Simple Summary
This study investigated the effects of progesterone treatment in vitro on apoptosis and steroidogenesis in porcine placental trophoblasts and the underlying molecular mechanisms. Trophoblasts were treated with different concentrations of progesterone for 48 h. Cell counts, steroidogenesis, and relevant gene and protein expression levels were measured. Progesterone inhibited trophoblast proliferation in a dose-dependent manner. High doses of progesterone significantly altered the expression levels of apoptosis-related and steroidogenesis-related genes and proteins, while low doses had a less pronounced effect. Thus, increased progesterone induces the apoptosis of porcine placental trophoblasts and induces abnormal steroidogenesis in the placenta. We believe that our study makes a significant contribution to the literature because it elucidates the effects of progesterone on porcine placental trophoblast functions.
Abstract
Placentation and placental steroidogenesis are important for pregnancy and maternal–fetal health. As pregnancy progresses, the main site of progesterone (P4) synthesis changes from the corpus luteum to the placenta, in which placental trophoblasts are the main cell type for P4 synthesis. Therefore, this study investigated the effects of P4 on apoptosis and steroidogenesis in porcine placental trophoblasts and the underlying molecular mechanisms. Porcine placental trophoblasts were treated with different concentrations of P4 for 48 h in a serum-free medium in vitro. Cell number, steroidogenesis, and relevant gene and protein expression levels were detected. A high dose of P4 (10.0 μM) significantly increased P4 (p < 0.01), androstenedione (p < 0.05), testosterone (p < 0.05), and estradiol (p < 0.05) production in porcine placental trophoblasts compared with that in control cells, while a low dose of P4 (1 × 10−3 μΜ) had no marked impact on steroid production. The mRNA expression of apoptosis-related genes (CASP3, CASP8, and Bax) (p < 0.05) and steroidogenesis-related genes (CYP11A1, CYP19A1, and StAR) (p < 0.01) was upregulated, and the expression of HSD3B and HSD17B4 was inhibited (p < 0.05) in the porcine placental trophoblasts treated with high doses of P4. Low doses of P4 had a lighter effect on gene expression than high doses. The expression of apoptosis-related proteins CASP3 (p < 0.05), and Bax (p < 0.01) and steroidogenesis-related proteins CYP19A1 (p < 0.05) and StAR (p < 0.01) was raised, but the proliferation-related protein CCND2 (p < 0.01) was downregulated in the pTr cells treated with high dose of P4. In comparison, a low dose of P4 inhibited the expression of Bax, CYP11A1 (all p < 0.01), and CCND2 (p < 0.05), but the expression of CASP3 (p < 0.05) and StAR (p < 0.01) was upregulated. In summary, excessive P4 can induce the apoptosis of porcine placental trophoblasts and lead to abnormal steroidogenesis in the placenta and hormone imbalance.
1. Introduction
Placental steroid hormones (progestogen, estrogen, testosterone, androgen, etc.) are mainly secreted by placental trophoblasts and are important biomarkers of pregnancy-associated diseases. They have a pivotal role in the maintenance of pregnancy, maternal adaptation to pregnancy, and fetal development [1,2]. Porcine placental trophoblast (pTr) cells are the chief cell types of the porcine placenta that perform endocrine functions and are an ideal model for studying the placental function and cell proliferation, migration, invasiveness, and steroid synthesis [3,4,5,6]. In early pregnancy, pTr cells adhere closely to maternal endometrial epithelial cells, forming the only bridge for maternal–fetal exchange [7,8]. Many nutrient transporters that affect placental nutrient transport efficiency are expressed in pTr cells and are regulated by maternal hormones, growth factors, and cytokines, suggesting that hormone levels may be related to placental nutrient transfer functions [9,10]. The structure of placental trophoblasts varies according to species and pregnancy period. Porcine placental trophoblasts consist of mononuclear trophoblast cells throughout pregnancy with no invasive capability, and only pigs and whales exhibit this feature [2,8]. Although the placental structures of interspecies are diverse, there are many common elements, especially those related to steroidogenesis and steroid hormone metabolism [11].
Progesterone is critical for the regulation of embryonic implantation [12] and promotes endometrial stromal differentiation, glandular secretion, and placentation during pregnancy [13]. With increasing gestational age, the placenta gradually replaces the ovary to become the largest organ for P4 secretion [14]. Except for humans, other mammals (mice, etc.) have higher progesterone levels in the early stages of fetal development. It is known that progesterone levels begin to decrease gradually in the middle and late stages of pregnancy [15,16,17]. However, there are reports that fetal blood P4 levels were independent of fetal age [18]. P4 can effectively maintain the growth and development of embryos, but excessive or insufficient P4 may lead to adverse pregnancy outcomes, such as threatened abortion [19]. P4 supplementation in early pregnancy is widely used to avoid abortion and prevent premature delivery, but changes in maternal P4 can also affect fetal P4 levels. For example, the application of exogenous P4 to the mother can significantly increase fetal serum P4 levels. Studies have found that P4 can promote uterine and placental angiogenesis, and when upregulated by trophoblast cells, it can express placental growth factor (PGIF), which is homologous to VEGF [15,20,21]. The secretory endometrium is vascular and glandular, and a lack of P4 can inhibit the transition of the endometrium to the secretory state [22]. P4 regulates the function of the exosomes derived from trophoblast cells to control intimal receptivity, ensuring the normal development of embryos during pregnancy [23]. P4 stimulates endometrial biosynthesis and promotes pregnancy via paracrine effects [24,25]. Endometrial stromal cells have been reported to increase the inhibitory effect of P4 on the proliferation of endometrial cancer cells through paracrine signal transduction; however, the mechanism is unclear [26]. The various biological effects of P4 are mainly mediated by the interaction between P4 and its receptor (PGR) [27], which promotes endometrial decidualization and maintains pregnancy mainly through its endocrine and immune functions [28]. Lissauer et al. [29] showed that P4 at a concentration of 10 μM had a more significant response to immune effects and inhibited the proliferation of human maternal T cells. The maternal immune system recognizes fetal antigens at the decidual–trophoblast interface [30], but the effect of P4 on pTr cell function remains unclear.
Therefore, this study intended to (1) assess the effects of the different concentrations of P4 on apoptosis and steroidogenesis in pTr cells and (2) assess the effect of P4 on relevant gene and protein expressions in pTr cells.
2. Materials and Methods
2.1. Ethics Statement
Living animals were not used in this study; therefore, ethical approval was not required.
