Simple Summary
Tick-borne pathogens are considered emergent because they cause several tick-borne diseases that threaten the health of humans and animals during tick feeding, including anaplasmosis, which is caused by Anaplasma spp. However, information on the carrier animals for Anaplasma spp. antibodies in the Qinghai–Tibetan Plateau Area is limited, and current data on the serodetection of anaplasmosis in plateau areas around the world are needed. Indirect ELISA and competitive ELISA are reliable serological tests that have been developed for the detection of Anaplasma infections in animals. Moreover, major surface protein 5 is a highly conserved surface protein of the Anaplasma genus that has proven effective as a diagnostic antigen and has been used in the serodetection of Anaplasma species infection in various animals with a high level of sensitivity. Hence, in this study, an rMSP5-ELISA was performed to analyze Anaplasma spp. IgG and IgM antibodies in potential carrier animals in the Qinghai–Tibetan Plateau Area. The results not only show the positive rate of IgG and IgM antibodies in the study animals but also indicate that there is a threat of tick biting and zoonotic pathogen infection in the vicinity of human activities in the tested areas. Our study should have major importance for identifying animals with Anaplasma spp. infection in the plateau area.
Abstract
Anaplasma genus infects the blood cells of humans and animals by biting, causing zoonotic anaplasmosis. However, limited data are available on carrier animals for Anaplasma spp. antibodies in the Qinghai–Tibetan Plateau Area. Therefore, a serological indirect ELISA diagnostic method based on the major surface protein 5 (MSP5), derived from Anaplasma phagocytophilum, was developed in this study to analyze both IgG and IgM antibodies of Anaplasma spp. in a total of 3952 animals from the Qinghai–Tibetan Plateau, including yaks (Bos grunniens), cows (Bos taurus), cattle (Bos taurus domesticus), Tibetan sheep (Ovis aries), horses (Equus ferus caballus), pigs (Sus domesticus), chickens (Gallus gallus domesticus), donkeys (Equus asinus), stray dogs (Canis sp.), and stray cats (Felis sp.). The results showed that recombinant MSP5 protein was expressed and was successfully used to establish the indirect ELISA methods. The overall positivity for Anaplasma IgG and IgM antibodies was 14.6% (578/3952) and 7.9% (312/3952), respectively, and a total of 123 animals (3.1%) were both IgG- and IgM-positive. Moreover, the most prevalent Anaplasma IgG positivity was exhibited by donkeys (82.5%), followed by stray dogs, Tibetan sheep, pigs, chickens, horses, yaks, cows, cattle, and stray cats. The analysis for IgM antibody positivity revealed that IgM positivity was the most prevalent in the stray dogs (30.1%), followed by horses, yaks, Tibetan sheep, cows, stray cats, and cattle. Moreover, the results revealed significant differences (p < 0.05) at different altitudes in Anaplasma-specific IgG in the yaks, Tibetan sheep, and horses, and in IgM in the yaks and Tibetan sheep. In conclusion, this study is the first to demonstrate that yaks, cows, cattle, Tibetan sheep, horses, donkeys, stray dogs, stray cats, pigs, and chickens living in the Qinghai–Tibet Plateau are carrier animals for Anaplasma spp. IgG or IgM antibodies. The current findings provide valuable current data on the seroepidemiology of anaplasmosis in China and for plateau areas of the world.
1. Introduction
Tick-borne pathogens (TBPs) are considered emergent because they cause severe tick-borne diseases that threaten the health of humans and animals during tick feeding [1,2,3]. Anaplasma spp. are obligate intracellular bacteria from the order Rickettsiales and the family Anaplasmataceae, and are serious TBPs that cause anaplasmosis in humans and animals by infecting red and white blood cells [4,5,6,7,8]. Anaplasmosis is characterized by hemolytic anemia in various animal species and is endemic in various tropical and subtropical regions worldwide [9,10,11,12,13,14,15,16,17,18,19].
The most important Anaplasma species that affect animals and humans are Anaplasma phagocytophilum, Anaplasma ovis, Anaplasma capra, Anaplasma marginale, Anaplasma bovis, and Anaplasma platys. A. phagocytophilum is an emergent tick-borne zoonotic pathogen transmitted by Ixodes tick species worldwide that affects humans, dogs, cats, horses, sheep, goats, cattle, donkeys, camels, and wild boars, causing human and animal granulocytic anaplasmosis [11,12,13,14,15,16,17,18,19]. The economic impacts of A. ovis and the unclassified species A. capra are more pronounced in sheep and goats [20]. A. marginale is the most important cause of anaplasmosis in bovines resulting in significant economic losses, while A. centrale causes mild disease [21]. A. bovis infects monocytes in small mammals and ruminants causing anaplasmosis [22], while A. platys affects platelets in dogs causing infectious canine cyclic thrombocytopenia [13]. With the increase in animal and tick populations, emergent tick-borne Anaplasma infections are diagnosed more frequently in humans and animals [23].
