Simple Summary
The present study aimed to determine benefits and risks of a dietary supplementation combining hydroxytyrosol and n-3 polyunsaturated fatty acids (PUFA) on prenatal development and metabolic traits in swine, a model of intrauterine growth restricted (IUGR) pregnancies. No effects were found regarding sows’ weight and adiposity. Treated sows had larger litters, with smaller fetuses. However, these animals had better development of some major organs. Fetuses from the treated group had better glycemic and lipidic indexes, but no effects on anti/prooxidant profiles were found.
Abstract
Maternal supplementation with antioxidants and n-3 PUFAs may be a promising strategy to reduce the risk of intrauterine growth restriction and preterm delivery, which may diminish the appearance of low-birth-neonates. A previous studies showed beneficial outcomes of the combination of hydroxytyrosol and linoleic acid, but there is no data of its prenatal effects. The present study aimed to determine the possible prenatal implications of such maternal supplementation at prenatal stages in swine, a model of IUGR pregnancies. Results showed effects on litter size, with treated sows having larger litters and, therefore, smaller fetuses. However, the brain/head weight ratio showed a positive effect of the treatment in development, as well as in some other major organs like lungs, spleen, or kidneys. On the other hand, treated piglets showed better glycemic and lipidemic profiles, which could explain postnatal effects. However, further research on the implications of the treatment on litter size and prenatal and postnatal development must be done before practical recommendation can be given.
1. Introduction
Maternal nutrition during pregnancy is critical for fetal growth, with major implications in the developmental competence and health status of the offspring during its lifetime [1]. Thus, in pigs, having in mind their importance for pork production and as a biomedical model, different nutrients for mothers are being tested to improve the fetal status. Maternal supplementation with polyphenols during pregnancy is being thoughtfully studied because of their antioxidant properties [2] and, more specifically, the interest in using hydroxytyrosol (a polyphenol present in olive oil and fruits) is increasing not only due to its antioxidant capacity, but also because of its metabolism-regulatory, anti-inflammatory, and immuno-modulatory properties [3]. Hydroxytyrosol has also been shown to diminish lipid peroxidation in swine fetuses, increasing their availability of omega-3 and omega-6 polyunsaturated fatty acids (n-3 and n-6 PUFA) [4]. Our group has previously shown the usefulness of this compound to counteract the appearance of intrauterine growth restriction (IUGR) and, therefore, to diminish the incidence of low birth weight (LBW) neonates and to favor the postnatal development of the piglets [4,5,6,7].
The positive role of hydroxytyrosol on fetal availability of n-3 and n-6 PUFA is pivotal, since PUFAs are molecules with key functions in metabolism, cell structure, and signaling being vital for the adequate fetal development during pregnancy (as reviewed in [8]). Notably, n-3 and n-6 PUFAs are indispensable for adequate tissue development during fetal stages and, therefore, pregnancy success [9]. Such importance is boosted by the fact that these fatty acids are deemed essential due to the inability of the animals to synthesize them. Thus, the fetus must obtain these essential fatty acids (EFA) through maternal transfer [10].
Having in mind the importance of the dietary intake of EFA by the mother on fetal development and homeostasis, maternal supplementation with α-linolenic acid (ALA, which is a precursor of other PUFAs), or directly with EPA and DHA [11], have increased in popularity [12]. However, there is evidence that excessive PUFAs intake may be detrimental for the fetal and newborn health status [13,14]. Therefore, there is a necessity of a systematic risk–benefit analysis and interventional research on PUFA supplementation during pregnancy, to further understand their effects at prenatal and postnatal stages in IUGR pregnancies, as recommended by the World Health Organization (WHO) (https://www.who.int/elena/titles/fish_oil_pregnancy/en/ (accessed on 17 January 2021)).
