Anthelmintic Activity, Cytotoxicity, and Phytochemical Screening of Plants Used to Treat Digestive Parasitosis of Small Ruminants in Benin (West Africa)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Identification and Collection
2.2. Phytochemical Screening of Plants
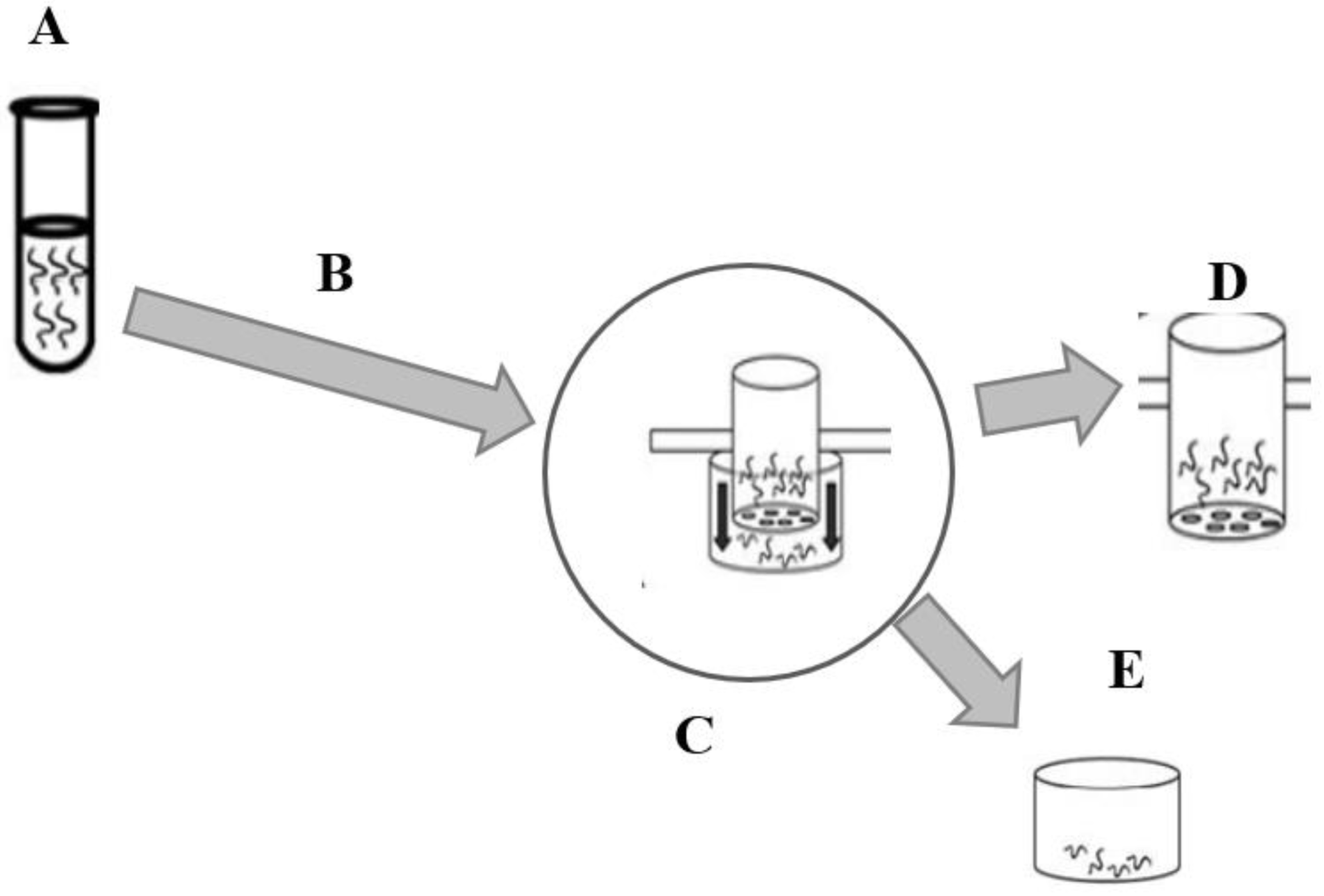
2.3. Extraction Procedure
2.4. Screening for Anthelmintic Activity of Extracts
2.4.1. Obtaining Infective Larvae
2.4.2. Larval Migration Inhibition Assay
2.5. Cytotoxicity Evaluation of Crude Extracts
2.6. Quantification of Secondary Metabolites
2.6.1. Total Phenol Content (TPC)
2.6.2. Total Flavonoid Content (TFC)
2.6.3. Condensed Tannin Content (CTC)
2.7. Statistical Analysis
3. Results
3.1. Secondary Metabolites of the Studied Plants
3.2. Extraction Yield
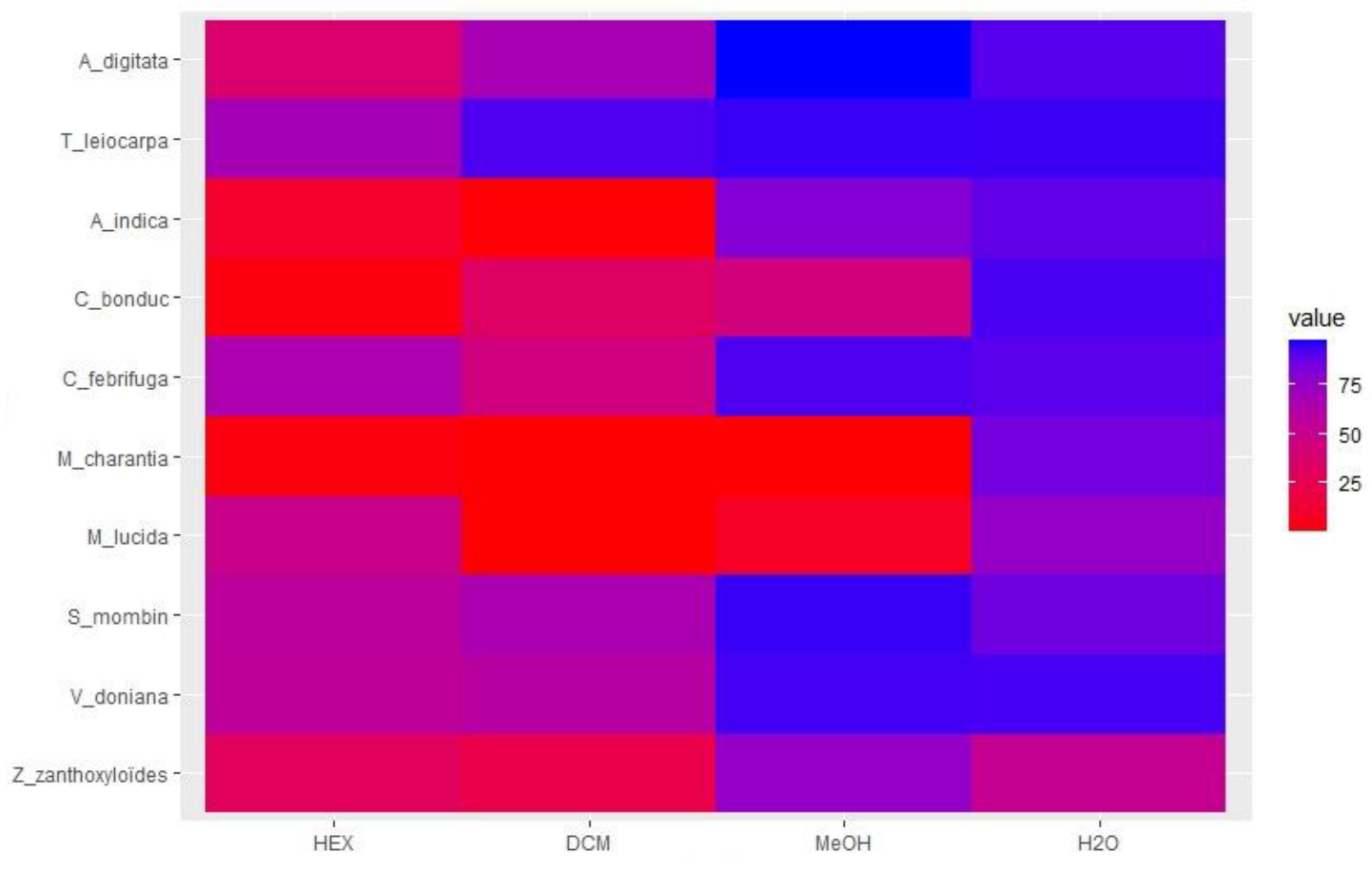
3.3. Anthelmintic Activity of the Extracts
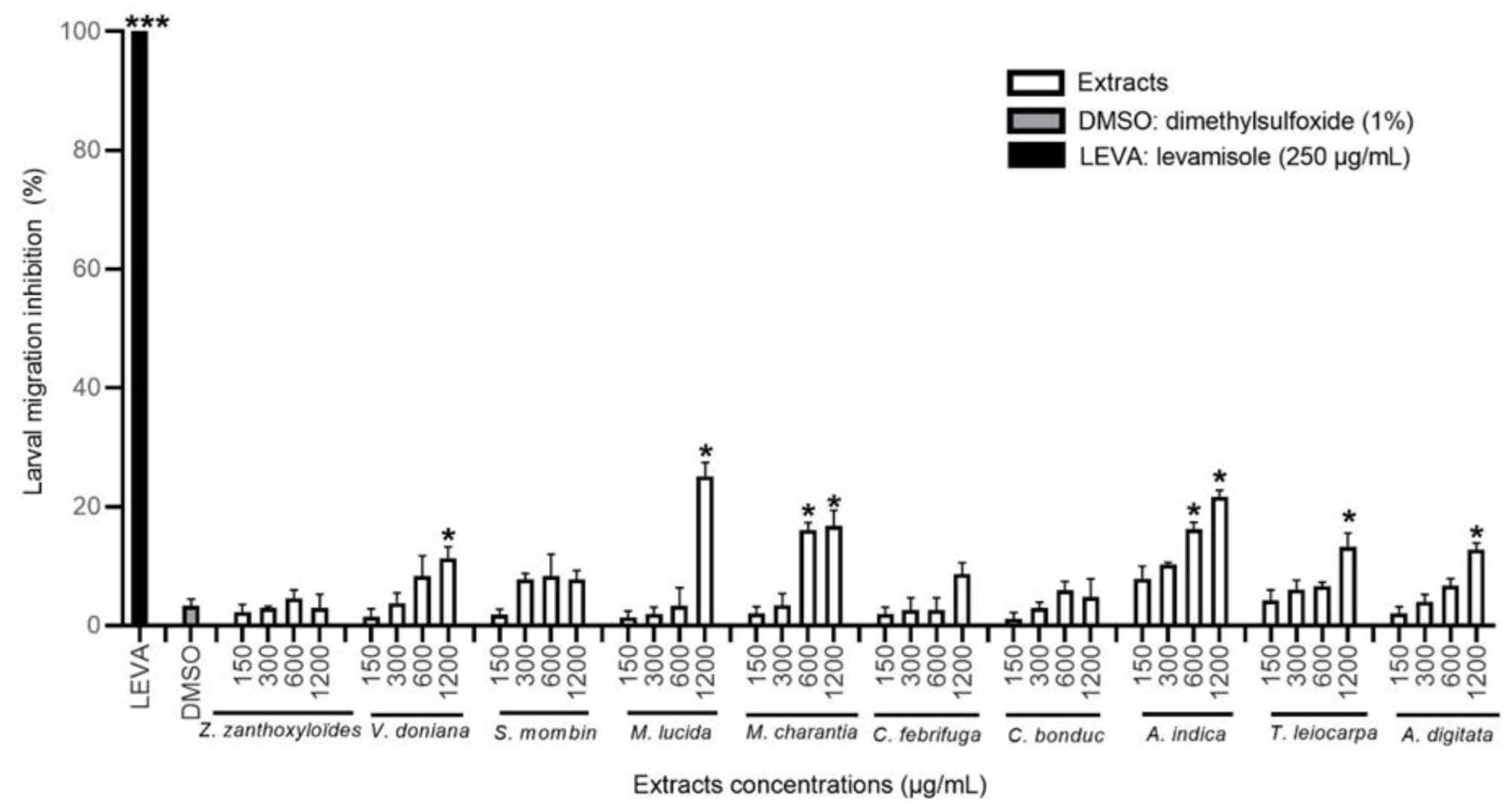
3.3.1. Effect of the HEX Extracts on the Migration of H. contortus Larvae
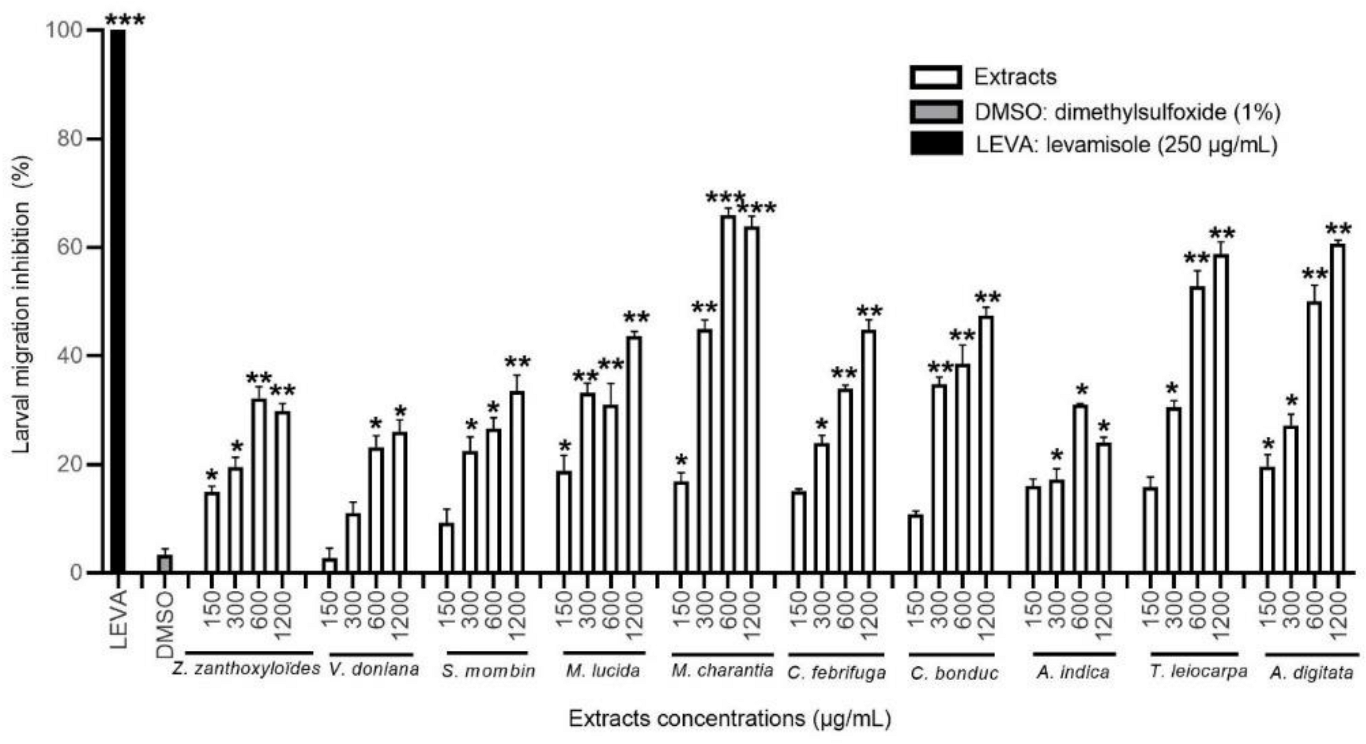
3.3.2. Effect of the DCM Extracts on the Migration of H. contortus Larvae
3.3.3. Effect of the MeOH Extracts on H. contortus Larval Migration
3.3.4. Effect of the H2O Extracts on H. contortus Larval Migration
3.4. Cytotoxicity of Extracts
3.5. Determination of TPC, TFC, and CTC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoste, H.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Mueller-Harvey, I.; Sotiraki, S.; Louvandini, H.; Thamsborg, S.M.; Terrill, T.H. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 2015, 212, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Hounzangbé-Adoté, M.S. L’élevage face à la pharmacopée en médecine vétérinaire au Sud du Bénin. Bull. Rech. Agron. 2001, 33, 1–9. [Google Scholar]
- Basabe, J.; Eiras, D.F.; Romero, J.R. Nutrition and gastrointestinal parasitism in ruminant production. Arch. Zootec. 2009, 58, 131–144. [Google Scholar]