2.2. Reagents
The following consumables were used in the cell culture: P4 and penicillin/streptomycin were obtained from Sigma-Aldrich (Burlington, MA, USA), Dulbecco’s modified Eagle’s medium (DMEM)/F-12, fetal bovine serum (FBS), and a 0.25% trypsin solution were obtained from Thermo Fisher Scientific (Waltham, MA, USA), and insulin–transferrin–selenium (ITS, 100×) was obtained from ScienCell (Carlsbad, CA, USA).
2.3. Cell Culture
Immortalized pTr cells (stem from Texas A&M University (College Station, TX, USA)) were grown in a DMEM/F-12 medium, with minor modifications, as previously reported [31]. The cells were cultured in dishes (d = 10 cm; Corning Inc., Corning, NY, USA) using 10 mL of DMEM/F-12 supplemented with FBS (10%), penicillin/streptomycin (1%), and ITS (1%) [8,32]. The medium was substituted every 48 h. When the confluence of cells reached approximately 80%, cells were collected in a 0.25% trypsin solution. After cell counting, an average of 1.0 × 106 cells were implanted into cell culture plates (12-well; Corning) with 2 mL of the medium (containing FBS, penicillin/streptomycin, and ITS) and cultured in an environment of 5% CO2 and 95% air at 37 °C for the first 48 h, with a medium change at 24 h. Then, according to the specific experiment, they were washed twice with a 1 mL serum-free medium (containing penicillin/streptomycin and ITS) and were treated in that serum-free medium for 48 h.
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) and Cell Counting
After treatment, ELISA kits (Jinenlai Biotech, Beijing, China) were used to detect the concentrations of four placental steroid hormones: P4 (GEL4686), androstenedione (A4, F8259), testosterone (T, GEL4562), and estradiol (E2; GEL4632). The sensitivities of the ELISA kit for P4, A4, T, and E2 were 80 pM, 30 pg/mL, 6 nM, and 8 pM, respectively. The average intra-assay coefficient of variation was 8.6–9.9%, and the average inter-assay coefficient was 9.4–11.1%.
For cell counting, the medium was gently removed from the wells, and the cells were rinsed with PBS, digested with trypsin, collected, and counted with an automatic cell counter (TZ20TM; Bio-Rad, Hercules, CA, USA) to count as previously described [33].
2.5. RNA Extraction and Quantitative Reverse-Transcriptase PCR (RT-qPCR)
The total RNA was extracted from cells using an RNAzol® RT reagent (1 mL/sample; Molecular Research Center, Inc., Cincinnati, OH, USA). The RNA was dissolved in DEPC-treated water (Tiangen Biotech, Beijing, China), quantified using an Eppendorf BioSpectrometer Kinetic (Eppendorf, Hamburg, Germany) at 260 nm, diluted to 300 ng/μL, and stored at −80 °C.
The primers for the amplification of cell-proliferation-related genes (CCND1, CCND2, Bax, and CDK4), apoptosis-related genes (CASP3, CASP8, and Bcl−2), steroidogenesis-related genes (CYP11A1, HSD17B4, HSD3B, StAR, and CYP19A1), and PGR were designed or retrieved according to the gene sequence of pigs. Primer sequences and estimated amplified fragments are listed in Table 1.
The total RNA extracted was reverse-transcribed into cDNA in a 10 μL reaction system by using a Script cDNA Synthesis Kit (Bio-Rad), and then the first-strand cDNA was directly used for RT-qPCR or stored at −80 °C [34].
RT-qPCR was conducted using a CFX96 Touch Real-Time PCR detection system (Bio-Rad) containing iTaqTM Universal SYBR® Green SuperMix (Bio-Rad) in a 10 µL reaction system [35]. The PCR procedure was the same as previously described [32]. Additionally, two negative controls, namely a no-template control and a no-reverse-transcriptase control, were included to confirm the absence of contaminants in the master mix and DNA contamination in RNA, respectively [34]. The relative abundance of the target mRNA transcripts was calculated as 2−∆∆Ct using the comparative threshold cycling method, normalized to GAPDH ribosomal RNA levels as previously described [34,35].


Table 1.
Primers for target genes of pTr cells.
Table 1.
Primers for target genes of pTr cells.
| Gene | Sequence of Primers (5′ to 3′) | Fragment (bp) | GenBank ID | Reference |
|---|---|---|---|---|
| CCND1 | F: GACCGCTTCCTGTCCCTGR: GTGGCACAGAGGGCGACGA | 317 | XM_021082686 | [8] |
| CCND2 | F: CGTCCAAGCTCAAAGAGACCR: CGAAGAATGTGCTCGATGAA | 169 | NM_214088 | [36] |
| CDK4 | F: GCATCCCAATGTTGTCCGR: GGGGTGCCTTGTCCAGATA | 125 | NM_001123097 | [8] |
| CASP8 | F: TCCTGAGCCTGGACTACATR: CTCCTCCTCATTGGTTTCC | 185 | NM_001031779.2 | [8] |
| CASP3 | F: GCCATGGTGAAGAAGGAAAAR: GTCCGTCTCAATCCCACAGT | 167 | NM_214131 | [8] |
| StAR | F: GGAAAAGACACAGTCATCACCCATR: CAGCCAGCACACACACGGAAC | 121 | NM_213755.2 | [8] |
| HSD17B4 | F: TGCCATGAGAGTTGTGAGGAAAR: CCTCAGGAGTCATTGGCTGATT | 127 | XM_021081514.1 | [8] |
| HSD3B | F: TCCACACCAGCAGCATAGAGR: ATACATGGGCCTCAGAGCAC | 206 | NM_001004049.2 | [8] |
| CYP19A1 | F: GTATATCGCCATGGTCATGR: AGCAGGCCGCTGGTCTCAT | 144 | NM_214429.1 | [8] |
| CYP11A1 | F: GCCGCATGGGACACTATTTTR: ATTTCCCAGGAGGCGGTAGA | 120 | NM_214427.1 | [8] |
| Bax | F: AAGCGCATTGGAGATGAACTR: CGATCTCGAAGGAAGTCCAG | 251 | XM_003127290.5 | [37] |
| Bcl–2 | F: TGTGTGGAGAGCGTCAACCGR: CCCATACAGCTCCACAAAGGCAT | 138 | XM_021099593.1 | [38] |
| PGR | F: GATTCAGAAGCCAGCCAGAGR: GATGCTTCATCCCCACAGAT | 83 | GQ903679 | [39] |
| GAPDH | F: AAGGAGTAAGAGCCCCTGGAR: TCTGGGATGGAAACTGGAA | 140 | NM_001206359.1 | [8] |
2.6. Western Blot Analysis
After treatment, the proteins were obtained according to cell lysate instructions (RIPA, R1091, Lablead, Beijing, China), and protein concentrations were determined using a BCA protein assay kit (B5000, Lablead, Beijing, China). Equal amounts of protein extracts (25 μg) were separated on SDS–PAGE gels and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were sealed in a blocking solution (P0252, Beyotime, Shanghai, China) for 30 min at room temperature and then incubated with the indicted primary antibodies at 4 °C, and the antibodies used in Western blot experiments are listed in Table 2. Then, the membranes were hatched with secondary antibodies (diluted with the blocking solution for 1:5000) for 1 h at room temperature. GAPDH or β-actin was used as the internal reference. The protein bands were developed using a gel imaging system (Bio-Rad) and quantitative analysis using ImageJ (NIH, USA).

