Effect of Long-Acting Selenium Preparation on Health and Productivity of Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Nutrition
2.2. Hematological, Biochemical and Immunological Blood Analyses
2.3. Statistical Analysis
3. Results
3.1. Feed Intake
3.2. Hematological, Biochemical and Immunological Blood Parameters in Ewes
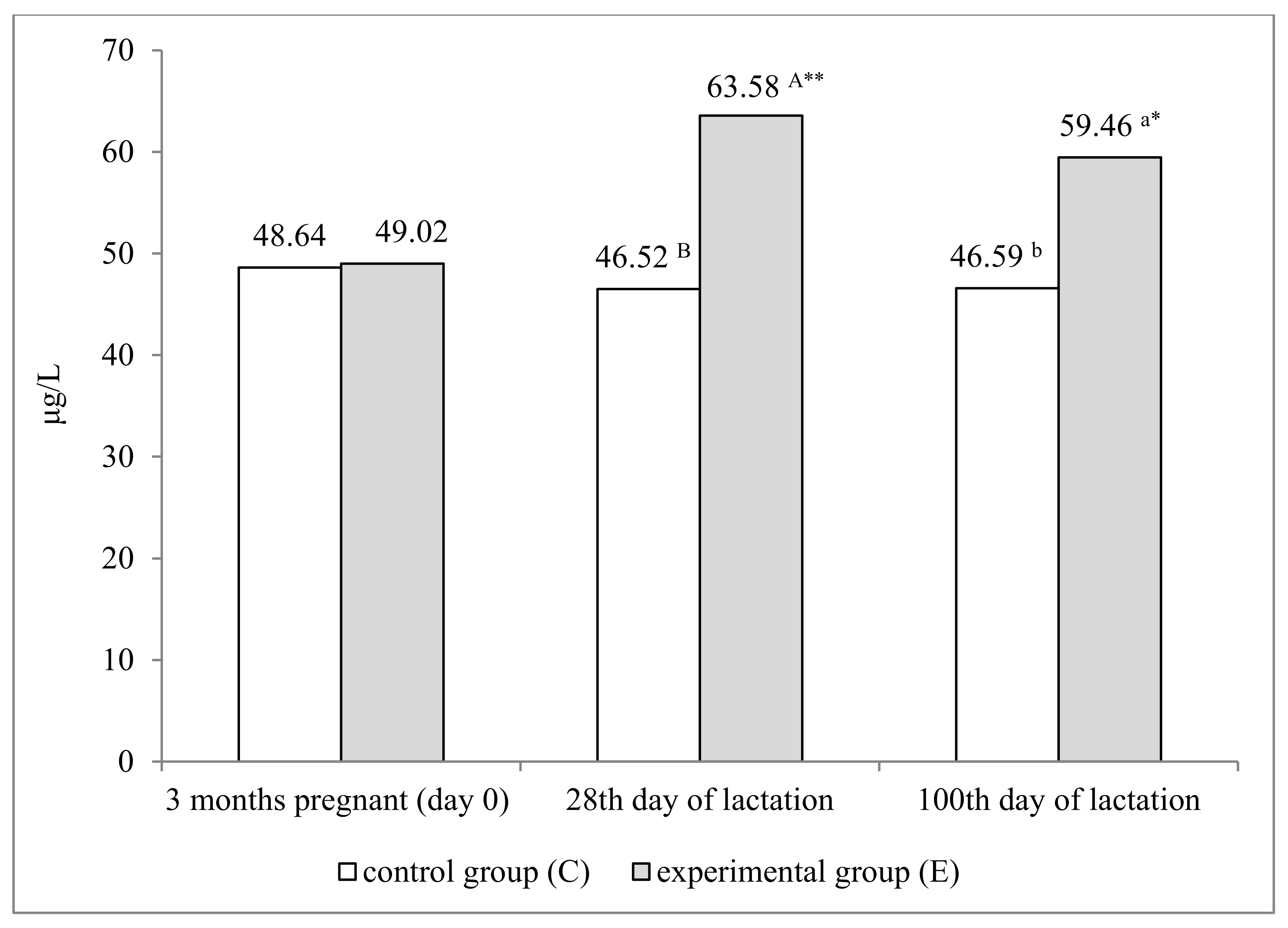
3.3. Blood Selenium Concentration in Ewes
3.4. The Growth Rate and Ultrasound Measurements of Lambs
4. Discussion
4.1. Hematological, Biochemical and Immunological Blood Parameters in Ewes
4.2. The Growth Rate and Ultrasound Measurements of Lambs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Čobanová, K.; Faix, Š.; Plachá, I.; Mihaliková, K.; Váradyová, Z.; Kišidayová, S.; Grešáková, L. Effects of Different Dietary Selenium Sources on Antioxidant Status and Blood Phagocytic Activity in Sheep. Biol. Trace Elem. Res. 2017, 175, 339–346. [Google Scholar] [CrossRef]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Faria, L.D.A.; Luz, P.H.D.C.; Abdalla, A.L. Selenium Fertilization in Tropical Pastures. In Importance of Selenium in the Environment and Human Health; IntechOpen: London, UK, 2020. [Google Scholar]
- Awual, R.; Yaita, T.; Suzuki, S.; Shiwaku, H. Ultimate selenium (IV) monitoring and removal from water using a new class of organic ligand based composite adsorbent. J. Hazard. Mater. 2015, 291, 111–119. [Google Scholar] [CrossRef]
- Galbraith, M.L.; Vorachek, W.R.; Estill, C.T.; Whanger, P.D.; Bobe, G.; Davis, T.Z.; Hall, J.A. Rumen Microorganisms Decrease Bioavailability of Inorganic Selenium Supplements. Biol. Trace Elem. Res. 2015, 171, 338–343. [Google Scholar] [CrossRef]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef]
- Żarczyńska, K.; Sobiech, P.; Snarska, A.; Babińska, I. Levels of selenium and vitamin E in the blood and morphological changes in the biceps femoris muscle during nutritional muscular dystrophy of calves. J. Elementol. 2019, 24, 1363–1370. [Google Scholar] [CrossRef]
- Sobolev, O.; Gutyj, B.; Petryshak, R.; Pivtorak, V.; Kovalskyi, Y.; Naumyuk, A.; Semchuk, I.; Mateusz, V.; Shcherbatyy, A.; Semeniv, B. Biological role of selenium in the organism of animals and humans. Ukr. J. Ecol. 2018, 8, 654–665. [Google Scholar] [CrossRef]
- Ceballos, A.; Kruze, J.; Barkema, H.; Dohoo, I.; Sánchez, J.; Uribe, D.; Wichtel, J.; Wittwer, F. Barium selenate supplementation and its effect on intramammary infection in pasture-based dairy cows. J. Dairy Sci. 2010, 93, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Annett, R.; Carson, A.; Fearon, A.; Kilpatrick, D. Effects of supplementation with fish oil and barium selenate on performance, carcass characteristics and muscle fatty acid composition of late season lamb finished on grass-based or concentrate-based diets. Animals 2011, 5, 1923–1937. [Google Scholar] [CrossRef]