- Hoste, H.; Chartier, C. Comparison of the effects on milk production of concurrent infection with Haemonchus contortus and Trichostrongylus colubriformis in high- and lowproducing dairy goats. Am. J. Vet. Res. 1993, 54, 1886–1893. [Google Scholar] [PubMed]
- Tchetan, E.; Olounlade, A.P.; Azando, E.V.B.; Quinet, M.; Marcotty, T.; Hounzangbe-Adoté, S.M.; Quetin-Leclercq, J.; Gbaguidi, F.A. La médecine ethnovétérinaire à la croisée de la recherche scientifique: Synthèse des connaissances et perspectives. Rev. D’élev. Méd. Vét. Pays Trop. 2021, 74, 167–175. [Google Scholar] [CrossRef]
- Ouachinou, J.M.A.S.; Dassou, G.H.; Idohou, R.; Adomou, A.C.; Yédomonhan, H. National inventory and usage of plant-based medicine to treat gastrointestinal disorders with cattle in Benin (West Africa). S. Afr. J. Bot. 2019, 122, 432–446. [Google Scholar] [CrossRef]
- Tchetan, E.; Olounlade, A.P.; Houehanou, T.D.; Azando, E.V.B.; Kaneho, J.A.; Houinato, M.R.B.; Hounzangbe-Adote, S.M.; Quetin-Leclercq, J.; Gbaguidi, F.A. Ethnoveterinary knowledge of sheep and goat farmers in Benin (West Africa): Effect of socioeconomic and environmental factors. Heliyon 2021, 7, e07656. [Google Scholar] [CrossRef] [PubMed]
- Koné, W.M.; Vargas, M.; Keiser, J. Anthelmintic activity of medicinal plants used in Côte d’Ivoire for treating parasitic diseases. Parasitol. Res. 2012, 110, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Diehl, M.S.; Kamanzi Atindehou, K.; Téré, H.; Betschart, B. Prospect for anthelminthic plants in the Ivory Coast using ethnobotanical criteria. J. Ethnopharmacol. 2004, 95, 277–284. [Google Scholar] [CrossRef]
- Ademola, I.O.; Fagbemi, B.O.; Idowu, S.O. Anthelminthic activity of extracts of Spondias mombin against gastrointestinal nematodes of sheep: Studies in vitro and in vivo. Trop. Anim. Health Prod. 2005, 37, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Chagas, A.C.S.; Vieira, L.S.; Freitas, A.R.; Araujo, M.R.A.; Araujo-Filho, J.A.; Araguao, W.R.; Navarro, A.M.C. Anthelmintic efficacy of neem (Azadirachta indica A. Juss) and the homeopathic product Fator Vermes1 in Morada Nova sheep. Vet. Parasitol. 