A main concerning issue in the use of PUFA supplementation during pregnancy is the negative effects on the oxidative/antioxidant status and homeostasis [15,16,17]. To counteract this oxidative effect of PUFAs, an antioxidant is needed. In this regard, our group has studied the combination of n-3 PUFA with hydroxytyrosol in the maternal diet [18]. The results obtained indicated that the offspring from supplemented sows had a lower mean weight and corpulence at birth, but a higher growth rate and, thus, higher weight and corpulence, increased muscle development with similar adiposity and better lipidemic profile at juvenile stages compared with mothers given a diet with no hydroxytyrosol and n-3 PUFA supplementation. Despite the promising results obtained, there is a scarcity of both the realistic benefits and the potential hazards of supplementation with PUFAs and hydroxytyrosol during pregnancy. Thus, the present trial aimed to determine the effects of a maternal dietary supplementation combining hydroxytyrosol and n-3 PUFAs on developmental patterns and metabolic traits of fetuses at IUGR risk at prenatal stages.
2. Materials and Methods
2.1. Ethic Statement
The experiment was performed according to the Spanish Policy for Animal Protection (RD 53/2013), which meets the European Union Directive 2010/63/UE on the protection of research animals. The INIA Committee of Ethics in Animal Research assessed and approved the experimental procedures (report CEEA 2013/036, 19 February 2014). Sows were housed in INIA animal facilities, which are in accordance with local, national, and European requirements for Scientific Procedures Establishments.
2.2. Animals and Experimental Procedures
The study involved a total of 131 fetuses (60 from the control group and 71 from the treated group), obtained from 14 primiparous Iberian sows pregnant after cycle synchronization with altrenogest (Regumate®, MSD, Boxmeer, The Netherlands) and artificial insemination with cooled semen from the same purebred boars.
Fetuses were obtained on gestational day 100 (which corresponds approximately with 90% of the 112-days gestation typical of this breed). On this day, blood samples were drawn from the orbital sinus of the sows, after 16 h fastening with sterile EDTA 10 mL vacuum tubes (Vacutainer™ Systems Europe, Meylan, France). Samples were immediately centrifuged at 1500× g for 15 min and afterwards, the plasma was separated and biobanked into polypropylene vials at −80 °C until they were assayed for metabolic biomarkers (i.e., glycemic values and lipid profiles) and antioxidant/oxidative status.
During pregnancy, sows were fed with a standard grain based-feed diet with the following mean component values: dry matter, 89.9%; crude protein, 12.28%; fat, 3.55%; metabolizable energy, 2910.44 Mcal/kg. From the start of the experimental period (insemination day, Day 0) to gestational day 35, food amount was adjusted to fulfill individual daily maintenance requirements based on data from the British Society of Animal Health [19]. Most abundant fatty acids (FA) in the diet were palmitic acid (20.6%), oleic acid (19.3%), and linolenic acid (41.5%).
On gestational day 35, all sows were weighed, and the feed amount was adjusted to fulfill 50% of daily maintenance requirements until delivery. This diet restriction has been previously found to increase the incidence of intrauterine growth restriction (IUGR; [20]). On this same day 35 of pregnancy, the females were pair-matched by body weight to obtain two homogeneous groups of seven sows. Therefore, there were no differences in mean body weight between groups (149.64 ± 7.47 kg vs. 146.57 ± 2.51 kg; p = 0.80), and maternal adiposity in terms of fat amount measured by P2 point (located at 4 cm from the midline and transversal to the head of the last rib) with a multifrequency linear-array ultrasonographic probe (SV1 Wireless scanner, SonopTek, Beijing, China) was also similar (46.67 ± 4.40 vs. 44.57 ± 2.80 cm; p = 0.86). One of the groups remained with the same diet (control group, Group C), whereas the other group (treated group, Group T) received an isocaloric diet including 4% of linseed oil and 1.5 mg hydroxytyrosol per kg of feed (Table 1; Natac S.L., Alcorcon, Madrid, Spain). The component values of the diet in the treated group were: dry matter: 89.7%; crude protein: 12.35%; fat: 6.23%; and metabolizable energy, 2.9 Mcal/kg. In the treatment diet, the most prominent fatty acids were palmitic acid (11.82%), linoleic acid (32.26%), and α linoleic acid (28.97%). There were traces of long-chain PUFA in the experimental diets which were probably caused by contamination during feed manufacturing or storage. Fatty acids methyl esters in the diet were identified by a gas chromatograph (Hewlett Packard HP-6890, Santa Clara, CA, USA) with a flame ionization detector and a capillary column (HP-Innowax, 30 m × 0.32 mm i.d. and 0.25 µm polyethylene glycol-film thickness;) [21]; after extraction and methylation by the one-step procedure proposed by Sukhija and Palmquist [22].