Table 2.
Primary antibodies used in Western blot experiments.
2.7. Experimental Design
Experiment 1 was designed to evaluate the effects of the different concentrations of P4 on the cell proliferation of pTr cells and the production of steroid hormones (P4, A4, T, and E2). The cells were cultured in a 10% fetal bovine serum medium for 48 h and washed twice with a 1 mL serum-free medium, and one of the following treatments was applied: 0.1 × 10−6 μM, 1 × 10−5 μM, 1 × 10−4 μM, 1 × 10−3 μM, 1 × 10−2 μM, 0.1 μM, or 10 μM of P4 for 48 h [8,32,40]. The cells were counted when the treatments were finished. The supernatants were collected to detect steroidogenesis. Based on their cell number, pTr cells were treated with a high dose (10 μM) or low dose (1 × 10−3 μM) of P4 for the effect of P4 on gene and protein expression in the pTr cells.
In Experiment 2, we intended to study the effects of P4 on pTr-cell-proliferation-related (CCND1, CCND2, Bax, and CDK4), apoptosis-related (CASP3, CASP8, and Bcl−2), steroid-synthesis-related (CYP11A1, CYP19A1, HSD17B4, HSD3B, and StAR), and PGR genes and relevant proteins. The cell culture was as described in Experiment 1; the cells were treated with either 1 × 10−3 μM or 10 μM P4 for 48 h, lysed, and subjected to RNA and protein extraction. The control group was treated with the same equivalence of the serum-free medium as the experimental group.
2.8. Statistical Analysis
After treatment, P4, A4, T, and E2 production in the cell medium were expressed as pM/mL, pg/mL, nM/mL, and pM/mL per 105 cells, respectively; these values were calculated as the number of cells at the end of treatment. Each experiment used three different pTr wells as experimental replicates, and each treatment was repeated three times. If the data did not distribute to a normal population, the log-transformation data were used for statistical analyses. The treatment effects on dependent variables (cell number, hormone production, target gene relative mRNA transcript abundance, and protein expression) were statistically analyzed using SPSS 25.0. The treatment effects were analyzed using ANOVA, and the data are shown as the mean ± SEM. Statistical significance was set at p < 0.05 and p < 0.01. All bar charts were produced using GraphPad Prism 8.0.2.
3. Results
3.1. Effect of P4 on Proliferation of pTr Cells
P4 inhibited pTr survival in a dose−dependent manner (Figure 1). After 48 h of treatment, the survival rate decreased as the P4 treatment concentration increased. The number of pTr cells decreased significantly when the P4 concentration exceeded 1 × 10−3 μM (p < 0.05). The cell counts decreased by 12.3%, 15.4%, 16.7%, and 21.0% at 1 × 10−3 μM, 0.01 μM, 0.1 μM, and 1 μM (p < 0.05), respectively, and decreased by 56.5% at 10.0 μM (p < 0.01), respectively. According to the observed changes in the cell, a P4 dose with a mild inhibitory effect (1 × 10−3 μM) was selected as the low dose, and 10.0 μM of P4 was selected as the high dose in the following experiments.
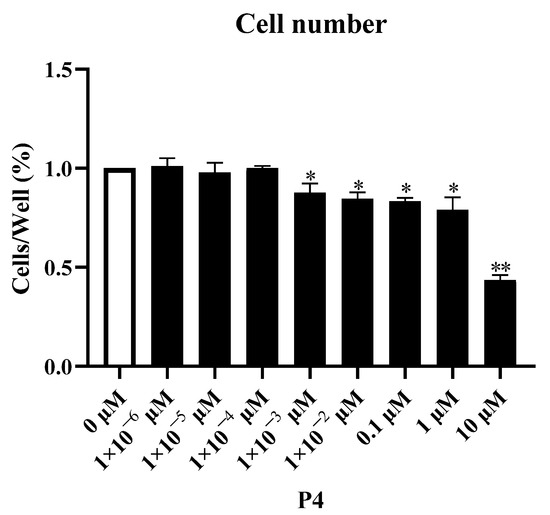
Figure 1.
Dose–response effect of P4 on porcine placental trophoblast cell numbers. The results represent the average ± SEM of three independent experiments; * indicates values significantly different from that of the control group (p < 0.05); ** indicates values significantly different from that of the control group (p < 0.01).
3.2. Effect of P4 on Steroid Hormone Synthesis in pTr Cells
P4 had a significant indigenous effect on steroidogenesis in pTr cells, especially at high doses (Figure 2). Compared with the control group (without P4) and treatment with a low dose of P4, the high dose (10.0 μM) of P4 promoted steroidogenesis in the pTr cells. The production of P4, A4, T, and E2 increased by 89% (p < 0.01), 434% (p < 0.05), 231% (p < 0.01), and 201% (p < 0.05), respectively. Low doses of P4 inhibited P4 production in pTr cells (p < 0.05) but had no significant effect on the synthesis of other hormones (p > 0.05).
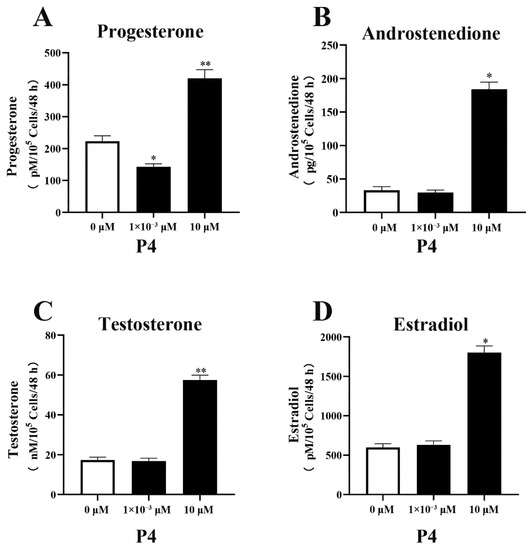
Figure 2.