Ndung’u et al. (1995) [24] and Molloy et al. (1999) [25] described competitive inhibition ELISA for the detection of anti-MSP5 antibodies, which is highly conserved and reactive to A. marginale, A. centrale, and A. ovis. Indirect ELISA is a reliable serological test similar to competitive ELISA that has been developed for the detection of Anaplasma infections in animals [26], and the key to these methods is the selection of antigens with strong specificity and high sensitivity. Major surface protein 5 (MSP5) is a highly conserved surface protein of the Anaplasma genus that has been proven effective as a diagnostic antigen, and this antigen has been used in the serodetection of Anaplasma species infection in various animals, including ruminants, equines, and canines, with a high level of sensitivity [27,28,29,30,31,32,33].
Although Anaplasma infections in animals have been characterized in some provinces in China [6,9,10,11,12], limited data are available on Anaplasma spp. antibodies in domestic and wild carrier animals in the Qinghai–Tibetan Plateau Area (QTPA). To investigate the serological epidemiology of Anaplasma spp. in a variety of animals that are adapted to the high altitude and cold climate of the QTPA, including yaks (Bos grunniens), cows (Bos taurus), cattle (Bos taurus domestica), Tibetan sheep (Ovis aries), horses (Equus ferus caballus), pigs (Sus domesticus), chickens (Gallus gallus domesticus), donkeys (Equus asinus), stray dogs (Canine sp.), and stray cats (Felis sp.), the serological indirect ELISA diagnostic method was developed in this study based on the MSP5 proteins derived from Anaplasma. This was to analyze both IgG and IgM antibodies of Anaplasma spp. in various animals. This assay may be of major importance in detecting Anaplasma spp. in animals of the plateau area.
2. Materials and Methods
2.1. Serum Collection
A total of 3952 serum samples were collected by random sampling from 10 animal species from Qinghai Province in the QTPA with geographical coordinates of 31°36′–39°19′ N and 89°35′–103°04′ E from June 2021 to February 2022. The animal species included 792 yaks, 489 cows, 451 cattle, 794 Tibetan sheep, 389 horses, 424 pigs, 220 chickens, 40 donkey, 226 stray dogs, and 127 stray cats. Information on the sampling sites is shown in Table S1. Fresh blood from different animals was collected from animals’ venous blood vessels using non-anticoagulated blood collection tubes. Subsequently, centrifugation was performed at 5000 rpm for 10 min. The serum from the supernatant was transferred to new collection tubes. Finally, all animal samples were stored in a −80 °C freezer until assayed. All procedures were carried out according to the ethical guidelines of Qinghai University.
2.2. Cloning and Expression of Anaplasma Major Surface Protein 5
The recombinant Anaplasma MSP5 protein was used to perform indirect ELISAs for detecting IgG and IgM antibodies against Anaplasma spp. The MSP5 gene was amplified by PCR from cDNA of Tibetan sheep blood, which was confirmed from a sample that was positive for A. phagocytophilum [10]. Primers that included a BamH I site (underlined) in the forward primer (FAsMSP5BamH1) 5′-CGC GGATCC TTC AGC AAA ATC GGC GAG AGG-3′ and a Not I site (underlined) in the reverse primer (RAsMSP5Not1) 5′-ATAAGAAT GCGGCCGC CTA AGA ATT AAG CAT GTG ACC-3′ for MSP5 were used [29]. The PCR products were digested with BamH I and Not I, and inserted into the pGEX-4T-1 plasmid vector, which was treated with the same restriction enzymes (Roche, Switzerland). IPTG at a final concentration of 0.2 mM was used to induce recombinant pGEX-4T-MSP5 expression in Escherichia coli BL21 (DE3) (New England BioLabs Inc., Ipswich, MA, USA) at 37 °C and 120 r for 12 h, and then the protein was purified with Glutathione Sepharose 4B beads (GE Healthcare Life Sciences, Pittsburgh, PA, USA) according to the manufacturer’s instructions. The final concentration of MSP5 protein was measured with a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Inc., Rockford, IL, USA) before being used.