2008, 151, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Azando, E.V.B.; Hounzangbé-Adoté, M.S.; Olounladé, P.A.; Brunet, S.; Fabre, N.; Valentin, A.; Hoste, H. Involvement of tannins and flavonoids in the in vitro effects of Newbouldia laevis and Zanthoxylum zanthoxyloïdes extracts on the exsheathment of third-stage infective larvae of gastrointestinal nematodes. Vet. Parasitol. 2011, 180, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Olounlade, P.A.; Azando, E.V.B.; Hounzangbé-Adoté, M.S.; TamHa, T.B.; Leroy, E.; Moulis, C.; Fabre, N.; Magnaval, J.F.; Hoste, H.; Valentin, A. In vitro anthelmintic activity of the essential oils of Zanthoxylum zanthoxyloïdes and Newbouldia laevis against Strongyloides ratti. Parasitol. Res. 2012, 110, 1427–1433. [Google Scholar] [CrossRef]
- Ndjonka, D.; Ajonina-Ekoti, I.; Djafsia, B.; Lüersen, K.; Abladam, E.; Liebau, E. Anogeissus leiocarpus extract on the parasite nematode Onchocerca ochengi and on drug resistant mutant strains of the free-living nematode Caenorhabditis elegans. Vet. Parasitol. 2012, 190, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Soro, D.; Koné, W.M.; Bonfoh, B.; Dro, B.; Toily, K.B.; Kamanzi, K. In vivo anthelmintic activity of Anogeissus leiocarpus Guill & Perr (Combretaceae) against nematodes in naturally infected sheep. Parasitol. Res. 2013, 112, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, M.; Varma, A. Medicinal Plants and Environmental Challenges; Springer Nature: Cham, Switzerland, 2018; pp. 1–415. [Google Scholar] [CrossRef]
