification in Different pH-Triggered Controlled Release Formulations for Oral Administration. Pharm. 2021, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Martos, P. Log transformation and the effect on estimation, implication, and interpretation of mean and measurement uncertainty in microbial enumeration. J. AOAC Int. 2019, 102, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevilla-Navarro, S.; Marín, C.; Cortés, V.; García, C.; Vega, S.; Catalá-Gregori, P. Autophage as a control measure for Salmonella in laying hens. Poult. Sci. 2018, 97, 4367–4373. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage biocontrol applications in food production and processing. Curr. Issues Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596. [Google Scholar] [CrossRef]
- Groves, P.J.; Williamson, S.L.; Ahaduzzaman, M.; Diamond, M.; Ngo, M.; Han, A.; Sharpe, S.M. Can a combination of vaccination, probiotic and organic acid treatment in layer hens protect against early life exposure to Salmonella Typhimurium and challenge at sexual maturity? Vaccine 2021, 39, 815–824. [Google Scholar] [CrossRef]
- Hashemzadeh, Z.; Karimi Torshizi, M.A.; Rahimi, S.; Razban, V.; Salehi, T.Z. Prevention of Salmonella Colonization in Neonatal Broiler Chicks by Using Different Routes of Probiotic Administration in Hatchery Evaluated by Culture and PCR Techniques. J. Agric. Sci. Technol. 2010, 12, 425–432. [Google Scholar]
- Kempf, F.; Menanteau, P.; Rychlik, I.; Kubasová, T.; Trotereau, J.; Virlogeux-Payant, I.; Schaeffer, S.; Schouler, C.; Drumo, R.; Guitton, E.; et al. Gut microbiota composition before infection determines the Salmonella super and low shedder phenotypes in chicken. Microb. Biotechnol. 2020, 13, 1611. [Google Scholar] [CrossRef]
- Alali, W.; Hofacre, C.; Mathis, G.; Faltys, G. Effect of essential oil compound on shedding and colonization of Salmonella enterica serovar Heidelberg in broilers. Poult. Sci. 2013, 92, 836–841. [Google Scholar] [CrossRef]
- Arsi, K.; Donoghue, A.M.; Woo-Ming, A.; Blore, P.J.; Donoghue, D.J. Intracloacal inoculation, an effective screening method for determining the efficacy of probiotic bacterial isolates against Campylobacter colonization in broiler chickens. J. Food Prot. 2015, 78, 209–213. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2013, 5, 108–119. [Google Scholar] [CrossRef]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of Bacteriophage Felix O1 into Chitosan-Alginate Microspheres for Oral Delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.H.; Islam, G.S.; Wu, Y.; Sabour, P.M.; Chambers, J.R.; Wang, Q.; Wu, S.X.Y.; Griffiths, M.W. Temporal distribution of encapsulated bacteriophages during passage through the chick gastrointestinal tract. Poult. Sci. 2016, 95, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G.; Petrone, V.; Escorcia, M.; Morishita, T.; Cobb, C.; Villaseñor, L.; Promsopone, B. Evaluation of avian-specific probiotic and Salmonella enteritidis-, Salmonella typhimurium-, and Salmonella heidelberg-specific antibodies on cecal colonization and organ invasion of Salmonella enteritidis in broilers. J. Food Prot. 2001, 64, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, S.D.; Hosseinidoust, Z. Phage therapy with a focus on the human microbiota. Antibiotics 2019, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Borda-Molina, D.; Seifert, J.; Camarinha-Silva, A. Current Perspectives of the Chicken Gastrointestinal Tract and Its Microbiome. Comput. Struct. Biotechnol. J. 2018, 16, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jahantigh, M. Antimicrobial resistance to Citrobacter spp. and Salmonella spp. isolated from goose eggs. Comp. Clin. Pathol. 2013, 22, 1–4. [Google Scholar] [CrossRef]
- Amalaradjou, M.A. Pre-harvest Approaches to Improve Poultry Meat Safety. In Food Safety in Poultry Meat Production; Venkitanarayanan, K., Thakur, S., Ricke, S.C., Eds.; Springer: Cham, Switzerland, 2019; pp. 95–122. [Google Scholar]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, T.H.; Kim, M.S.; Lee, D.H.; Lee, Y.N.; Park, J.K.; Youn, H.N.; Lee, H.J.; Yang, S.Y.; Cho, Y.W.; Lee, J.B.; et al. Use of bacteriophage for biological control of Salmonella Enteritidis infection in chicken. Res. Vet. Sci. 2012, 93, 1173–1178. [Google Scholar] [CrossRef]
- Adhikari, P.A.; Cosby, D.E.; Cox, N.A.; Lee, J.H.; Kim, W.K. Effect of dietary bacteriophage supplementation on internal organs, fecal excretion, and ileal immune response in laying hens challenged by Salmonella Enteritidis. Poult. Sci. 2017, 96, 3264–3271. [Google Scholar] [CrossRef]
- Vaz, C.S.L.; Voss-Rech, D.; Alves, L.; Coldebella, A.; Brentano, L.; Trevisol, I.M. Effect of time of therapy with wild-type lytic bacteriophages on the reduction of Salmonella Enteritidis in broiler chickens. Vet. Microbiol. 2020, 240, 108527. [Google Scholar] [CrossRef]
- Toro, H.; Price, S.B.; McKee, S.; Hoerr, F.J.; Krehling, J.; Perdue, M.; Bauermeister, L. Use of Bacteriophages in Combination with Competitive Exclusion to Reduce Salmonella from Infected Chickens. Avian Dis. 2005, 49, 118–124. [Google Scholar] [CrossRef]
- Kim, K.H.; Ingale, S.L.; Kim, J.S.; Lee, S.H.; Lee, J.H.; Kwon, I.K.; Chae, B.J. Bacteriophage and probiotics both enhance the performance of growing pigs but bacteriophage are more effective. Anim. Feed Sci. Technol. 2014, 196, 88–95. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Rebenaque, L.; Malik, D.J.; Catalá-Gregori, P.; Marin, C.; Sevilla-Navarro, S. Gastrointestinal Dynamics of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages in Broiler Production. Animals 2022, 12, 144. https://doi.org/10.3390/ani12020144
Lorenzo-Rebenaque L, Malik DJ, Catalá-Gregori P, Marin C, Sevilla-Navarro S. Gastrointestinal Dynamics of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages in Broiler Production. Animals. 2022; 12(2):144. https://doi.org/10.3390/ani12020144
Chicago/Turabian StyleLorenzo-Rebenaque, Laura, Danish J. Malik, Pablo Catalá-Gregori, Clara Marin, and Sandra Sevilla-Navarro. 2022. "Gastrointestinal Dynamics of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages in Broiler Production" Animals 12, no. 2: 144. https://doi.org/10.3390/ani12020144
APA StyleLorenzo-Rebenaque, L., Malik, D. J., Catalá-Gregori, P., Marin, C., & Sevilla-Navarro, S. (2022). Gastrointestinal Dynamics of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages in Broiler Production. Animals, 12(2), 144. https://doi.org/10.3390/ani12020144