2.3. Development of Indirect ELISAs for Detecting IgG and IgM Antibodies against Anaplasma spp.
Here, IgG and IgM antibodies against Anaplasma spp. from the study animals were detected by indirect ELISA tests based on recombinant MSP5 protein. The 0.1 μg/mL recombinant proteins were diluted in coating buffer (0.05 M carbonate–bicarbonate, pH 9.6) and incubated at 4 °C overnight to perform indirect ELISA analysis. Briefly, the next day, the ELISA plates were washed with PBS-T (0.05% Tween-20) three times, blocked with 3% skimmed milk for 1 h, then washed once. Collected sera were diluted 1:100 and incubated for 1 h at 37 °C, and the plates were washed with PBS-T six times. Then, the secondary antibodies (Table 1) were diluted 1:3000–4000 and incubated for another 1 h at 37 °C. After washing six times, the ABTS, (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) substrate was added to the plates and incubated for 1 h at RT. The results were measured at OD 450 nm. The soluble GST protein was used as the control under consistent experimental conditions with rMSP5-ELISA. Moreover, the animal sera were confirmed as positive or negative against Anaplasma IgG or IgM antibodies by a commercial cELISA kit (Anaplasma antibody test kit, cELISA; VMRD Inc., Pullman, WA, USA) for ruminants, a commercial ELISA (Ehrlichia equi Antibody Kit, Helica Biosystems, Inc., Santa Ana, CA, USA) for equines, rapid in-house immunochromographic assays (SNAP® 4Dx® from IDEXX® Laboratories, Westbrook, ME, USA) for dogs, and SPF animal sera for the remaining animal species in the study (Chunsheng, Wuhan, China) were used as controls. All positive and negative sera were kept in our laboratory at Qinghai University.

Table 1.
Secondary antibodies used in this study.
2.4. Data Analysis
The cut-off points were calculated by using the OD 450 values for Anaplasma spp. negative sera. Briefly, cut-off values = X + 3SD (X: mean values of OD 415 nm of negative controls, SD: standard deviation of OD 415 nm of negative controls). The OD 415 values of the tested animals were greater than the respective cut-off values judged as positive.
Four possible results for each type of animal in this study could be shown: IgG positivity, IgM positivity, both IgG and IgM positivity, or both IgG and IgM negativity. Therefore, the statistics in this study were presented as the after mentioned four possible results for each animal species. Moreover, this study differentiated all animals into three altitude groups: 2000–3000, 3000–4000, and 4000–5000 m altitudes to analyze the influence of the different heights above sea level on Anaplasma infections in the sampling areas.
To graph and analyze the data, GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA) was used. The antibody prevalence and 95% confidence intervals per pathogen species were calculated using the OpenEpi program (http://www.openepi.com/Proportion/Proportion.htm) Version 3.01 accessed on 15 August 2022. The chi-squared test was used to compare proportions of positivity at different altitudes and among different animal species. The differences were considered to be statistically significant when the resulting p-values were lower than 0.05.
3. Results
3.1. Establishment of rMSP5-ELISA
In this study, the recombinant Anaplasma MSP5 protein was expressed (Figure 1), and used to establish indirect ELISA methods for identifying carrier animals of Anaplasma spp. infection in the Qinghai–Tibetan Plateau. To perform the rMSP5-based indirect ELISA in this study, the negative sera confirmed by the kits for Anaplasma antibody tests and the SPF animal sera were used to determine OD 415 values and to calculate cut-off values for IgG and IgM antibodies in the yaks, cows, cattle, Tibetan sheep, horses, pigs, chickens, donkeys, stray dogs, and stray cats (Figure 2A).
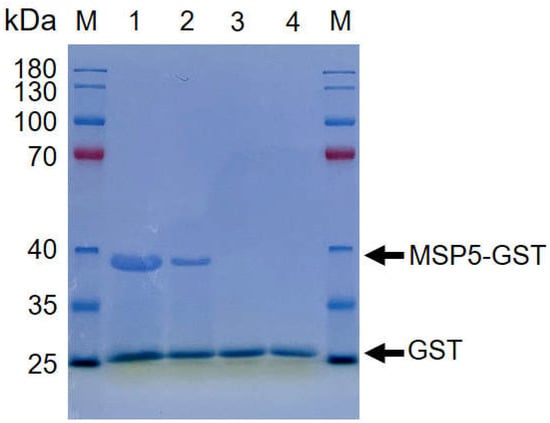
Figure 1.
The recombinant MSP5 protein of Anaplasma was expressed. SDS-PAGE analysis was performed by adding 20 μL samples diluted with the loading buffer is 2 X SDS. The results showed the rMSP5-GST in fractions 1 (Protein concentration is 2 mg/μg), 2 (Protein concentration is 1 mg/μg) and the GST in fractions 3 (Protein concentration is 2 mg/μg), 4 (Protein concentration is 1 mg/μg).
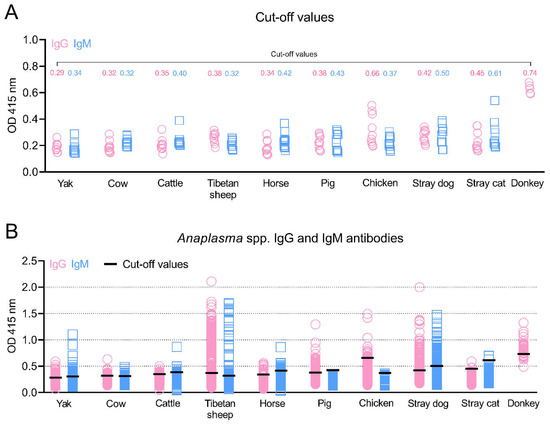
Figure 2.