Effect of P4 on P4 (A), A4 (B), T (C), and E2 (D) levels in pTr cells after 48 h treatment. The results represent the average ± SEM of three independent experiments; * indicates values significantly different from that of the control group (p < 0.05); ** indicates values significantly different from that of the control group (p < 0.01).
3.3. Effects of P4 on Gene Expression in pTr Cells
3.3.1. Cell-Proliferation-/Apoptosis-Related Gene Expression
The different concentrations of P4 had different effects on the expression of genes related to proliferation and apoptosis in pTr cells (Figure 3). The administration of P4 increased the expression of the apoptotic genes CASP3 and CASP8 (p < 0.05); only a high dose of P4 increased Bax expression, while a low dose of P4 inhibited Bax expression (p < 0.05). Regarding cell-proliferation-related genes, low doses of P4 inhibited CCND1 expression (p < 0.05), and high-dose P4 significantly inhibited CCND2 and Bcl−2 gene expression (p < 0.05) but had no significant effect on CCND1 expression (p > 0.05). P4 treatment had no significant effect on CDK4 expression (p > 0.05). High-dose P4 significantly increased the Bax/Bcl−2 ratio in pTr cells (p < 0.01).
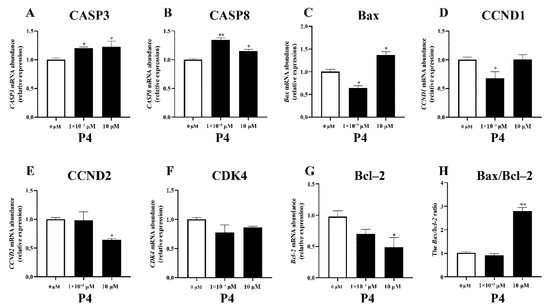
Figure 3.
Effects of P4 on mRNA transcription abundance of apoptosis-related genes (A–C), proliferation-related genes (D–G), and Bax/Bcl–2 ratio (H) in pTr cells treated with P4 for 48 h. The results represent the average ± SEM of three independent experiments; * indicates values significantly different from that of the control group (p < 0.05); ** indicates values significantly different from that of the control group (p < 0.01).
3.3.2. Expression of Steroidogenesis-Related Genes
The different concentrations of P4 had different effects on the expression of steroidogenesis-related genes in pTr cells (Figure 4). A high dose of P4 increased the expression of CYP11A1, CYP19A1, StAR (all p < 0.01), and PGR (p < 0.05) and inhibited the expression of HSD3B and HSD17B4 (p < 0.05). A low dose of P4 had no significant effect on gene expression, except on the CYP19A1 gene, suggesting that CYP19A1 gene expression may be sensitive to P4.
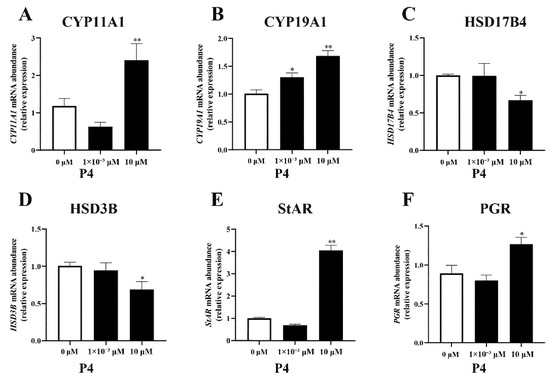
Figure 4.
Effect of P4 on mRNA abundance of steroidogenesis−related genes CYP11A1 (A), CYP19A1 (B), HSD17B4 (C), HSD3B (D), StAR (E), and PGR (F) mRNA transcripts in pTr cells after 48 h treatment. The results represent the average ± SEM of three independent experiments; * indicates values significantly different from that of the control group (p < 0.05); ** indicates values significantly different from that of the control group (p < 0.01).
3.4. Effects of P4 on Protein Expression in pTr Cells
The different concentrations of P4 had different effects on protein expression in pTr cells (Figure 5). A high dose of P4 enhanced the protein abundance of CASP3, CYP19A1 (all p < 0.05), and Bax, StAR (all p < 0.01) and inhibited CCND2 (p < 0.01). A low dose of P4 enhanced the protein of CASP3 (p < 0.05) and StAR (p < 0.01) but inhibited Bax, CYP11A1 (all p < 0.01), and CCND2 (p < 0.05).
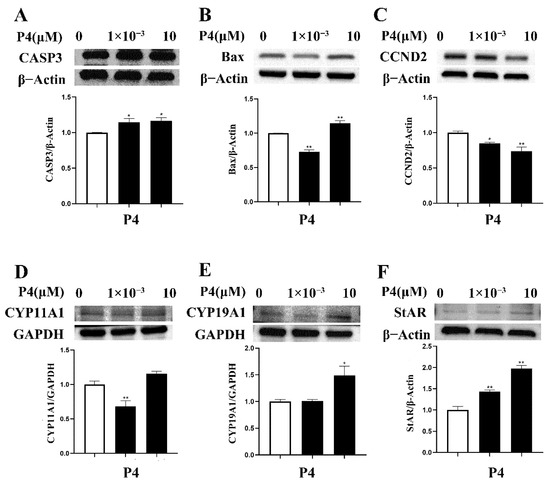
Figure 5.
Effect of P4 on protein abundance of CASP3 (A), Bax (B), CCND2 (C), CYP11A1 (D), CYP19A1 (E), and StAR (F) in pTr cells after 48 h treatment. The results represent the average ± SEM of three independent experiments; * indicates values significantly different from that of the control group (p < 0.05); ** indicates values significantly different from that of the control group (p < 0.01). Original Western blot bands and intensity ratio are shown in Figure S1 and Table S1.
4. Discussion
In this research, pTr cells were challenged with the different concentrations of P4 to preliminarily investigate the effect of P4 on cell proliferation as well as steroidogenesis. P4 concentration was selected based on the results of this study and from the published literature [41,42,43]. The results showed that a high dose of P4 induced apoptosis in pTr cells and upregulated the expression of steroid-hormone-synthesis-related genes, resulting in increased steroidogenesis in pTr cells in vitro.