Table 1.
Estimated analysis (g/kg), ingredient (g/kg; left table), and fatty acid composition (g/100 g total fatty acids) of the experimental diets of Control sows (group C) and treated with hydroxytyrosol and n-3-PUFA (Treated; group T).
2.3. Sampling of Fetuses and Placentas
Sows were euthanized in compliance with RD 53/2013. Afterwards, the entire genital tracts were collected for morphometric evaluation and fetal sampling. The content of the uterus was exposed, and fetal sex was determined by visual inspection immediately after recovery. A sample of fetal blood was drawn from the heart and/or umbilical cord using EDTA syringes and processed as previously described for sows.
Head size (biparietal diameter and occipito–nasal length), trunk length (crown–rump length) and corpulence (thoracic and abdominal circumferences) were measured in all individuals. Then, the head was separated from the trunk at the atlanto–occipital union and weighted. Total viscera were extracted from the body and carcass and total viscera were weighted separately. Main organs (brain, heart, lungs, liver, kidneys, intestine, and spleen) were also weighted individually and, finally, ratios of head-to-body weight, brain-to-head weight, and weight of total viscera and individual organs relative to viscera weight were calculated [5,23].
2.4. Evaluation of Maternal and Fetal Anti/Prooxidant and Metabolic Status
Values for total antioxidant capacity were determined using the ferric reducing antioxidant power assay (FRAP) as previously described [24], whilst lipid peroxidation was assessed by measuring malondialdehyde (MDA; µmol/L) using the thiobarbituric acid reaction [25].
Parameters related to glycemic profile (glucose and fructosamine) and lipid metabolism (total cholesterol, high and low-density lipoprotein cholesterol [HDL-c and LDL-c, respectively] and triglycerides), lactate and urea were measured in maternal and fetal plasma using a clinical chemistry analyzer (Konelab 20, Thermo Scientific, Vantaa, Finland), according to manufacturer’s instructions [26].
2.5. Statistical Analysis
Data were analyzed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). Data from sows were analyzed via Student’s t-test and a general linear model (GLM) for repeated measures (body weight and subcutaneous adiposity). Verification of normal distribution was done with a Shapiro–Wilk test. The equality of variance was studied with a F-test. Effects of diet (control vs. treatment) sex (female vs. male) on developmental traits, adiposity, fatty acid composition, oxidative stress, and metabolic status were assessed using two-way ANOVA and Student’s t-test. Due to the strong bias between treatment and litter size, litter size was also considered an effect. To group animals by their litter size, we calculated the total mean number of fetuses per litter (9.25 ± 1.42 piglets per sow), and then defined small litter as those with ≤9 piglets and large litters as those with >9 piglets. Chi-squared and Fisher tests were used to ascertain differences in the proportions of small/large litters, females/males, and IUGR e between treatments.
Relationships between maternal metabolic biomarkers and features of fetuses were explored using Pearson correlation. Based on previous studies [27], fetuses with severe growth restriction (IUGR) were defined as those having a body weight lower than one standard deviation of the litter mean value. Statistical significance was considered when p < 0.05, whereas a trend was considered when 0.1 < p < 0.05.
3. Results
3.1. Effects of Dietary Hydroxytyrosol and n-3 PUFA on the Sows
There were no main differences of the treatment on the morphometrics and metabolic features of the sows. At the day of sampling (Day 100 of pregnancy), body weight and adiposity (in terms of total subcutaneous fat and outer and inner layers apart) and plasma indexes for pro-/antioxidant status and metabolism of glucose and lipids were similar in both control sows (Group C) and sows treated with hydroxytyrosol and n-3-PUFA (Group T), as depicted in Supplementary Table S1.