Anaplasma spp. IgG and IgM antibodies in various animals in the Qinghai–Tibet Plateau Area were detected by the indirect ELISA methods based on MSP5 antigens in this study. The cut-off values (A) and positive animals for IgG and IgM antibodies (B) are shown. OD: Optical Density.
3.2. Positivity for Anaplasma IgG and IgM Antibodies
As shown in Figure 2B and Table 2, the overall positivity for Anaplasma IgG and IgM antibodies was 14.6% (578/3952) and 7.9% (312/3952), respectively, and a total of 123 animals (3.1%) were both IgG- and IgM-positive. A total of 10.5% (83/792) of yaks, 6.7% (33/489) of cows, 4.4% (20/451) of cattle, 29.8% (237/794) of Tibetan sheep, 11.8% (46/389) of horses, 23.8% (101/424) of pigs, 8.6% (19/220) of chickens, 82.5% (33/40) of donkeys, 49.1% (111/226) of stray dogs, and 4.7% (6/127) of stray cats were positive for at least one indicator (IgG or IgM). Moreover, the donkey was the animal with the highest IgG positivity prevalence (82.5%), followed by stray dogs, Tibetan sheep, pigs, chickens, horses, yaks, cows, cattle, and stray cats. IgM antibody positivity was most prevalent in stray dogs (30.1%), followed by horses, yaks, Tibetan sheep, cows, stray cats, and cattle. No pigs or chickens were positive for IgM antibodies against Anaplasma.

Table 2.
Seropositive rates of Anaplasma IgG and IgM antibodies in animals in the QTPA.
3.3. Influence of Altitude on the Positivity of Anaplasma IgG and IgM Antibodies
The results from the analysis of the influence of altitude revealed significant differences (p < 0.05) in Anaplasma-specific IgG prevalence at different altitudes in the yaks, Tibetan sheep, and horses, while IgM prevalence was significantly different in the yaks and Tibetan sheep at different altitudes (Table 3). The results revealed significant differences (p < 0.05) at different altitudes in Anaplasma-specific IgG prevalence in the yaks, Tibetan sheep, and horses and in IgM prevalence in the yaks and Tibetan sheep. There were no differences at different altitudes in the other animal species.

Table 3.
Analysis of influence of altitude on positivity of Anaplasma IgG and IgM antibodies.
4. Discussion
The Qinghai–Tibetan Plateau Area (QTPA) is the largest plateau that has the highest average altitude on the planet, and is located in Northwestern China [10]. It has a unique and vigorous natural ecosystem due to its high altitude (with an average elevation of more than 2000 m above sea level) and cold climate (an average annual temperature below 10 °C) [11]. In specific areas, there are specialized tick species that can transmit unique pathogens. However, there are few studies on the detection of antibodies against tick-borne diseases in the Qinghai–Tibet Plateau, which is an area with much livestock production. Anaplasma species are zoonotic pathogens with ticks as vectors and with mammalian reservoir hosts [34]. Their transmission is closely related to the activity of ticks, and a variety of specialized species of Ixodes are distributed across the Qinghai–Tibet Plateau, such as Haemaphysalis qinghaiensis, Dermacentor nuttalli, and Dermacentor silvarum [11,12]. Importantly, the zoonotic pathogen has been detected and characterized in these ixodid ticks [35]. The Anaplasma genus infects the blood cells of humans and animals through biting, causing zoonotic diseases, while grazing and straying increase animal exposure to ticks. In this study, an rMSP5-ELISA was performed to analyze Anaplasma spp. IgG and IgM antibodies in potential carrier animals that live in proximity to humans in the QTPA. This study provides valuable current data on the epidemiology of anaplasmosis in China and in the plateau areas in the world.
MSP5 is a transmembrane protein of 210 amino acid residues that is present in all recognized Anaplasma species and that is highly conserved among different strains of A. marginale, A. centrale, A. ovis and A. phagocytophilum [19]. It has been used in the sero determination of Anaplasma infection in animals, and among the methods of serodetection, indirect ELISA and competitive ELISA are considered reliable [27,28,29,30,31,32,33,36]. The MSP5 protein in this study was expressed with 182 amino acids whose signal peptide was removed to develop an indirect ELISA for detecting antibodies against A. marginale, A. centrale, A. ovis, and A. phagocytophilum and to screen carrier animals for infection with Anaplasma species. Furthermore, some studies have shown that the antibodies against A. marginale MSP5 are recognized in both acute stages of infection and chronically infected carrier cattle [27,29]. In the current study, rMSP5-ELISA was used to detect IgG and IgM antibodies and revealed that the overall positivity for these antibodies was 14.6% and 7.9%, respectively, suggesting that tested animal species were in the acute stages of infection or are carriers of antibodies for anaplasmosis. Of course, the presence of these antibodies is inseparable from the participation of the vector ticks.