- Waterman, C.; Smith, R.A.; Pontiggia, L.; DerMarderosian, A. Anthelmintic screening of Sub-Saharan African plants used in traditional medicine. J. Ethnopharmacol. 2010, 127, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Ajaiyeoba, E.O.; Abiodun, O.O.; Falade, M.O.; Ogbole, N.O.; Ashidia, J.S.; Happi, C.T.; Akinboy, D.O. In vitro cytotoxicity studies of 20 plants used in Nigerian antimalarial ethnomedicine. Phytomedicine 2006, 13, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Misra, L.N.; Vyry Wouatsa, N.A.; Kumar, S.; VenkateshKumar, R.; Tchoumbougnang, F. Antibacterial, cytotoxic activities and chemical composition of fruits of two Cameroonian Zanthoxylum species. J. Ethnopharmacol. 2013, 148, 74–80. [Google Scholar] [CrossRef]
- Basaran, G.S.; Bekci, H.; Baldemir, A.; İlgün, S.; Cumaoğlu, A. Cytotoxic Effects of Functional Foods Momordica charantia L. and Lycium barbarum L. Extracts on Prostate Cancer Cells. Proceedings 2017, 1, 1044. [Google Scholar] [CrossRef]
- Miwa, N.; Mizuno, S.; Okamoto, S. Differential cytotoxicity to human lung normal diploid, virus-transformed and carcinoma cells by the antitumor antibiotics, auromomycin and macromomycin, and their non-protein chromophores. J. Antibiot. 1983, 36, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Stępnik, M.; Arkusz, J.; Smok-Pieniążek, A.; Bratek-Skicki, A.; Salvati, A.; Lynch, I.; Dawson, K.A.; Gromadzińska, J.; De Jong, W.H.; Rydzyński, K. Cytotoxic effects in 3T3-L1 mouse and WI-38 human fibroblasts following 72 hour and 7 day exposures to commercial silica nanoparticles. Toxicol. Appl. Pharmacol. 2012, 263, 89–101. [Google Scholar] [CrossRef] [PubMed]
- West-Africa-ECOWAS-Region-Members-Countries. Available online: https://www.researchgate.net/publication/332562556/figure/download/fig1/AS:750477507575809@1555939189146/West-Africa-ECOWAS-region-members-countries-Source-Google-map.png (accessed on 2 September 2022).
- Communauté Economique des Etats de l’Afrique de l’Ouest. Available online: https://www.populationdata.net/wp-content/uploads/2016/11/Communaute-economique-des-Etats-de-lAfrique-de-lOuest-CEDEAO.png (accessed on 2 September 2022).