3.2. Effects of Dietary Hydroxytyrosol and n-3 PUFA on the Fetuses
3.2.1. Effects on Litter Features
There was a trend (p = 0.075), for a higher mean litter size in the Group T (9.86 ± 0.55 vs. 8.42 ± 0.76 piglets/sow in the Group C), and there was a higher proportion of large litters in the Group T compared with Group C (19 vs. 77%, p < 0.00001). However, no significant differences were found in the number of ovulations (12.33 ± 1.0 in Group C vs. 12.71 ± 0.86 in the Group T; p = 0.78) or the ratio of viable fetuses compared to the number of ovulations (71.48 ± 0.03 vs. 79.16 ± 0.06% in Groups C and T, respectively; p = 0.33). Finally, the percentage of females and males were 47% and 52%, respectively, in Group C, whereas there was 58% of females and 42% of males in the treated group, which means a tendency of different proportions of females and males in the Group T (p = 0.08). Further information about litter size and composition per sow can be found at Supplementary Table S2.
3.2.2. Effects on Body Weight, Size, and Composition
The uterine weight was higher in the sows from the Group C (16.86 ± 0.70 kg vs. 16.14 ± 1.04 kg; p < 0.05) despite of a trend for a higher number of fetuses in the Group T. This outcome was due to the higher weight of the control fetuses and placentas when compared to their treated counterparts (938.1 ± 16. 86 g vs. 803.6 ± 18.26 g; p < 0.0001 for fetuses and 654.0 ± 18.58 g vs. 519.7 ± 16.97 g; p < 0.01 for placentae), without sex-related differences. Overall, there were no sex-related differences, but, conversely, when each treatment was studied separately, female fetuses from treated mothers were lighter than their brothers; no differences were found in the control groups (Figure 1).
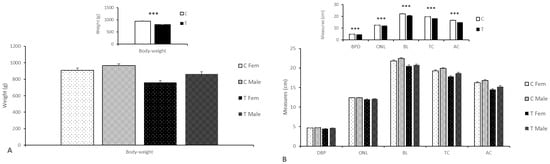
Figure 1.
Mean (±S.E.M.) body weights (panel A) and sizes (panel B) ± S.E.M. of fetuses born from control sows (Group C) or sows treated with hydroxytyrosol and n-3 PUFA from Day 35 to Day 100 of gestation (Group T). Main graphs represent sex-and group-differences, while insets represent only group-related differences. C = control; T = treated; BPD = biparietal diameter; ONL = occipito–nasal length; TL = trunk length; TC = thoracic circumference; AC = abdominal circumference. *** p < 0.001.
The percentage of fetuses with a body weight lower than 1 SD from mean litter weight (defined as IUGR) was 22.0% (13 of 59; 9 females and 4 males) in the Group C, whilst in the Group T such percentage was 14.5% (10 of 69 fetuses; 8 females and 2 males), with no significance (p = 0.38). To avoid sex effects (since males are usually bigger than females at this gestational stage and the number of males and females were not equally distributed between groups), we studied each sex separately. When we analyzed only females, there were 17.8% of IUGR in the Group C (5 of 28 female fetuses) and 17.5% in the Group T (7 of 40 female fetuses). When only males were analyzed, Group C had 5 IUGR fetuses out of 31 males (16.1%) and Group T showed 5 IUGR fetuses out of 29 males (17.2%). No significance was achieved in either sex.
Assessment of body size showed that control fetuses were larger than treated fetuses (Figure 1 and Supplementary Table S3), with a longer and wider trunk (p < 0.0001), without sex-related differences.
These differences were also driven by litter size. Thus, fetuses from small litters were heavier, longer, and wider than those from large ones (p < 0.05 for body weight, trunk length, and abdominal circumference and p < 0.1 for occipito–nasal length). However, even in litters with similar size, fetuses in the Group C were bigger than fetuses from Group T (p < 0.05).
Similarly to results observed in the entire body weight, the assessment of the weights of carcasses and total viscera were higher in the control group (525.3 ± 10.14 g vs. 442.3 ± 10.8 g; p < 0.01 for carcasses and 165.8 ± 3.814 g vs. 138.8 ± 3.458 g; p < 0.01 for total viscera; Table 2 and Supplementary Table S3). Sex related effects were only found in the Group T, with females having lighter carcass and viscera than males of the same group (p < 0.001 for both). On the other hand, it should be highlighted that carcasses were heavier in the Group C compared to Group T when assessing small litters (p < 0.0001), but heavier in the Group T compared to Group C when assessing large litters (p < 0.05).

Table 2.