- Brzostowski, H.; Milewski, S.; Tański, Z. Schlachtwert und Fleischqualität von Lämmern der Schafrasse Skudden. Arch. Anim. Breed. 2010, 53, 578–588. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005; pp. 210–219. [Google Scholar]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 1995; p. 518. [Google Scholar]
- Lie, R.F.; Schmitz, J.M.; Pierre, K.J.; Gochman, N. Cholesterol oxidase-based determination, by continuous-flow analysis, of total and free cholesterol in serum. Clin. Chem. 1976, 22, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.W.; Artiss, J.D.; Strandbergh, D.R.; Zak, B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 1983, 29, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Choi, Y.-K.; Im, H.-S.; Yarimaga, O.; Yoon, E.; Kim, H.-S. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry, 2nd ed.; WB Saunders: Philadelphia, PA, USA, 1994; pp. 816–818. [Google Scholar]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 1995; p. 286. [Google Scholar]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th ed.; WB Saunders: St. Louis, MO, USA, 2006; p. 2290. [Google Scholar]
- Siwicki, A.K.; Anderson, D.P. Immunostimulation in fish: Measuring the effects of stimulants by serological and immunological methods. US Fish Wildl. Serv. IFI Olszt. 1993, 1, 17. [Google Scholar]
- Siwicki, A.K.; Studnicka, M. Ceruloplasmin activity in carp (Cyprinus carpio). Isr. J. Aquacult. Bamidgeh. 1986, 38, 126–129. [Google Scholar]
- Chung, S.; Secombes, C. Analysis of events occurring within teleost macrophages during the respiratory burst. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 89, 539–544. [Google Scholar] [CrossRef]
- Rook, G.; Steele, J.; Umar, S.; Dockrell, H. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by γ-interferon. J. Immunol. Methods 1985, 82, 161–167. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Junkuszew, A.; Ringdorfer, F. Computer tomography and ultrasound measurement as methods for the prediction of the body composition of lambs. Small Rumin. Res. 2005, 56, 121–125. [Google Scholar] [CrossRef]
- Shi, L.; Ren, Y.; Zhang, C.; Yue, W.; Lei, F. Effects of organic selenium (Se-enriched yeast) supplementation in gestation diet on antioxidant status, hormone profile and haemato-biochemical parameters in Taihang Black Goats. Anim. Feed Sci. Technol. 2018, 238, 57–65. [Google Scholar] [CrossRef]