- Houghton, P.J.; Raman, A. Laboratory Handbook for Fractionation of Natural Extracts; Chapman and Hall: London, UK, 1998; 199p. [Google Scholar]
- Tona, L.; Kambu, K.; Ngimbi, N.; Cimanga, K.; Vlietinck, A.J. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 1998, 61, 57–65. [Google Scholar] [CrossRef]
- Ouachinou, J.M.A.S.; Dassou, G.H.; Agbankpé, A.J.; Koudoro, Y.A.; Agbangnan, P.; Triana, N.H.; Favi, G.A.; Djidohokpin, D.; Adomou, A.C. Variation of secondary metabolite contents and activities against bovine diarrheal pathogens among Zygophyllaceae species in Benin and implications for conservation. Planta Med. 2022, 88, 1–8. [Google Scholar] [CrossRef]
- Tchetan, E.; Azando, E.V.B.; Olounladé, P.A.; Alowanou, G.G.; Hounzangbé-Adoté, S.M. In vitro effects of tannin and extracts of Bridelia ferruginea and Mitragyna inermis on the exsheathment of infective larvae of Haemonchus contortus. Int. J. Vet. Sci. Med. 2020, 8, 93–99. [Google Scholar] [CrossRef]
- Rabel, B.; McGregor, P.; Dough, G. Improved bioassay for estimation of effects of ovine gastrointestinal inhibitoty mucus and on nematode larval migration anthelminthic. Int. J. Parasitol. 1994, 24, 671–676. [Google Scholar] [CrossRef]
- Alowanou, G.G.; Olounladé, P.A.; Akouèdegni, G.C.; Faihun, A.M.L.; Koudandé, D.O.; Hounzangbé-Adoté, S. In vitro anthelmintic effects of Bridelia ferruginea, Combretum glutinosum, and Mitragyna inermis leaf extracts on Haemonchus contortus, an abomasal nematode of small ruminants. Parasitol. Res. 2019, 118, 1215–1223. [Google Scholar] [CrossRef]
- Rendon, D.A.Z. Optimization of a Larval Migration Inhibition Assay (LMIA) for Determining In Vitro Susceptibility of Equine Cyathostomins to Macrocyclic Lactone Anthelmintics, and Evaluation of Variation in Species-Specific Drug Tolerance Levels Using a 454 Large-Scale Parallel Pyrosequencing System. Master’s Thesis, University of Georgia, Athens, GA, USA, 2012. [Google Scholar] [CrossRef]
- Stevigny, C.; Block, S.; De Pauw-Gillet, M.C.; de Hoffmann, E.; Llabrès, G.; Adjakidjé, V.; Quetin-Leclercq, J. Cytotoxic aporphine alkaloids from Cassytha filiformis. Planta Med. 2002, 68, 1042–1044. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; de Mello, J.C.P. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Arora, S.; Itankar, P. Extraction, isolation and identification of flavonoid from Chenopodium album aerial parts. J. Tradit. Complement. Med. 2018, 8, 476–482. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Shea, M.P.; Shanmuganayagam, D.; Krueger, C.G.; Howell, A.B.; Reed, J.D. Comparison of isolated cranberry (Vaccinium macrocarpon Ait.) proanthocyanidins to catechin and procyanidins A2 and B2 for use as standards in the 4-(Dimethylamino)cinnamaldehyde assay. J. Agric. Food Chem. 2012, 60, 4578–4585. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing: R Foundation for Statistical Computing, Vienna, Austria. 2013. Available online: http://www.R-project.org/ (accessed on 18 September 2018).
- Coles, C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Zabré, G.; Kaboré, A.; Bayala, B.; Katikic, L.M.; Costa-Júnior, L.M.; Tamboura, H.H.; Beleme, A.M.G.; Abdalla, A.L.; Niderkorn, V.; Hoste, H.; et al. Comparison of the in vitro anthelmintic effects of Acacia nilotica and Acacia raddiana. Parasite 2017, 24, 44. [Google Scholar] [CrossRef]
- Brunet, S.; Aufrère, J.; El Babili, F.; Fouraste, I.; Hoste, H. The kinetics of exsheathment of infective nematode larvae is disturbed in the presence of a tannin-rich plant extract (sainfoin) both in vitro and in vivo. Parasitology 2007, 134, 1253–1262. [Google Scholar] [CrossRef]
- Dilrukshi Herath, H.M.P.; Prestona, S.; Hofmanna, A.; Davisc, R.A.; Koehlera, A.V.; Changa, B.C.H.; Jabbara, A.; Gassera, R.B. Screening of a small, well-curated natural product-based library identifies two rotenoids with potent nematocidal activity against Haemonchus contortus. Vet. Parasitol. 2017, 244, 172–175. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.T.; Sinkkonen, J.; Salminen, J.P.; Hoste, H. Ellagitannins Inhibit the Exsheathment of Haemonchus contortus and Trichostrongylus colubriformis larvae: The efficiency increases together with the molecular size. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar] [CrossRef]