Mean (± S.E.M.) values of weight of different body parts and major organs and their ratios at Day 100 of gestation in different treatments (fetuses from control sows, Group C and from sows treated with hydroxytyrosol, and n-3-PUFA from Day 35 to Day 100 of gestation (Group T) and by litter sizes (small ≤ 9 fetuses, large > 9 fetuses).
Fetuses from mothers of Group C had heavier weights of the heads and major organs (p < 0.05, for all and p < 0.1; for brain; Table 2 and Supplementary Table S3). Independently of treatment group, fetuses from small litters had a heavier head, spleen, and intestine than fetuses from large ones (p < 0.05).
The assessment of the different ratios between main organs and structures (Table 2 and Supplementary Table S4) showed that fetuses in the Group T showed higher brain/head weight ratio (p < 0.0001); carcass/total weight ratio (p < 0.01); total viscera weight/total weight ratio (p < 0.05). Such effect was also found in the ratios of spleen and kidneys to total viscera weight (p < 0.05, for both). There were significant interactions with litter size in brain/head ratio, liver/total viscera weight and intestines/total viscera weight, being higher in small litters in the case of Group C and in large litters in Group T (p < 0.05 for all).
3.2.3. Effects on Fetal Pro-Antioxidant and Metabolic Status
There were no significant differences between groups when either antioxidant capacity (in terms of ferric reducing antioxidant power assay; FRAP) or lipid peroxidation (in terms of malondialdehyde; MDA) were analyzed at plasma samples. We observed higher MDA levels in females (Group C: 2.2 ± 0.1 vs. 2.0 ± 0.1 and Group T: 2.0 ± 0.1 vs. 1.7 ± 0.1; p < 0.05 for both) independently of treatment and in small litters compared to large ones (2.1 ± 0.1 vs. 1.8 ± 0.1; p < 0.05), independently of treatment and sex.
The assessment of the glycemic profile showed higher plasma glucose concentrations in the Group T (p < 0.05; Figure 2 and Supplementary Table S5) and higher glucose levels in females than in their male counterparts in both groups (p < 0.01 for all). Conversely, the assessment of the lipids’ profile showed lower cholesterol levels (p < 0.05) in the fetuses of the Group T, especially when comparing females and large litters from both treatment groups (p < 0.01 for both). There were significant litter-size by treatment interactions (p < 0.05 for fructosamine, glucose, triglycerides, and lactate; p < 0.0001 for urea). Thus, glucose, HDL-c, lactate, and triglyceride concentrations were higher in small litters of Group T and large litters of Group C when compared to small litters of Group C and large litters of Group T, respectively. On the other hand, fructosamine and urea were higher in small litters of Group C and large litters of Group T when compared with small litters of Group T and large litters of Group C, respectively.
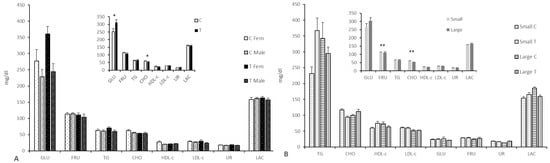
Figure 2.
Mean (±S.E.M.) plasma concentrations of indexes for glucids (A) and lipids metabolism (B) at Day 100 of pregnancy in fetuses from control sows (Group C) and sows treated with hydroxytyrosol and n-3-PUFA from Day 35 to Day 100 of gestation (Group T). Main graphs represent sex-and group-differences, while insets represent only group-related differences. TG = triglycerides; CHO = cholesterol; HDL-c = high density lipoprotein cholesterol; LDL = low density lipoprotein cholesterol; GLU = Glucose; FRUC = fructosamine; UR = urea; LAC = lactate. Asterisks denote significant differences (* for p < 0.05 and ** for p < 0.01).
The assessment of possible feto-maternal interactions in the metabolic profile of the Group C showed no significant relationship for glycemic and lipid profiles. The lactate correlation in this group showed a negative value (r = −0.264; p < 0.05), with higher values in the fetuses. Similarly, no significant correlations were found in the glycemic or lipidic profiles in the Group T, with only negative relationships assessed in lactate and urea (r = −0.262, p < 0.05 for lactate and r = −0.510, p < 0.01 for urea). No significant correlations were found in the MDA and FRAP levels of Group C and T.