- Pisek, L.; Trávniček, J.; Salat, J.; Kroupová, V.; Soch, M. Changes in white blood cells in sheep blood during selenium supplementation. Vet. Med. 2008, 53, 255–259. [Google Scholar] [CrossRef]
- Soliman, E.B.; Aki, A.E.M.; Kassab, A.Y. Combined effect of vitamin E and selenium on some productive and physiological characteristics of ewes and their lambs during suckling period. Egypt. J. Sheep Goat Sci. 2012, 7, 31–42. [Google Scholar]
- Milewski, S.; Sobiech, P.; Błażejak-Grabowska, J.; Wójcik, R.; Żarczyńska, K.; Miciński, J.; Ząbek, K. The Efficacy of a Long-Acting Injectable Selenium Preparation Administered to Pregnant Ewes and Lambs. Animals 2021, 11, 1076. [Google Scholar] [CrossRef]
- Abdel-Raheem, S.; Mahmoud, G.; Senosy, W.; El-Sherry, T. Influence of Vitamin E and Selenium Supplementation on the Performance, Reproductive Indices and Metabolic Status of Ossimi Ewes. Slov. Vet. Res. 2019, 56, 353–363. [Google Scholar] [CrossRef]
- El-Shahat, K.H.; Abdel Monem, U.M. Effects of dietary supplementation with vitamin E and/or selenium on metabolic and reproductive performance of Egyptian Baladi ewes under subtropical conditions. World Appl. Sci. J. 2011, 12, 1492–1499. [Google Scholar]
- Ziaei, N. Effect of selenium and vitamin E supplementation on reproductive indices and biochemical metabolites in Raieni goats. J. Appl. Anim. Res. 2014, 43, 426–430. [Google Scholar] [CrossRef]
- Saba, F.E.; Saleh, A.A.K.; Al Moafy, A.A. Effect of supplementation with different types of selenium on lactation performance and some blood parameters of Farafra and Saidi ewes and performance of their lambs. Egypt. J. Sheep Goat Sci. 2019, 14, 19–30. [Google Scholar] [CrossRef]
- Novoselec, J.; Šperanda, M.; Klir, Ž.; Mioč, B.; Steiner, Z.; Antunović, Z. Blood biochemical indicators and concentration of thyroid hormones in heavily pregnant and lactating ewes depending on selenium supplementation. Acta Veter. Brno 2017, 86, 353–363. [Google Scholar] [CrossRef]
- Sobiech, P.; Kuleta, Z. Usefulness of some biochemical indicators in detection of early stages of nutritional muscular dystrophy in lambs. Small Rumin. Res. 2002, 45, 209–215. [Google Scholar] [CrossRef]
- Sousa, C.P.; Azevedo, J.T.; Silva, A.M.; Viegas, C.A.; Reis, R.L.; Gomes, M.; Dias, I.R. Serum total and bone alkaline phosphatase levels and their correlation with serum minerals over the lifespan of sheep. Acta Vet. Hung. 2014, 62, 205–214. [Google Scholar] [CrossRef][Green Version]