- Hounzangbé-Adoté, M.S.; Paolini, V.; Fouraste, I.; Moutairou, K.; Hoste, H. In vitro effect of four tropical plants on three life-cycle stages of the parasitic nematode, Haemonchus contortus. Res. Vet. Sci. 2005, 78, 155–160. [Google Scholar] [CrossRef]
- Kabore, A.; Belem, A.M.G.; Tamboura, H.H.; Traore, A.; Sawadogo, L. In vitro anthelmintic effect of two medicinal plants (Anogeissus leiocarpus and Daniellia oliveri) on Haemonchus contortus, an abosomal nematode of sheep in Burkina Faso. Afr. J. Biotechnol. 2009, 8, 4690–4695. [Google Scholar]
- Waterman, C.; Patel, Z.; Kim, S.; Rivera, A.; Pontiggia, L.; Grace, M.H.; Smith, R. Anthelmintic activity of Punicalagin from Anogeissus leiocarpus. Univers. J. Plant Sci. 2015, 3, 67–71. [Google Scholar] [CrossRef][Green Version]
- Ndjonka, D.; Abladam, E.D.; Djafsia, B.; Ajonina-Ekoti, I.; Achukwi, M.D.; Liebau, E. Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J. Helminthol. 2014, 88, 481–488. [Google Scholar] [CrossRef]
- Ikawa, M.; Schaper, T.D.; Dollard, C.A.; Sasner, J.J. Utilization of Folin-Ciocalteu phenol reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Almeida, G.S.I.M.; Barreiros, L.; Reis, S.; Segundo, M.A. Automatic aluminum chloride method for routine estimation of total flavonoids in red wines and teas. Food Anal. Methods 2012, 5, 530–539. [Google Scholar] [CrossRef]
- Gbolade, A.A.; Adeyemi, A.A. Anthelmintic activities of three medicinal plants from Nigeria. Fitoterapia 2008, 79, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Shahadat, H.M.; Mostofa, M.; Mamun, M.A.A.; Hoque, M.E.; Awal, M.A. Comparative efficacy of korolla (Momordica charantia) extract and Ivermec® pour on with their effects on certain blood parameters and body weight gain in indigenous chicken infected with Ascaridia galli. Bangladesh J. Vet. Med. 2008, 6, 153–158. [Google Scholar] [CrossRef]
- Cimanga, R.K.; Tona, G.L.; Mesia, G.K.; Kambu, O.K.; Bakana, D.P.; Kalenda, P.D.T.; Penge, A.O.; Muyembe, J.J.T.; Totté, J.; Pieters, L.; et al. Bioassay-guided isolation of antimalarial triterpenoid acids from the leaves of Morinda lucida. Pharm. Biol. 2006, 44, 677–681. [Google Scholar] [CrossRef]
- Suzuki, M.; Tung, N.H.; Kwofie, K.D.; Adegle, R.; Amoa-Bosompem, M.; Sakyiamah, M.; Ayertey, F.; Owusu, K.B.A.; Tuffour, I.; Atchoglo, P.; et al. New anti-trypanosomal active tetracyclic iridoid isolated from Morinda lucida Benth. Bioorganic Med. Chem. Lett. 2015, 25, 3030–3033. [Google Scholar] [CrossRef]
- Amoa-Bosompem, M.; Ohashi, M.; Mosore, M.T.; Agyapong, J.; Tung, N.H.; Kwofie, K.D.; Ayertey, F.; Owusu, K.B.A.; Tuffour, I.; Atchoglo, P.; et al. In vitro anti-Leishmania activity of tetracyclic iridoids from Morinda lucida, benth. Trop. Med. Health 2016, 44, 1–5. [Google Scholar] [CrossRef]
- Wadkar, G.H.; Kane, S.R.; Matapati, S.S.; Hogade, M.G. In vitro anthelmintic activity of Caesalpinia bonducella (Linn). Flem. leaves. J. Pharm. Res. 2010, 3, 926–927. [Google Scholar]
- Mann, A.; Yahaya, Y.; Banso, A.; Ajayi, G.O. Phytochemical and antibacterial screening of Anogeissus leiocarpus against some microorganisms associated with infectious wounds. Afr. J. Microbiol. 2008, 2, 60–62. [Google Scholar]
- Musila, M.F.; Dossaji, S.F.; Nguta, J.M.; Lukhoba, C.W.; Munyao, J.M. In vivo antimalarial activity, toxicity and phytochemical screening of selected antimalarial plants. J. Ethnopharmacol. 2013, 146, 557–561. [Google Scholar] [CrossRef]


| Authenticated Number | Scientific Name | Family |
|---|---|---|
| AAC 1504/HNB | Terminalia leiocarpa (DC.) Baill. | Combretaceae |
| AAC 1510/HNB | Momordica charantia L. | Cucurbitaceae |
| AAC 1508/HNB | Caesalpinia bonduc (L.) Roxb. | Fabaceae |
| AAC 1505/HNB | Adansonia digitata L. | Malvaceae |
| AAC 1509/HNB | Azadirachta indica A. Juss. | Meliaceae |