CYP11A1 is the initial step in catalyzing steroid biosynthesis, and StAR facilitates cholesterol delivery from the adventitia to the intima [44]. StAR transports cholesterol to the mitochondria, where CYP11A1 converts it to pregnenolone, which synthesizes steroid hormones in response to the enzymes involved in steroid synthesis [45]. In this study, in order to explain how P4 regulates steroid synthesis in pTr cells, RT-qPCR was used to quantify the mRNA transcription abundance of CYP11A1, CYP19A1, HSD3B, HSD17B4, and StAR genes, and Western blot was used to quantify the protein expression of CYP11A1, CYP19A1, and StAR. The results indicated that a high dose of P4 may affect the synthesis of steroid hormones in pTr cells by changing the expression of the genes and proteins related to steroid synthesis. Our study found that after a high-dose P4 treatment, P4 levels in pTr cells were significantly upregulated, and the corresponding expression of StAR and CYP11A1 were also significant raised. Miao et al. [46] found that StAR expression was positively correlated with P4 levels in goat ovarian granulosa cells, which is consistent with our results in pTr cells. CYP11A1 converts cholesterol to P4 [47], suggesting that StAR and CYP11A1 may jointly regulate P4 production. As the short half-life of progesterone [48], the added progesterone could be ignored in the final detection results. Increased A4 and T production are related to the increased expression of StAR, CYP11A1, and HSD3B [49,50]; however, our study found that HSD3B expression significantly decreased after the high-dose P4 treatment, which is inconsistent with previous studies and requires further study. Pregnenolone is a precursor of several steroid hormones (P4, A4, T, etc.), which are produced through CYP11A1 transformation in the inner membrane of the mitochondria [45,51,52,53,54]. However, the CYP11A1 was not augmented with a high dose of P4, indicating that a high dose of P4 may cause increases in P4, T, and A4 synthesis by affecting the gene and protein expression of StAR. HSD17B4 is not only related to steroid synthesis but also proved to be a novel proliferation-promoting gene, whose overexpression or knockout can promote or inhibit, respectively, the proliferation of the human hepatocellular carcinoma cell line HepG2 [55]. Therefore, the inhibition of pTr proliferation by high doses of P4 may also be related to a decrease in HSD17B4 expression. CYP19A1 is a key enzyme in estrogen synthesis and is related to the mRNA expression of proliferation-related genes (CCND1 and CDK2). When CYP19A1 was knocked down, proliferation-related genes were upregulated [56], which provides another explanation for the inhibition of proliferation-related genes in pTr cells after P4 treatment. In addition, the placenta is the main site of estrogen production and the most active site for CYP19A1 expression [57]. Decreased estrogen levels are associated with downregulated CYP19A1 expression in mouse granulosa cells [58]. Our results showed that a high dose of P4 promoted E2 synthesis, possibly due to the upregulation of CYP19A1 gene expression. This is consistent with our research indicating that a high dose of P4 promoted E2 synthesis in pTr cells and upregulated the gene and protein expression of CYP19A1, suggesting that E2 synthesis is mainly related to the gene and protein expression of CYP19A1. During pregnancy, an appropriate increase in E2 contributes to placental vascular function, while excessive E2 secretion may lead to oxidative stress [59]. The imbalance of coordination between P4 and E2 may lead to inflammation and reduce the endometrial receptivity to the embryo. In early pregnancy, E2 induces endometrial epithelial proliferation, while P4 inhibits E2-induced proliferation [60]. Studies have reported that the decrease in P4 before delivery may be due to the transformation of P4 into E2 in the placenta, which may be related to species [28].
P4 inhibits proliferation and induces the apoptosis of breast cancer cells at relatively high physiological concentrations (approximately 10 μM) [40]. Studies have shown that P4 can inhibit the invasion of human trophoblast cells and reduce the number of invasive cells with the increase in P4 [61]. In addition, P4 inhibits the proliferation of endometrial cancer cells via a paracrine action [26]. Horita et al. [62] reported that P4 increased p53 gene expression and induced apoptosis in breast cancer cells. The apoptotic cascade may be triggered by both the extrinsic and intrinsic pathways. In the extrinsic pathway, apoptotic factors (tumor necrosis factor, etc.) bind to membrane receptors and activate CASP8 and CASP3 to induce DNA fragmentation and cell death. In the intrinsic pathway, CASP3 is activated by increasing the ratio of pro-apoptotic proteins (Bax) to anti-apoptotic proteins (Bcl−2) and finally induces apoptosis [63,64]. The results of the present study are consistent with these findings, indicating that a high dose of P4 may increase the Bax/Bcl−2 ratio by changing the balance between Bax and Bcl−2 and stimulate the upregulation of CASP3 and CASP8, resulting in the apoptosis of pTr cells. Formby and Wiley [43] found that P4 inhibited the proliferation of breast cancer cells in a dose-dependent manner by activating the apoptotic pathway (P53 was upregulated, and Bcl−2 was downregulated) in vitro. Cyclin D1 (CCND1) is a G1/S phase-specific cell cycle regulator that promotes cell proliferation by binding and activating CDK4 and CDK6 [8]. In early pregnancy, CCND1 and CDK4 regulate cell cycle progression and promote the cell cycle transition from G1 to S in pTr cells [65]. It has been found that in the process of placentation, if adverse pregnancy occurs, the expression of CCND1 is upregulated, such as in preeclampsia [66]. In this study, CASP3 and CASP8 gene expression significantly increased, CCND1 and CCND2 gene expression significantly decreased, and CDK4 did not show significant changes in the pTr cells treated with high doses of P4. It is speculated that a high dose of P4 inhibits cell cycle progression by reducing the expression of CCND1, suppressing its interaction with CDK4, and leading to cell death by increasing the expression of CASP3 and CASP8. In addition, P4 upregulated the expression levels of apoptosis-related proteins (CASP3 and Bax), inhibited the expression of proliferation-related protein (CCND2), suggesting that P4 induced the apoptosis of pTr cells probably because it upregulated the expression of apoptosis-related genes and proteins and downregulated the expression of proliferation-related genes and proteins.
There is a complex endocrine–paracrine–autocrine regulatory system in the placenta [67,68]. As pregnancy progresses, the placenta becomes the principal source of steroid hormone synthesis [8,32]. An imbalance of steroid hormones is related to many pregnancy complications, such as preeclampsia, caused by insufficient P4 [69]. A premature increase in P4 levels is related to abnormal implantation and decreased pregnancy rates [70,71,72,73]. Due to the paracrine effect of P4 on endometrial cells, the proliferation of endometrial cancer cells is inhibited [26,74]. P4 generally performs genomic and non-genomic functions by inducing PGR gene expression and activating its receptors [75]. This study detected that high doses of P4 upregulated the gene expression of PGR, suggesting that P4 binds to its receptor to regulate pTr cell function.
5. Conclusions
According to the results of the current study, a high dose of P4 can increase steroidogenesis through the upregulation of the expression of steroid-hormone-synthesis-related genes and proteins, thus inducing apoptosis in pTr cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12192704/s1, Figure S1: Effect of P4 on protein abundance of CASP3 (A), Bax (B), CCND2 (C), CYP11A1 (D), CYP19A1 (E), and StAR (F) in pTr cells after 48 h treatment. Table S1: The intensity ratio of bands in Western blot experiments.