Bovine anaplasmosis, primarily caused by A. marginale, which can primarily cause acute anaplasmosis in adult bovines, is considered one of the most serious tick-borne diseases in ruminants [30,31]. A. centrale, a species closely related to A. marginale, is used to prevent acute anaplasmosis in several countries worldwide [30]. This study tested a total of 1732 bovines and found that 5.0% (87/1732) were Anaplasma IgG positive, 4.2% (73/1732) were Anaplasma IgM positive and 1.4% (24/1732) were both IgG and IgM positive, indicating that bovines are among those animals that can develop acute infections and middle and late infections in this plateau area. Moreover, sheep and goats can become infected with obligate intracellular bacteria of the genera Anaplasma, A. phagocytophilum and A. ovis, leading to the development of ovine and caprine anaplasmosis [18,28,32]. The present results showed not only a high IgG-positive rate (25.8%) but also a 5.8% IgM antibody positivity in Tibetan sheep. These results are consistent with our previous studies showing that pathogens, including A. ovis, A. bovis, A. capra, and A. phagocytophilum, were molecularly detected in yak and Tibetan sheep blood DNA samples at current sampling sites [10,11].
Tick-borne diseases in horses and donkeys mainly include equine piroplasmosis caused by Babesia caballi and Theileria equi, Lyme borreliosis caused by Borrelia burgdorferi, and equine granulocytic anaplasmosis caused by A. phagocytophilum, which are widely reported in countries across the world [7,15,16]. However, limited research has been found on the analysis of equine granulocytic anaplasmosis in the equines of China [15,16]. Horses and donkeys from areas in which A. phagocytophilum is endemic have a high seroprevalence of antibodies against A. phagocytophilum [7]. However, the difference is that the current study found a high Anaplasma spp. IgG positive rate (82.5%) only in donkeys, with low IgG positivity (6.9%) in horses, while IgM antibodies representing acute infection were detected in horses (6.9%) in this study. To analyze these findings, two points need to be considered: (a) equine granulocytic ehrlichiosis is a seasonal vector-borne disease closely associated with the activity of ticks from mid-spring to the end of summer, consistent with current sampling time points, and antibody positivity corresponds to the prevailing distribution of ticks of Ixodes genus; (b) A. phagocytophilum was detected in Ixodes ticks throughout China [37,38], including in the Qinghai–Tibet Plateau [35].
Furthermore, canine anaplasmosis caused by A. phagocytophium and A. platys is a vector-borne disease transmitted mainly via ticks, and has been extensively studied worldwide, including in China, by using molecular biology detection methods and through analysis of serological antibodies [13,39,40,41]. This study detected both Anaplasma spp. IgG and IgM antibodies in stray dogs and found that out of 226 dogs, 103 or 68 were positive for IgG or IgM antibodies, respectively, and 60 dogs were positive for both IgG and IgM antibodies. The results were significantly higher than the 10.1% average IgG positivity for dogs across ten provinces of China [40], which may be due to the dog samples in the current study being from stray dogs that are more likely to exposed to ticks. Moreover, cases of A. phagocytophilum infection in cats have been detected by molecular assays and reported in many countries [14]. The current results revealed that 3 of 127 cats were positive for Anaplasma IgG or IgM antibodies. This is the first report of Anaplasma infection in cats in China. Cats have been found to show a lower number of A. phagocytophilum infections in comparison with dogs [14], consistent with our results. In addition, our study investigated Anaplasma antibody levels in serum samples from pigs and chickens, which are two animal types for which there are few reports of Anaplasma infection or even detection of antibody levels. Although molecular tests in past studies may have identified unrecognized infections in wild boars and birds [17,42], our results detected only IgG antibodies and no IgM positivity in pigs and chickens of the QTPA.
The results of the current study revealed the higher Anaplasma IgG and IgM positive rates for yaks and higher IgG positive rates for Tibetan sheep at 3000–4000 m altitudes than at 2000–3000 m altitudes, while there were higher IgM positive rates for Tibetan sheep and higher IgG and IgM positive rates for horses at 2000–3000 m altitudes than at 3000–4000 m. This may be consistent with our unpublished investigations, which have shown that altitude did not significantly affect the distribution of ticks in the altitude region of 2000–5000 m in the Qinghai–Tibet Plateau, China. However, the distribution of ticks was related to season and humidity. The distribution of ticks significantly affects the prevalence of tick-borne pathogens in humans and animals.