- Muñoz, C.; Carson, A.F.; McCoy, M.A.; Dawson, L.E.R.; Irwin, D.; Gordon, A.W.; Kilpatrick, D.J. Effect of supplementation with barium selenate on the fertility, prolificacy and lambing performance of hill sheep. Vet. Rec. 2009, 164, 265–271. [Google Scholar] [CrossRef]
- Erdoğan, S.; Karadaş, F.; Yılmaz, A.; Karaca, S. The effect of organic selenium in feeding of ewes in late pregnancy on selenium transfer to progeny. Rev. Bras. Zootec. 2017, 46, 147–155. [Google Scholar] [CrossRef]
- Humann-Ziehank, E.; Tegtmeyer, P.C.; Seelig, B.; Roehrig, P.; Ganter, M. Variation of serum selenium concentrations in German sheep flocks and implications for herd health management consultancy. Acta Vet. Scand. 2013, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk, B.; Tomza-Marciniak, A.; Dobrzanski, Z.; Szewczuk, M.; Stankiewicz, T.; Gaczarzewicz, D.; Lachowski, W. The effect of selenized yeast supplementation on some performance parameters in sheep. Turk. J. Vet. Anim. Sci. 2013, 37, 61–67. [Google Scholar] [CrossRef]
- Karimi-Poor, M.; Tabatabaine, S.N.; Zamani, F.; Pirestani, A.; Bahrami, Y. Investigation of selenium concentration of sheep’s diet blood and milk in different regions from a central state of Iran. Ann. Biol. Res. 2011, 2, 51–61. [Google Scholar]
- Hamam, A. Effect of Vitamin E and Selenium Supplements on the Antioxidant Markers and Immune Status in Sheep. J. Biol. Sci. 2007, 7, 870–878. [Google Scholar] [CrossRef]
| Specification | CJ Concentrate | Barley Straw | Meadow Hay |
|---|---|---|---|
| Dry matter | 88.65 | 95.21 | 84.95 |
| Crude ash | 5.59 | 4.81 | 9.79 |
| Crude protein | 19.32 | 4.22 | 17.25 |
| Crude fat | 3.42 | 1.84 | 1.82 |
| Crude fiber | 6.92 | 45.4 | 28.62 |
| Gross energy MJ kg−1 | 16.14 | 16.89 | 16.22 |
| Specification | Group | SEM | p-Value | |
|---|---|---|---|---|
| C | E | |||
| Ewes | ||||
| Pregnancy: Dry matter | 1187.14 | 1200.33 | 1.064 | 0.376 |
| Crude protein | 100.34 | 99.24 | 0.824 | 0.213 |
| Crude fiber | 395.64 | 400.03 | 0.664 | 0.145 |
| PDIN | 61.91 | 62.60 | 0.654 | 0.433 |
| PDIE | 79.83 | 79.83 | 0.654 | 0.934 |
| Lactation: Dry matter | 1534.25 | 1551.30 | 1.064 | 0.385 |
| Crude protein | 149.72 | 148.08 | 2.478 | 0.425 |
| Crude fiber | 462.88 | 468.02 | 0.868 | 0.356 |
| PDIN | 92.29 | 93.32 | 1.256 | 0.215 |
| PDIE | 112.76 | 112.76 | 0.224 | 0.995 |
| Lambs | ||||
| Rearing period: Dry matter | 548.63 | 554.73 | 4.856 | 0.426 |
| Crude protein | 70.46 | 69.69 | 0.551 | 0.174 |
| Crude fiber | 108.03 | 109.23 | 1.580 | 0.572 |
| PDIN | 43.46 | 43.94 | 0.270 | 0.562 |
| PDIE | 49.69 | 49.69 | 0.456 | 0.941 |