Author Contributions
Y.L. (Yueshuai Liu): formal analysis, writing—review and editing. H.D.: data curation, visualization. Y.Y.: investigation, visualization. Y.L. (Yan Liu): supervision, writing—review and editing, funding acquisition. X.C.: investigation, visualization. T.F.: conceptualization, methodology, writing—review and editing, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the National Natural Science Foundation of China (31972575), the Beijing Municipal Natural Science Foundation (6202009), the Fundamental Research Funds for the Central Universities of Northwest Minzu University (31920220039), and the Research project of Beijing Academy of Agriculture and Forestry Sciences (CZZJ202205).
Institutional Review Board Statement
Live animals were not used in this study; therefore, ethical approval was not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cameo, P.; Bischof, P.; Calvo, J.C. Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol. Reprod. 2003, 68, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Chavatte-Palmer, P.; Tarrade, A. Placentation in different mammalian species. Ann. Endocrinol. 2016, 77, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Rielland, M.; Hue, I.; Renard, J.P.; Alice, J. Trophoblast stem cell derivation, cross-species comparison and use of nuclear transfer: New tools to study trophoblast growth and differentiation. Dev. Biol. 2008, 322, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, X.; Yin, Y.; Li, X.; Gao, H.; Bazer, F.W.; Wu, G. Putrescine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. Biol. Reprod. 2014, 91, 106. [Google Scholar] [CrossRef]
- Kim, J.; Song, G.; Wu, G.; Bazer, F.W. Functional roles of fructose. Proc. Natl. Acad. Sci. USA 2012, 109, E1619–E1628. [Google Scholar] [CrossRef]
- Guo, P. Effects of Arginine on Reproductive Performance in Gilts and the Proliferation of Porcine Placenta Trophectoderm Cells; Northeast Agricultural University: Harbin, China, 2017. (In Chinese) [Google Scholar]
- Suleman, M.; Malgarin, C.M.; Detmer, S.E.; Harding, J.; MacPhee, D.J. The porcine trophoblast cell line PTr2 is susceptible to porcine reproductive and respiratory syndrome virus-2 infection. Placenta 2019, 88, 44–51. [Google Scholar] [CrossRef]
- Wei, S.L.; Yang, Y.Z.; Xiao, Y.X.; Liu, Y.; Tian, J.H.; Spicer, L.J.; Feng, T. Effects of N-carbamylglutamate on steroidogenesis and relative abundances of mRNA transcripts in pig placental trophoblasts. Anim. Reprod. Sci. 2020, 221, 106569. [Google Scholar] [CrossRef]
- Wei, S.L.; Zheng, C.; Liu, Y.; Feng, T. Progress in research on the porcine placental barrier. Animal Husbandry & Veterinary Medicine. 2021, 53, 148–154. (In Chinese) [Google Scholar]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental responses to changes in the maternal environment determine fetal growth. Front Physiol. 2016, 7, 12. [Google Scholar] [CrossRef]
- Strauss, J.F.; Martinez, F.; Kiriakidou, M. Placental steroid hormone synthesis: Unique features and unanswered questions. Biol. Reprod. 1996, 54, 303–311. [Google Scholar] [CrossRef]
- Cui, X.; Sun, J.; Liang, C.; Zheng, Q.; Yang, X.; Liu, S.; Yan, Q. Progesterone promotes embryo adhesion by upregulating c-Fos/c-Jun transcription factor-mediated poFUT1 expression. Biol. Reprod. 2019, 101, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Dias, A. Pregnancy in pigs: The journey of an early life. Domest. Anim. Endocrinol. 2022, 78, 106656. [Google Scholar] [CrossRef] [PubMed]
- Song, H.B. Prospective Study of Dynamic Monitoring of Serum β-hCG and Progesterone to Predict Early Pregnancy Outcome. Master’s Thesis, Qingdao University, Qingdao, China, January 2020. [Google Scholar]
- Solano, M.E.; Arck, P.C. Steroids, Pregnancy and Fetal Development. Front. Immunol. 2019, 10, 3017. [Google Scholar] [CrossRef]
- Johansson, E.D.; Jonasson, L.E. Progesterone levels in amniotic fluid and plasma from women. I. Levels during normal pregnancy. Acta. Obstet. Gynecol. Scand. 1971, 50, 339–343. [Google Scholar] [CrossRef]
- Virgo, B.B.; Bellward, G.D. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology 1974, 95, 1486–1490. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Skinner, M.K. Progesterone regulation of primordial follicle assembly in bovine fetal ovaries. Mol. Cell. Endocrinol. 2009, 313, 9–16. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhang, D.W.; Mai, H.M.; Luo, J.Y.; Chen, H.M. Serum progesterone and insulin-like growth factor in predicting adverse outcomes in early pregnancy. Med. Innov. China 2019, 16, 30–33. (In Chinese) [Google Scholar]
- Breier, G.; Albrecht, U.; Sterrer, S.; Risau, W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992, 114, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Rätsep, M.T.; Felker, A.M.; Kay, V.R.; Tolusso, L.; Hofmann, A.P.; Croy, B.A. Uterine natural killer cells: Supervisors of vasculature construction in early decidua basalis. Reproduction 2015, 149, R91–R102. [Google Scholar] [CrossRef]
- Jewson, M.; Purohit, P.; Lumsden, M.A. Progesterone and abnormal uterine bleeding/menstrual disorders. Best Pract. Res. Clin. Obst. Gynaecol. 2020, 69, 62–73. [Google Scholar] [CrossRef]
- Su, Y.; Li, Q.; Zhang, Q.; Li, Z.; Yao, X.; Guo, Y.; Xiao, L.; Wang, X.; Ni, H. Exosomes derived from placental trophoblast cells regulate endometrial epithelial receptivity in dairy cows during pregnancy. J. Reprod. Dev. 2022, 68, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Obr, A.E.; Grimm, S.L.; Bishop, K.A.; Pike, J.W.; Lydon, J.P.; Edwards, D.P. Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells. Mol. Endocrinol. 2013, 27, 1808–1824. [Google Scholar] [CrossRef]
- Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Spencer, T.E.; Wu, G. Mechanisms for the establishment and maintenance of pregnancy: Synergies from scientific collaborations. Biol. Reprod. 2018, 99, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xie, L.; Xu, Z.; Hao, M.; Yang, B.; Shan, W.; Wang, Y.; Lv, Q.; Chen, X. NrCAM secreted by endometrial stromal cells enhances the progestin sensitivity of endometrial cancer cells through epigenetic modulation of PRB. Cancer Gene Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.A.; Edwards, D.P. Mechanism of action of progesterone antagonists. Exp. Biol. Med. 2002, 227, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Feinshtein, V.; Ben-Zvi, Z.; Sheiner, E.; Amash, A.; Sheizaf, B.; Holcberg, G. Progesterone levels in cesarean and normal delivered term placentas. Arch. Gynecol. Obstet. 2010, 281, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Lissauer, D.; Eldershaw, S.A.; Inman, C.F.; Coomarasamy, A.; Moss, P.A.; Kilby, M.D. Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur. J. Immunol. 2015, 45, 2858–2872. [Google Scholar] [CrossRef]
- Lissauer, D.M.; Piper, K.P.; Moss, P.A.; Kilby, M.D. Fetal microchimerism: The cellular and immunological legacy of pregnancy. Expert Rev. Mol. Med. 2009, 11, e33. [Google Scholar] [CrossRef]
- Cai, S.; Zhu, J.; Zeng, X.; Ye, Q.; Ye, C.; Mao, X.; Zhang, S.; Qiao, S.; Zeng, X. Maternal N-Carbamylglutamate Supply during Early Pregnancy Enhanced Pregnancy Outcomes in Sows through Modulations of Targeted Genes and Metabolism Pathways. J. Agric. Food Chem. 2018, 66, 5845–5852. [Google Scholar] [CrossRef]
- Ding, H.; Yang, Y.; Wei, S.; Spicer, L.J.; Kenéz, Á.; Xu, W.; Liu, Y.; Feng, T. Influence of N-acetylcysteine on steroidogenesis and gene expression in porcine placental trophoblast cells. Theriogenology 2021, 161, 49–56. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, J.H.; Xu, X.L.; Chen, Z.L.; Spicer, L.J.; Feng, T. Effects of N-carbamylglutamate and L-arginine on gonadotrophin-releasing hormone (GnRH) gene expression and secretion in GT1-7 cells. Reprod. Fertil. Dev. 2018, 30, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Schütz, L.F.; Morrell, B.C.; Perego, M.C.; Spicer, L.J. Effects of N-carbamylglutamate and L-arginine on steroidogenesis and gene expression in bovine granulosa cells. Anim. Reprod. Sci. 2018, 188, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Wang, S.; Liu, H.; Zhang, D.; Wang, Y.; Ji, H. Swine-derived probiotic lactobacillus plantarum modulates porcine intestinal endogenous host defense peptide synthesis through TLR2/MAPK/AP-1 signaling pathway. Front. Immunol. 2019, 10, 2691. [Google Scholar] [CrossRef] [PubMed]
- Chermuła, B.; Jeseta, M.; Sujka-Kordowska, P.; Konwerska, A.; Jankowski, M.; Kranc, W.; Kocherova, I.; Celichowski, P.; Antosik, P.; Bukowska, D.; et al. Genes regulating hormone stimulus and response to protein signaling revealed differential expression pattern during porcine oocyte in vitro maturation, confirmed by lipid concentration. Histochem. Cell Biol. 2020, 154, 77–95. [Google Scholar] [CrossRef]
- Kang, H.G.; Lee, S.; Jeong, P.S.; Kim, M.J.; Park, S.H.; Joo, Y.E.; Park, S.H.; Song, B.S.; Kim, S.U.; Kim, M.K.; et al. Lycopene improves in vitro development of porcine embryos by reducing oxidative stress and apoptosis. Antioxidants 2021, 10, 230. [Google Scholar] [CrossRef]
- Zhu, Z.; Pan, Q.; Zhao, W.; Wu, X.; Yu, S.; Shen, Q.; Zhang, J.; Yue, W.; Peng, S.; Li, N.; et al. BCL2 enhances survival of porcine pluripotent stem cells through promoting FGFR2. Cell Prolif. 2021, 54, e12932. [Google Scholar] [CrossRef]
- Mathew, D.J.; Sellner, E.M.; Green, J.C.; Okamura, C.S.; Anderson, L.L.; Lucy, M.C.; Geisert, R.D. Uterine progesterone receptor expression, conceptus development, and ovarian function in pigs treated with RU 486 during early pregnancy. Biol. Reprod. 2011, 84, 130–139. [Google Scholar] [CrossRef]
- Formby, B.; Wiley, T.S. Bcl-2, survivin and variant CD44 v7-v10 are downregulated and p53 is upregulated in breast cancer cells by progesterone: Inhibition of cell growth and induction of apoptosis. Mol. Cell Biochem. 1999, 202, 53–61. [Google Scholar] [CrossRef]
- Li, M.Q.; Xie, F.; Shi, J.W.; Yang, H.L.; Lai, Z.Z.; Shen, H.H.; Ruan, L.Y.; Wang, Y.; Qiu, X.M. Aspirin enhances the protective effect of progesterone on trophoblast cell from oxidative stress and apoptosis. Reprod. Dev. Med. 2021, 5, 1–8. [Google Scholar]
- Pei, J.; Liu, Z.; Wang, C.; Chu, N.; Liu, L.; Tang, Y.; Liu, H.; Xiang, Q.; Cheng, H.; Li, M.; et al. Progesterone attenuates sirt1-deficiency-mediated pre-eclampsia. Biomolecules 2022, 12, 422. [Google Scholar] [CrossRef]
- Formby, B.; Wiley, T.S. Progesterone inhibits growth and induces apoptosis in breast cancer cells: Inverse effects on Bcl-2 and p53. Ann. Clin. Lab Sci. 1998, 28, 360–369. [Google Scholar] [PubMed]
- Sasaki, G.; Zubair, M.; Ishii, T.; Mitsui, T.; Hasegawa, T.; Auchus, R.J. The contribution of serine 194 phosphorylation to steroidogenic acute regulatory protein function. Mol. Endocrinol. 2014, 28, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wan, W.; Zhu, K.; Pan, M.; Zhao, X.; Ma, B.; Wei, Q. Effects of 4-vinylcyclohexene diepoxide on the cell cycle, apoptosis, and steroid hormone secretion of goat ovarian granulosa cells. Vitr. Cell Dev. Biol. Anim. 2022, 58, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Castaño, P.; Parween, S.; Pandey, A.V. Bioactivity of curcumin on the cytochrome p450 enzymes of the steroidogenic pathway. Int. J. Mol. Sci. 2019, 20, 4606. [Google Scholar] [CrossRef]
- Salem, H.F. Sustained-release progesterone nanosuspension following intramuscular injection in ovariectomized rats. Int. J. Nanomed. 2010, 5, 943–954. [Google Scholar] [CrossRef]