5. Conclusions
The results of this study showed the positive rates of IgG and IgM antibodies in the study animals. Our study first demonstrated that yaks, cows, cattle, Tibetan sheep, horses, donkeys, stray dogs, stray cats, pigs, and chickens living at different altitudes on the Qinghai–Tibet Plateau were animals susceptible to Anaplasma spp. infection, indicating that there is a threat of tick bites and zoonotic pathogen infection in the vicinity of human and animal activities in the tested areas. These findings suggest the importance and urgency of preventing tick bites. However, the investigated IgG and IgM antibodies may vary according to several factors, such as the time of exposure, the pathogens or strains of Anaplasma spp. involved, and the infection status of the animals. Future studies on the interactions among vectors, animals, and pathogens utilizing molecular and serological analyses are recommended to fully elucidate the dynamics of Anaplasma spp. and other tick-borne pathogens in this plateau area.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12192723/s1, Table S1: Sampling sites in the study, Figure S1: The original results of the recombinant MSP5 protein of Anaplasma was expressed.
Author Contributions
Conceptualization, Y.S.; Data curation, J.Z. and H.M.; Formal analysis, J.A. and T.Q.; Funding acquisition, Y.S.; Investigation, J.Z., H.M., J.A. and T.Q.; Methodology, J.Z., H.M., J.A. and T.Q.; Project administration, Y.S.; Writing—original draft, J.L.; Writing—review and editing, Y.S., M.K. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Natural Science Foundation of Qinghai Province of China (grant numbers 2022-ZJ-940Q).
Institutional Review Board Statement
All procedures were carried out according to the ethical guidelines of Qinghai University. The animal study protocol was approved by the Ethics Committee of Qinghai University at 17-March-2022 (License number: SL-2022007).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
We declare that we have no competing interest.
References
- Anderson, J.F.; Magnarelli, L.A. Biology of ticks. Infect. Dis. Clin. North. Am. 2008, 22, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G. Tick-borne infections of animals and humans: A common ground. Int. J. Parasitol. 2014, 44, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Lagunova, E.K.; Liapunova, N.A.; Tuul, D.; Otgonsuren, G.; Nomin, D.; Erdenebat, N.; Abmed, D.; Danchinova, G.A.; Sato, K.; Kawabata, H.; et al. Co-infections with multiple pathogens in natural populations of Ixodes persulcatus ticks in Mongolia. Parasit. Vectors 2022, 15, 236. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.; Tkachev, S.; Tikunova, N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect. Genet. Evol. 2021, 91, 104833. [Google Scholar] [CrossRef]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef]
- Wang, J.; Kelly, P.; Zhang, J.; Shi, Z.; Song, C.; Zheng, X.; Zhang, Y.; Hao, Y.; Dong, H.; El-Mahallawy, H.S.; et al. Detection of Dirofilaria immitis antigen and antibodies against Anaplasma phagocytophilum, Borrelia burgdorferi and Ehrlichia canis in dogs from ten provinces of China. Acta. Parasitol. 2018, 63, 412–415. [Google Scholar] [CrossRef]
- Laamari, A.; Azzag, N.; Tennah, S.; Derdour, S.Y.; China, B.; Bouabdallah, R.; Ghalmi, F. Seroprevalence of Antibodies Against Anaplasma phagocytophilum and Borrelia burgdorferi in Horses (Equus caballus) from Northern Algeria. J. Vet. Res. 2020, 64, 413–419. [Google Scholar] [CrossRef]
- Selim, A.; Attia, K.A.; Alsubki, R.A.; Albohairy, F.; Kimiko, I.; Said, M.B. The first study on the seroprevalence of Anaplasma spp. in small ruminants and assessment of associated risk factors in North Egypt. Vet. World 2022, 15, 1221–1227. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, C.; Gong, P.; Pei, Z.; Peng, Y.; Jian, F.; Wang, R.; Zhang, L.; Qi, M.; Ning, C. Molecular detection and phylogeny of Anaplasma spp. closely related to Anaplasma phagocytophilum in small ruminants from China. Ticks. Tick. Borne. Dis. 2022, 13, 101992. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Ma, P.; Wei, Y.; Li, R.; Chen, Z.; Tian, S.; Qi, T.; Yang, J.; Sun, Y.; et al. Molecular detection of Anaplasma spp., Babesia spp. and Theileria spp. in yaks (Bos grunniens) and Tibetan sheep (Ovis aries) on the Qinghai-Tibetan Plateau, China. Parasit. Vectors 2021, 1, 613. [Google Scholar] [CrossRef]