| AAC 1503/HNB | Crossopteryx febrifuga (Afzel. ex G. Don) Benth. | Rubiaceae |
| AAC 1501/HNB | Zanthoxylum zanthoxyloïdes (Lam.) Zepern. & Timler | Rutaceae |
| AAC 1507/HNB | Morinda lucida Benth. | Rubiaceae |
| AAC 1511/HNB | Vitex doniana Sweet | Verbenaceae |
| AAC 1506/HNB | Spondias mombin L. | Anacardiaceae |
| Secondary Metabolites | Anacardiaceae | Combretaceae | Cucurbitaceae | Fabaceae | Malvaceae | Meliaceae | Rubiaceae | Rutaceae | Verbenaceae | |
|---|---|---|---|---|---|---|---|---|---|---|
| S. mombin | T. leiocarpa | M. charantia | C. bonduc | A. digitata | A. indica | C. febrifuga | M. lucida | Z. zanthoxyloïdes | V. doniana | |
| Total tannins | + | + | + | + | + | + | + | + | + | + |
| Catechic tannins | - | - | - | - | - | - | - | - | - | + |
| Gallic tannins | + | + | + | + | + | + | + | + | + | + |
| Anthocyanins | - | - | - | - | + | - | + | - | - | - |
| Leucoanthocyanins | - | - | - | - | + | - | + | - | - | + |
| Reducing compounds | + | + | + | + | + | + | + | + | + | + |
| Mucilage | - | - | - | - | + | - | + | - | - | - |
| Saponosides | - | + | + | - | - | + | + | + | - | + |
| Cyanogenic derivatives | - | - | - | - | - | - | - | - | - | - |
| Triterpenoids | + | - | + | + | - | + | - | + | + | + |
| Steroids | - | + | - | - | - | - | - | - | - | - |
| Coumarins | + | - | + | - | - | - | - | - | + | - |
| Quinone derivatives | + | - | - | - | - | - | - | + | + | + |
| Free anthracene | - | - | - | - | - | - | - | - | - | - |
| O-Heterosides | - | - | - | - | - | - | - | - | - | - |
| C-Heterosides | - | - | - | - | - | - | - | - | - | - |
| Alkaloids | + | - | - | + | - | - | - | - | + | + |
| Flavonoids | + | + | + | + | - | + | + | + | - | + |
| Solvents | Hexane | Dichloromethane | Methanol | Water | |
|---|---|---|---|---|---|
| Plants | |||||
| Z. zanthoxyloïdes | 1.4 | 0.6 | 3.2 | 6.8 | |
| V. doniana | 3.2 | 0.9 | 4.7 | 8.4 | |
| S. mombin | 1.7 | 2.5 | 4.9 | 8.7 | |
| M. lucida | 4.3 | 2.1 | 9.6 | 13.5 | |
| M. charantia | 0.5 | 1.4 | 4.8 | 14.8 | |
| C. febrifuga | 1.7 | 3.5 | 11.8 | 10.7 | |
| C. bonduc | 5.3 | 6.6 | 4.7 | 9.8 | |
| A. indica | 0.8 | 8.7 | 3.6 | 10.7 | |
| T. leiocarpa | 0.9 | 16.2 | 5.6 | 10.2 | |
| A. digitata | 1.5 | 7.5 | 3.7 | 2.0 | |
| Plants | Solvent | Migration Inhibition Rate (%) | Cell Viability (%) | |||
|---|---|---|---|---|---|---|
| Concentrations Tested (µg/mL) | ||||||
| 150 | 300 | 600 | 1200 | |||
| Zanthoxylum zanthoxyloïdes | HEX | 2.3 ± 1.3 | 3.0 ± 0.3 | 4.5 ± 1.5 | 3.0± 2.3 | 30.3 ± 18.2 |
| DCM | 4.7 ± 1.7 | 7.0 ± 1.0 | 17.9 ± 1.0 | 47.7 ± 3.1 | 22.7 ± 14.3 | |
| MeOH | 14.9 ± 1.0 | 19.5 ± 1.8 | 32.2 ± 2.1 | 29.9 ± 1.3 | 75.4 ± 2.1 | |
| H2O | 2.3 ± 1.4 | 12.6 ± 2.7 | 12.6 ± 2.7 | 18.4 ± 2.5 | 52.1 ± 10.9 | |
| Vitex doniana | HEX | 1.5 ± 1.3 | 3.8 ± 1.8 | 8.3 ± 3.5 | 11.3 ± 2.0 | 55.2 ± 10.3 |
| DCM | 2.9 ± 1.3 | 10.8 ± 2.0 | 13.3 ± 0.9 | 26.6 ± 1.2 | 59.2 ± 2.6 | |
| MeOH | 2.8 ± 1.8 | 11.0 ± 2.1 | 23.2 ± 2.1 | 25.9 ± 2.2 | 93.4 ± 11.9 | |
| H2O | 9.1 ± 2.5 | 26.6 ± 0.6 | 27.7 ± 2.2 | 71.3 ± 2.0 | 92.9 ± 4.1 | |
| Spondias mombin | HEX | 1.8 ± 1.0 | 7.7 ± 1.1 | 8.3 ± 3.7 | 7.7 ± 1.6 | 56.6 ± 8.4 |
| DCM | 31.6 ± 1.7 | 25.1 ± 1.4 | 36.2 ± 0.6 | 38.8 ± 1.4 | 66.3 ± 4.9 | |
| MeOH | 9.3 ± 2.5 | 22.6 ± 2.5 | 26.6 ± 2.0 | 33.5 ± 2.9 | 94.4 ± 10.6 | |
| H2O | 2.5 ± 2.2 | 11.4 ± 1.1 | 13.9 ± 3.3 | 18.9 ± 2.7 | 85.4 ± 4.0 | |
| Morinda lucida | HEX | 1.3 ± 1.2 | 1.9 ± 1.2 | 3.3 ± 3.0 | 25.2 ± 2.2 | 49.3 ± 2.0 |
| DCM | 13.7 ± 2.4 | 17.1 ± 1.5 | 20.0 ± 1.0 | 22.3 ± 3.0 | 0.6 ± 0.1 | |
| MeOH | 18.9 ± 2.8 | 33.2 ± 1.8 | 31.1 ± 3.9 | 43.7 ± 0.8 | 9.2 ± 8.3 | |
| H2O | 4.3 ± 1.0 | 10.1 ± 0.9 | 20.9 ± 0.6 | 45.3 ± 2.9 | 74.8 ± 13.3 | |
| Momordica charantia | HEX | 2.0 ± 1.2 | 3.36 ± 2.0 | 16.1 ± 1.2 | 16.8 ± 2.6 | 2.9 ± 1.1 |
| DCM | 13.7 ± 2.5 | 20.0 ± 0.5 | 28.6 ± 2.1 | 35.4 ± 0.9 | 0.7 ± 0.2 | |
| MeOH | 16.8 ± 1.7 | 44.9 ± 1.7 | 65.9 ± 1.3 | 63.9 ± 1.9 | 0.8 ± 0.3 | |
| H2O | 2.3 ± 0.8 | 9.4 ± 2.2 | 15.1 ± 1.3 | 18.7 ± 2.1 | 84.9 ± 4.3 | |
| Crossopteryx febrifuga | HEX | 1.9 ± 1.3 | 2.6 ± 2.0 | 2.6 ± 2.0 | 8.6 ± 2.0 | 64.6 ± 21.7 |