- Sun, D.; Cui, Y.; Jin, B.; Zhang, X.; Yang, X.; Gao, C. Effects of the yangjing capsule extract on steroidogenesis and apoptosis in mouse leydig cells. Evid Based Complement Alternat. Med. 2012, 2012, 985457. [Google Scholar] [CrossRef]
- Fox, C.W.; Zhang, L.; Sohni, A.; Doblado, M.; Wilkinson, M.F.; Chang, R.J.; Duleba, A.J. Inflammatory Stimuli Trigger Increased Androgen Production and Shifts in Gene Expression in Theca-Interstitial Cells. Endocrinology 2019, 160, 2946–2958. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ji, M.; Wen, X.; Chen, D.; Huang, F.; Guan, X.; Tian, J.; Xie, J.; Shao, J.; Wang, J.; et al. Effects of midazolam on the development of adult leydig cells from stem cells in vitro. Front Endocrinol. 2021, 12, 765251. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction. 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- Lavoie, H.A.; King, S.R. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp. Biol. Med. 2009, 234, 880–907. [Google Scholar] [CrossRef] [PubMed]
- Noyola-Martínez, N.; Halhali, A.; Zaga-Clavellina, V.; Olmos-Ortiz, A.; Larrea, F.; Barrera, D. A time-course regulatory and kinetic expression study of steroid metabolizing enzymes by calcitriol in primary cultured human placental cells. J. Steroid Biochem. Mol. Biol. 2017, 167, 98–105. [Google Scholar] [CrossRef]
- Lu, X.; Ma, P.; Kong, L.; Wang, X.; Jiang, L. Vitamin K2 inhibits hepatocellular carcinoma cell proliferation by binding to 17β-hydroxysteroid dehydrogenase 4. Front. Oncol. 2021, 11, 757603. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Duan, A.; Ma, X.; Liang, S.; Deng, T. Knockdown of CYP19A1 in buffalo follicular granulosa cells results in increased progesterone secretion and promotes cell proliferation. Front. Vet. Sci. 2020, 7, 539496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Huang, J.; Gu, X.; Li, L.; Han, J. Downregulation of aromatase plays a dual role in preeclampsia. Mol. Hum. Reprod. 2021, 27, gaab013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Tasaki, T.; Tsukamoto, M.; Wang, K.Y.; Azuma, K. Deficiency of Wnt10a causes female infertility via the β-catenin/Cyp19a1 pathway in mice. Int. J. Med. Sci. 2022, 19, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Han, S.; Xu, P.; Wang, Y.; Cheng, T.; Hu, C. Estrogen and preeclampsia: Potential of estrogens as therapeutic agents in preeclampsia. Drug Des. Devel. Ther. 2021, 15, 2543–2550. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef]
- Jo, Y.S.; Lee, G.S.; Nam, S.Y.; Kim, S.J. Progesterone Inhibits Leptin-Induced Invasiveness of BeWo Cells. Int. J. Mol. Sci. 2015, 12, 773–779. [Google Scholar] [CrossRef]
- Horita, K.; Inase, N.; Miyake, S.; Formby, B.; Toyoda, H.; Yoshizawa, Y. Progesterone induces apoptosis in malignant mesothelioma cells. Anticancer Res. 2001, 21, 3871–3874. [Google Scholar]
- Sugino, N.; Okuda, K. Species-related differences in the mechanism of apoptosis during structural luteolysis. J. Reprod. Dev. 2007, 53, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- De Falco, M.; Fedele, V.; Cobellis, L.; Mastrogiacomo, A.; Giraldi, D.; Leone, S.; De Luca, L.; Laforgia, V.; De Luca, A. Pattern of expression of cyclin D1/CDK4 complex in human placenta during gestation. Cell Tissue Res. 2004, 317, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.M.; Giuffrida, D.; Masturzo, B.; Mele, P.; Piccoli, E.; Eva, C.; Todros, T.; Rolfo, A. Altered expression of G1/S phase cell cycle regulators in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise. Cell Cycle 2017, 16, 200–212. [Google Scholar] [CrossRef]
- Zhou, Q.; Acharya, G. Editorial: Placental hormones and pregnancy-related endocrine disorders. Front Endocrinol. 2022, 13, 905829. [Google Scholar] [CrossRef]
- Alvarado-Flores, F.; Kaneko-Tarui, T.; Beyer, W.; Katz, J.; Chu, T.; Catalano, P.; Sadovsky, Y.; Hivert, M.F.; O’Tierney-Ginn, P. Placental miR-3940-3p is associated with maternal insulin resistance in late pregnancy. J. Clin. Endocrinol. Metab. 2021, 106, 3526–3535. [Google Scholar] [CrossRef]
- Shin, Y.Y.; An, S.M.; Jeong, J.S.; Yang, S.Y.; Lee, G.S.; Hong, E.J.; Jeung, E.B.; Kim, S.C.; An, B.S. Comparison of steroid hormones in three different preeclamptic models. Mol. Med. Rep. 2021, 23, 252. [Google Scholar] [CrossRef]
- Lawrenz, B.; Melado, L.; Fatemi, H. Premature progesterone rise in ART-cycles. Reprod. Biol. 2018, 18, 1–4. [Google Scholar] [CrossRef]
- Huang, B.; Ren, X.; Wu, L.; Zhu, L.; Xu, B.; Li, Y.; Ai, J.; Jin, L. Elevated Progesterone Levels on the Day of Oocyte Maturation May Affect Top Quality Embryo IVF Cycles. PLoS ONE 2016, 11, e0145895. [Google Scholar] [CrossRef]
- Adda-Herzog, E.; Poulain, M.; de Ziegler, D.; Ayoubi, J.M.; Fanchin, R. Premature progesterone elevation in controlled ovarian stimulation: To make a long story short. Fertil. Steril. 2018, 109, 563–570. [Google Scholar] [CrossRef]
- Kalakota, N.R.; George, L.C.; Morelli, S.S.; Douglas, N.C.; Babwah, A.V. Towards an Improved Understanding of the Effects of Elevated Progesterone Levels on Human Endometrial Receptivity and Oocyte/Embryo Quality during Assisted Reproductive Technologies. Cells 2022, 11, 1405. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Slayden, O.D. Progesterone-induced gene expression in uterine epithelia: A myth perpetuated by conventional wisdom. Biol. Reprod. 2008, 79, 1008–1009. [Google Scholar] [CrossRef] [PubMed]
- Diep, C.H.; Ahrendt, H.; Lange, C.A. Progesterone induces progesterone receptor gene (PGR) expression via rapid activation of protein kinase pathways required for cooperative estrogen receptor alpha (ER) and progesterone receptor (PR) genomic action at ER/PR target genes. Steroids 2016, 114, 48–58. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).