- Li, J.; Jian, Y.; Jia, L.; Galon, E.M.; Benedicto, B.; Wang, G.; Cai, Q.; Liu, M.; Li, Y.; Ji, S.; et al. Molecular characterization of tick-borne bacteria and protozoans in yaks (Bos grunniens), Tibetan sheep (Ovis aries) and Bactrian camels (Camelus bactrianus) in the Qinghai-Tibetan Plateau Area, China. Ticks. Tick. Borne. Dis. 2020, 11, 101466. [Google Scholar] [CrossRef]
- Qi, Y.; Ai, L.; Zhu, C.; Lu, Y.; Lv, R.; Mao, Y.; Lu, N.; Tan, W. Co-existence of Multiple Anaplasma Species and variants in ticks feeding on hedgehogs or cattle poses potential threats of anaplasmosis to humans and livestock in Eastern China. Front. Microbiol. 2022, 13, 913650. [Google Scholar] [CrossRef]
- Elhamiani Khatat, S.; Daminet, S.; Kachani, M.; Leutenegger, C.M.; Duchateau, L.; El Amri, H.; Hing, M.; Azrib, R.; Sahibi, H. Anaplasma spp. in dogs and owners in north-western Morocco. Parasit. Vectors 2017, 10, 202. [Google Scholar] [CrossRef]
- Schäfer, I.; Kohn, B. Anaplasma phagocytophilum infection in cats: A literature review to raise clinical awareness. J. Feline. Med. Surg. 2020, 22, 428–441. [Google Scholar] [CrossRef]
- Saleem, S.; Ijaz, M.; Farooqi, S.H.; Ghaffar, A.; Ali, A.; Iqbal, K.; Mehmood, K.; Zhang, H. Equine Granulocytic Anaplasmosis 28 years later. Microb. Pathog. 2018, 119, 1–8. [Google Scholar] [CrossRef]
- Dzięgiel, B.; Adaszek, Ł.; Kalinowski, M.; Winiarczyk, S. Equine granulocytic anaplasmosis. Res. Vet. Sci. 2013, 95, 316–320. [Google Scholar] [CrossRef]
- Myczka, A.W.; Szewczyk, T.; Laskowski, Z. The Occurrence of Zoonotic Anaplasma phagocytophilum Strains, in the Spleen and Liver of Wild Boars from North-West and Central Parts of Poland. Acta. Parasitol. 2021, 66, 1082–1085. [Google Scholar] [CrossRef]
- Mason, K.L.; Gonzalez, M.V.; Chung, C.; Mousel, M.R.; White, S.N.; Taylor, J.B.; Scoles, G.A. Validation of an improved Anaplasma antibody competitive ELISA for detection of Anaplasma ovis antibody in domestic sheep. J. Vet. Diagn. Investig. 2017, 29, 763–766. [Google Scholar] [CrossRef]
- Bisen, S.; Aftab, A.; Jeeva, K.; Silamparasan, M.; Yadav, S.; Chandra, D.; Sankar, M.; Garg, R.; Raina, O.K. Molecular and serological detection of Anaplasma infection in carrier cattle in north India. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100550. [Google Scholar] [CrossRef]
- Noaman, V.; Sazmand, A. Anaplasma ovis infection in sheep from Iran: Molecular prevalence, associated risk factors, and spatial clustering. Trop. Anim. Health Prod. 2021, 54, 6. [Google Scholar] [CrossRef]
- Iqbal, N.; Mukhtar, M.U.; Yang, J.; Sajid, M.S.; Niu, Q.; Guan, G.; Liu, Z.; Yin, H. First Molecular Evidence of Anaplasma bovis and Anaplasma phagocytophilum in Bovine from Central Punjab, Pakistan. Pathogens 2019, 8, 155. [Google Scholar] [CrossRef]
- Rajput, Z.I.; Hu, S.H.; Arijo, A.G.; Habib, M.; Khalid, M. Comparative study of Anaplasma parasites in tick carrying buffaloes and cattle. J. Zhejiang Univ. Sci. B 2005, 6, 1057–1062. [Google Scholar] [CrossRef]
- Ismail, N.; McBride, J.W. Tick-Borne Emerging Infections: Ehrlichiosis and Anaplasmosis. Clin. Lab. Med. 2017, 37, 317–340. [Google Scholar] [CrossRef]
- Ndung’u, L.W.; Aguirre, C.; Rurangirwa, F.R.; McElwain, T.F.; McGuire, T.C.; Knowles, D.P.; Palmer, G.H. Detection of Anaplasma ovis infection in goats by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1995, 33, 675–679. [Google Scholar] [CrossRef]
- Molloy, J.B.; Bowles, P.M.; Knowles, D.P.; McElwain, T.F.; Bock, R.E.; Kingston, T.G.; Blight, G.W.; Dalgliesh, R.J. Comparison of a competitive inhibition ELISA and the card agglutination test for detection of antibodies to Anaplasma marginale and Anaplasma centrale in cattle. Aust. Vet J. 1999, 77, 245–249. [Google Scholar] [CrossRef]
- Aubry, P.; Geale, D.W. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef]