| Parameter | 3rd Month of Pregnancy (Day 0) | 28th Day of Lactation | 100th Day of Lactation | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | E | C | E | C | E | Treatment | Time | Treatment × Time | ||
| WBC (109/L) | 7.29 | 7.32 | 7.34 | 7.58 | 7.38 | 7.84 * | 1.086 | 0.036 | 0.749 | 0.789 |
| RBC (1012/L) | 11.42 | 11.45 | 11.46 | 11.59 | 11.43 | 11.65 * | 0.571 | 0.044 | 0.509 | 0.722 |
| HGB (g/dL) | 11.75 | 11.83 | 11.86 | 11.92 | 11.83 | 11.96 | 0.346 | 0.513 | 0.931 | 0.983 |
| HCT (%) | 33.75 | 34.03 | 33.94 | 34.28 | 34.18 | 34.45 | 3.840 | 0.916 | 0.425 | 0.917 |
| MCV (fl) | 32.04 | 31.12 | 30.76 | 30.33 | 31.03 | 30.81 | 0.360 | 0.830 | 0.343 | 0.965 |
| MCH (pg) | 10.21 | 10.34 | 10.24 | 10.29 | 10.29 | 10.30 | 0.142 | 0.609 | 0.691 | 0.881 |
| MCHC (g/dL) | 34.10 | 34.12 | 34.33 | 33.98 | 34.28 | 34.39 | 0.500 | 0.971 | 0.838 | 0.963 |
| PLT (109/L) | 512.62 | 509.38 | 516.22 | 518.47 | 519.03 | 523.87 | 19.28 | 0.876 | 0.803 | 0.961 |
| Parameter | 3rd Month of Pregnancy (Day 0) | 28th Day Of Lactation | 100th Day of Lactation | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | E | C | E | C | E | Treatment | Time | Treatment × Time | ||
| GLU (mmol/L) | 2.23 | 2.27 | 2.26 | 2.24 | 2.24 | 2.28 | 0.126 | 0.251 | 0.936 | 0.396 |
| TP (g/L) | 66.28 | 66.39 | 67.02 | 68.51 | 66.98 | 67.66 | 8.45 | 0.474 | 0.962 | 0.203 |
| Chol (mmol/L) | 2.15 | 2.16 | 2.06 | 2.18 | 2.13 | 2.14 | 0.053 | 0.204 | 0.439 | 0.886 |
| TG (mmol/L) | 0.56 | 0.58 | 0.52 | 0.59 | 0.63 | 0.66 | 0.006 | 0.158 | 0.690 | 0.568 |
| Ca (mmol/L) | 2.31 | 2.30 | 2.33 | 2.34 | 2.30 | 2.29 | 0.019 | 0.223 | 0.816 | 0.482 |
| Mg (mmol/Ll) | 1.06 | 1.09 | 1.04 | 1.12 | 1.06 | 1.07 | 0.008 | 0.560 | 0.735 | 0.512 |
| Pinorg (mmol/L) | 2.64 | 2.63 | 2.78 | 2.59 | 2.64 | 2.79 | 0.472 | 0.281 | 0.770 | 0.198 |
| AST (U/L) | 125.80 | 122.43 | 128.70 | 123.98 | 125.55 | 120.27 | 21.39 | 0.275 | 0.879 | 0.169 |
| ALP (U/L) | 151.15 | 152.66 | 152.98 | 153.53 | 151.67 | 152.77 | 20.92 | 0.645 | 0.581 | 0.824 |
| LDH (U/L) | 1332.60 | 1228.04 | 1354.55 | 1245.56 | 1355.20 | 1226.98 | 74.54 | 0.553 | 0.187 | 0.690 |
| GGT (U/L) | 53.95 | 53.87 | 54.02 | 52.50 | 52.77 | 54.17 | 9.57 | 0.582 | 0.864 | 0.518 |
| Parameter | 3rd Month of Pregnancy (Day 0) | 28th Day of Lactation | 100th Day of Lactation | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | E | C | E | C | E | Treatment | Time | Treatment × Time | ||
| Lysozyme activity (mg/L) | 1.09 | 1.08 | 1.08 b | 1.13 a | 1.12 B | 1.32 A,** | 0.101 | 0.001 | 0.005 | 0.360 |
| Ceruloplasmin activity (mg/L) | 49.58 | 49.33 | 50.02 | 51.22 | 50.67 | 52.35 | 8.27 | 0.125 | 0.126 | 0.428 |