| DCM | 3.9 ± 2.3 | 21.1 ± 2.2 | 37.5 ± 2.3 | 50.8 ± 1.6 | 44.7 ± 19.1 | |
| MeOH | 15.1 ± 0.4 | 23.9 ± 1.4 | 33.9 ± 0.6 | 44.8 ± 1.9 | 91.8 ± 4.5 | |
| H2O | 5.3 ± 0.9 | 4.8 ± 1.8 | 13.3 ± 2.5 | 22.3 ± 1.9 | 90.0 ± 3.8 | |
| Caesalpinia bonduc | HEX | 1.3 ± 1.0 | 2.9 ± 1.0 | 5.9 ± 1.6 | 4.7 ± 3.1 | 2.9 ± 1.4 |
| DCM | 2.3 ± 2.6 | 17.9 ± 2.3 | 18.2 ± 3.3 | 34.9 ± 2.2 | 32.9 ± 10.2 | |
| MeOH | 10.8 ± 0.6 | 34.8 ± 1.3 | 38.6 ± 3.4 | 47.5 ± 1.4 | 42.6 ± 11.7 | |
| H2O | 8.6 ± 1.4 | 21.5 ± 2.7 | 28.8 ± 1.4 | 39.9 ± 2.0 | 92.7 ± 7.4 | |
| Azadirachta indica | HEX | 7.8 ± 2.2 | 10.2 ± 0.4 | 16.3 ± 1.1 | 21.7 ± 1.1 | 11.1 ± 1.3 |
| DCM | 12.6 ± 2.0 | 16.1 ± 1.1 | 18.4 ± 1.8 | 27.6 ± 1.9 | 1.3 ± 0.2 | |
| MeOH | 16.1 ± 1.2 | 17.2 ± 2.0 | 31.0 ± 0.2 | 24.1 ± 0.9 | 80.4 ± 12.3 | |
| H2O | 10.2 ± 1.4 | 16.4 ± 0.8 | 21.8 ± 2.0 | 21.8 ± 1.3 | 88.7 ± 6.1 | |
| Terminalia leiocarpa | HEX | 4.2 ± 1.8 | 6.0 ± 1.6 | 6.6 ± 0.6 | 13.3 ± 2.3 | 68.3 ± 10.0 |
| DCM | 47.8 ± 1.2 | 52.3 ± 0.5 | 57.7 ± 1.5 | 63.5 ± 2.3 | 91.6 ± 7.6 | |
| MeOH | 15.9 ± 1.8 | 30.6 ± 1.2 | 52.8 ± 2.9 | 58.7 ± 2.2 | 94.4 ± 3.3 | |
| H2O | 5.9 ± 1.6 | 26.2 ± 1.6 | 33.3 ± 3.1 | 34.1 ± 2.4 | 94.1 ± 2.7 | |
| Adansonia digitata | HEX | 2.0 ± 1.2 | 4.0 ± 1.2 | 6.7 ± 1.2 | 12.8 ± 1.2 | 37.2 ± 6.8 |
| DCM | 3.9 ± 2.9 | 6.4 ± 2.2 | 14.8 ± 1.4 | 23.2 ± 2.3 | 66.4 ± 11.3 | |
| MeOH | 19.7 ± 2.1 | 27.2 ± 2.1 | 50.0 ± 3.0 | 60.6 ± 0.7 | 97.2 ± 5.9 | |
| H2O | 11.4 ± 2.1 | 31.6 ± 1.1 | 54.4 ± 2.3 | 77.2 ± 1.9 | 90.8 ± 4.2 | |
| Identification | TPC (mg GAE/g of Extract) | TFC (mg QE/g of Extract) | CTC (mg CE/g of Extract) |
|---|---|---|---|
| T. leiocarpa (HEX) | 9.9 ± 1.5 | 9.6 ± 1.2 | 0.7 ± 0.1 |
| T. leiocarpa (DCM) | 268.7 ± 7.9 | 48.6 ± 1.3 | 0.6 ± 0.1 |
| T. leiocarpa (MeOH) | 265.4 ± 6.9 | 51.3 ± 0.5 | 1.7 ± 0.5 |
| T. leiocarpa (H2O) | 453.5 ± 2.8 | 67.9 ± 0.1 | 6.1 ± 0.3 |
| C. febrifuga (HEX) | 9.9 ± 0.4 | 2.4 ± 1.2 | 0.4 ± 0.0 |
| C. febrifugea (DCM) | 34.1 ± 2.1 | 6.39 ± 0.13 | 0.2 ± 0.0 |
| C. febrifuga (MeOH) | 98.3 ± 1.5 | 21.3 ± 0.3 | 5.2 ± 0.2 |
| C. febrifuga (H20) | 123.5 ± 6.5 | 14.4 ± 0.3 | 8.5 ± 0.7 |
| M. lucida (HEX) | 14.0 ± 1.7 | 8.6 ± 3.4 | 0.5 ± 0.1 |
| M. lucida (DCM) | 22.4 ± 1.9 | 5.4 ± 0.9 | 0.1 ± 0.0 |
| M. lucida (MeOH) | 42.5 ± 1.4 | 10.5 ± 1.5 | 0 |
| M. lucida (H2O) | 30.7 ± 0.9 | 9.9 ± 1.5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchetan, E.; Olounladé, P.A.; Azando, E.V.B.; Khaliq, H.A.; Ortiz, S.; Houngbeme, A.; Alowanou, G.G.; Koura, B.I.; Akouedegni, G.C.; Houinato, M.R.B.; et al. Anthelmintic Activity, Cytotoxicity, and Phytochemical Screening of Plants Used to Treat Digestive Parasitosis of Small Ruminants in Benin (West Africa). Animals 2022, 12, 2718. https://doi.org/10.3390/ani12192718
Tchetan E, Olounladé PA, Azando EVB, Khaliq HA, Ortiz S, Houngbeme A, Alowanou GG, Koura BI, Akouedegni GC, Houinato MRB, et al. Anthelmintic Activity, Cytotoxicity, and Phytochemical Screening of Plants Used to Treat Digestive Parasitosis of Small Ruminants in Benin (West Africa). Animals. 2022; 12(19):2718. https://doi.org/10.3390/ani12192718
Chicago/Turabian StyleTchetan, Esaïe, Pascal Abiodoun Olounladé, Erick Virgile Bertrand Azando, Hafiz Abdul Khaliq, Sergio Ortiz, Alban Houngbeme, Géorcelin Goué Alowanou, Bossima Ivan Koura, Guénolé Coovi Akouedegni, Marcel Romuald Benjamin Houinato, and et al. 2022. "Anthelmintic Activity, Cytotoxicity, and Phytochemical Screening of Plants Used to Treat Digestive Parasitosis of Small Ruminants in Benin (West Africa)" Animals 12, no. 19: 2718. https://doi.org/10.3390/ani12192718
APA StyleTchetan, E., Olounladé, P. A., Azando, E. V. B., Khaliq, H. A., Ortiz, S., Houngbeme, A., Alowanou, G. G., Koura, B. I., Akouedegni, G. C., Houinato, M. R. B., Hounzangbe-Adote, S. M., Gbaguidi, F. A., & Quetin-Leclercq, J. (2022). Anthelmintic Activity, Cytotoxicity, and Phytochemical Screening of Plants Used to Treat Digestive Parasitosis of Small Ruminants in Benin (West Africa). Animals, 12(19), 2718. https://doi.org/10.3390/ani12192718