- de Echaide, S.T.; Knowles, D.P.; McGuire, T.C.; Palmer, G.H.; Suarez, C.E.; McElwain, T.F. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5. J. Clin. Microbiol. 1998, 36, 777–782. [Google Scholar] [CrossRef]
- Dreher, U.M.; de la Fuente, J.; Hofmann-Lehmann, R.; Meli, M.L.; Pusterla, N.; Kocan, K.M.; Woldehiwet, Z.; Braun, U.; Regula, G.; Staerk, K.D.; et al. Serologic cross-reactivity between Anaplasma marginale and Anaplasma phagocytophilum. Clin. Diagn. Lab. Immunol. 2005, 12, 1177–1183. [Google Scholar] [CrossRef][Green Version]
- Alleman, A.R.; Barbet, A.F.; Sorenson, H.L.; Strik, N.I.; Wamsley, H.L.; Wong, S.J.; Chandrashaker, R.; Gaschen, F.P.; Luckshander, N.; Bjöersdorff, A. Cloning and expression of the gene encoding the major surface protein 5 (MSP5) of Anaplasma phagocytophilum and potential application for serodiagnosis. Vet. Clin. Pathol. 2006, 35, 418–425. [Google Scholar] [CrossRef]
- Primo, M.E.; Thompson, C.S.; Valentini, B.S.; Sarli, M.; Novoa, M.B.; Mangold, A.J.; de Echaide, S.T. Development of a novel fusion protein with Anaplasma marginale and A. centrale MSP5 improved performance of Anaplasma antibody detection by cELISA in infected and vaccinated cattle. PLoS ONE. 2019, 14, e0211149. [Google Scholar] [CrossRef]
- Sarangi, L.N.; Rana, S.K.; Prasad, A.; Ponnanna, N.M.; Sharma, G.K. Prevalence of antibodies to Anaplasma in cattle and buffaloes of different organized herds in India. J. Parasit. Dis. 2021, 45, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Rubel, W.; Schoneberg, C.; Wolf, A.; Ganter, M.; Bauer, B.U. Seroprevalence and risk factors of Anaplasma spp. in German Small Ruminant Flocks. Animals 2021, 11, 2793. [Google Scholar] [CrossRef] [PubMed]
- Sarli, M.; Thompson, C.S.; Novoa, M.B.; Valentini, B.S.; Mastropaolo, M.; Echaide, I.E.; de Echaide, S.T.; Primo, M.E. Development and evaluation of a double-antigen sandwich ELISA to identify Anaplasma marginale-infected and A. centrale-vaccinated cattle. J. Vet. Diagn. Invest. 2020, 32, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet. Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Han, R.; Yang, J.F.; Mukhtar, M.U.; Chen, Z.; Niu, Q.L.; Lin, Y.Q.; Liu, G.Y.; Luo, J.X.; Yin, H.; Liu, Z.J. Molecular detection of Anaplasma infections in ixodid ticks from the Qinghai-Tibet Plateau. Infect. Dis. Poverty. 2019, 8, 12. [Google Scholar] [CrossRef]
- de Echaide, S.T.; Bono, M.F.; Lugaresi, C.; Aguirre, N.; Mangold, A.; Moretta, R.; Farber, M.; Mondillo, C. Detection of antibodies against Anaplasma marginale in milk using a recombinant MSP5 indirect ELISA. Vet. Microbiol. 2005, 106, 287–292. [Google Scholar] [CrossRef]
- Cao, W.C.; Zhan, L.; He, J.; Foley, J.E.; DE Vlas, S.J.; Wu, X.M.; Yang, H.; Richardus, J.H.; Habbema, J.D. Natural Anaplasma phagocytophilum infection of ticks and rodents from a forest area of Jilin Province, China. Am. J. Trop. Med. Hyg. 2006, 75, 664–668. [Google Scholar] [CrossRef]
- Jiang, J.F.; Jiang, B.G.; Yu, J.H.; Zhang, W.Y.; Gao, H.W.; Zhan, L.; Sun, Y.; Zhang, X.A.; Zhang, P.H.; Liu, W.; et al. Anaplasma phagocytophilum infection in ticks, China-Russia border. Emerg. Infect. Dis. 2011, 17, 932–934. [Google Scholar] [CrossRef]
- Cazan, C.D.; Ionică, A.M.; Matei, I.A.; D’Amico, G.; Muñoz, C.; Berriatua, E.; Dumitrache, M.O. Detection of Leishmania infantum DNA and antibodies against Anaplasma spp., Borrelia burgdorferi s.l. and Ehrlichia canis in a dog kennel in South-Central Romania. Acta. Vet. Scand. 2020, 62, 42. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Xu, B.; Lu, Q.; Li, L.; Chang, L.; Zhang, X.; Fan, D.; Li, G.; Jin, Y.; et al. Anaplasma phagocytophilum infection in domestic animals in ten provinces/cities of China. Am. J. Trop. Med. Hyg. 2012, 87, 185–189. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, D.; Mao, J.; Zhang, Z.; Yu, J. The occurrence of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis and Anaplasma phagocytophilum in dogs in China. J. Helminthol. 2012, 86, 185–189. [Google Scholar] [CrossRef]
- Daniels, T.J.; Battaly, G.R.; Liveris, D.; Falco, R.C.; Schwartz, I. Avian reservoirs of the agent of human granulocytic ehrlichiosis? Emerg. Infect. Dis. 2002, 8, 1524–1525. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).