| Gamma globulin level (g/L) | 28.93 | 29.02 | 29.60 b | 31.04 a | 29.89 B | 33.34 A,** | 9.48 | 0.005 | 0.001 | 0.836 |
| RBA (OD 620 nm) | 0.48 | 0.49 | 0.50 | 0.53 | 0.49 B | 0.56 A | 0.051 | 0.001 | 0.273 | 0.939 |
| PKA (OD 620 nm) | 0.40 | 0.39 | 0.39 B | 0.45 A,* | 0.40 b | 0.44 a,* | 0.048 | 0.001 | 0.048 | 0.207 |
| MTT-Con A (RI) | 1.20 | 1.22 | 1.23 B | 1.36 A,* | 1.24 B | 1.36 A,* | 0.069 | 0.001 | 0.029 | 0.336 |
| MTT-LPS (RI) | 1.09 | 1.08 | 1.02 b | 1.10 a,** | 1.04 b | 1.12 a,** | 0.041 | 0.038 | 0.001 | 0.936 |
| Specification | Group | SEM | p-Value | |
|---|---|---|---|---|
| C | E | |||
| Body weight (kg), days of age | ||||
| 2 | 2.09 | 2.07 | 0.09 | 0.89 |
| 28 | 9.53 B | 11.17 A | 0.33 | 0.01 |
| 100 | 13.79 b | 16.09 a | 0.52 | 0.02 |
| Daily gains (g), days of age | ||||
| 2–28 | 286.15 A | 350.00 B | 12.65 | 0.01 |
| 28–100 | 59.17 | 68.33 | 7.25 | 0.53 |
| 2–100 | 119.39 a | 143.06 b | 5.59 | 0.03 |
| Ultrasound measurements | ||||
| MLD USG scanning: - depth (cm), days of age | ||||
| 28 | 0.83 | 0.89 | 0.03 | 0.60 |
| 100 | 1.10 b | 1.22 a | 0.05 | 0.01 |
| - width (cm), days of age | ||||
| 28 | 2.09 | 2.13 | 0.07 | 0.20 |
| 100 | 2.68 b | 2.76 a | 0.09 | 0.02 |
| - area (cm2), days of age | ||||
| 28 | 2.82 | 2.89 | 0.15 | 0.26 |
| 100 | 4.28 b | 4.37 a | 0.24 | 0.04 |
| Fat thickness over the loin-eye area (cm), days of age | ||||
| 28 | 0.08 | 0.09 | 0.01 | 0.18 |
| 100 | 0.15 | 0.17 | 0.01 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błażejak-Grabowska, J.; Milewski, S.; Ząbek, K.; Sobiech, P.; Wójcik, R.; Żarczyńska, K.; Miciński, J. Effect of Long-Acting Selenium Preparation on Health and Productivity of Sheep. Animals 2022, 12, 140. https://doi.org/10.3390/ani12020140
Błażejak-Grabowska J, Milewski S, Ząbek K, Sobiech P, Wójcik R, Żarczyńska K, Miciński J. Effect of Long-Acting Selenium Preparation on Health and Productivity of Sheep. Animals. 2022; 12(2):140. https://doi.org/10.3390/ani12020140
Chicago/Turabian StyleBłażejak-Grabowska, Justyna, Stanisław Milewski, Katarzyna Ząbek, Przemysław Sobiech, Roman Wójcik, Katarzyna Żarczyńska, and Jan Miciński. 2022. "Effect of Long-Acting Selenium Preparation on Health and Productivity of Sheep" Animals 12, no. 2: 140. https://doi.org/10.3390/ani12020140
APA StyleBłażejak-Grabowska, J., Milewski, S., Ząbek, K., Sobiech, P., Wójcik, R., Żarczyńska, K., & Miciński, J. (2022). Effect of Long-Acting Selenium Preparation on Health and Productivity of Sheep. Animals, 12(2), 140. https://doi.org/10.3390/ani12020140