4. Discussion
In the present study, we aimed to determine the effects of a maternal supplementation with n-3 PUFA and hydroxytyrosol, from day 35 of gestation onwards, on body composition and metabolic and oxidative stress status in sows and their progeny using an animal model that has previously shown to increase the appearance of IUGR offspring [4,5,6,7].
The maternal features such as body weight, oxidative stress, or adiposity did not differ between control and treatment groups, which agrees with previous data in the same swine model of IUGR when only supplemented with dietary hydroxytyrosol [5] or in other animal models in which high dietary levels of n-3 PUFA were given to the dams [27].
The number of fetuses showed a higher litter size due to a significantly higher proportion of large litters in the treated group and a higher ratio of viable fetuses to ovulations, which may reflect a higher offspring viability. In a previous study from our group, hydroxytyrosol did not showed any effect on litter size [6]. On the other hand, Webel et al. [28] described a larger litter size in sows after n-3 PUFA treatment, whilst other studies did not report any difference [29]. Such a higher litter size in Group T plus a trend for a higher proportion of females than males in this group, with lower body weight than their male littermates (a difference not found in the Group C), may have influenced the lower body weight and size found in our treated group compared to controls.
Prolificacy is a well-known factor limiting body weight and size since the uterine space is limited, especially in the Iberian pig [30], because each piglet and its correspondent placenta have less space to develop, and, therefore, reach a smaller size and weight [31]. This outcome has been previously described in pigs treated with hydroxytyrosol but there were no deleterious outcomes at birth and during postnatal phases [4,6] and also in our previous study combining n-3 PUFA and hydroxytyrosol [18].
The results on maternal supplementation with n-3 PUFA in previous studies are contradictory, with some of them showing numerically higher body weight at birth [32,33] and other studies showing no effect [34]. These controversial results have been also reported in humans (reviewed by Grieger and Clifton, [35]). These incongruences could be explained by the different source and concentration of n-3 PUFA supplemented, as well as plausible implications of lifestyle, so further research on this regard is needed before any recommendation.
Similarly to the higher birth-weight, the weights of viscera were heavier in the fetuses from Group C than those from Group T. However, the ratios of brain to head, carcass to body weight, and total viscera to body weight ratio (caused by a higher relative weight of intestine and spleen) were higher in the treated fetuses. The effects on the brain, at the light of the review performed by Innis ([36]) may be related to the positive impacts of n-3 PUFA on brain development and to the neuroprotective properties of hydroxytyrosol [37,38,39]. These differences in the relative weight of brain and intestine may explain the higher average daily weight (ADWG) and growth rate found in a previous study in Iberian pigs with the same maternal supplementation [18]. Moreover, maternal supplementation had different effects depending on the litter size in our study. Thus, differences between control and treated animals were more evident when comparing small litters than when comparing large ones, in agreement with previous studies [6]. Therefore, these results suggest better results of the hydroxytyrosol and n-3 PUFA supplementation in larger litters.
Differences in developmental patterns between Groups C and T may be related to metabolic features; specifically, glucose (higher in treated fetuses). Glucose is the metabolite that crosses the placenta at easiest [40,41], thus, any improvement in placental circulation increases the glucose availability to the fetus. This is vital on fetal development (specially the brain) [42,43] and neonatal survival, since the newborn pig depends on its glucose energy reserves to move to the mother and suck, and to regulate body temperature [44]. It is also important to note that glucose levels were different between groups depending on litter size: higher in the small litters of the Group T and higher in the large litters of the Group C. Conversely, fructosamine (a molecule indicative of precedent glucose availability [45]) was higher in the small litters of the Group C and in the large litters of the Group T. Thus, the treatment affected the glycolytic profile differently depending on the litter size, resulting in better glucose metabolism in large litters. Assessment of lipid metabolism showed that cholesterol levels were lower in the Group T, in accordance with previous research [46,47]. The interaction between treatment and litter size also affected other metabolites related to lipid metabolism, like HDL-c and triglycerides (higher in small litters in the Group T and in large litters in the Group C when compared with the opposite treatment and the same litter size), whereas the opposite was found in LDL-c. Previous research found that both n-3-PUFA and hydroxytyrosol have important effects on lipid metabolism, with n-3 PUFA reducing plasma concentrations of total cholesterol and LDL-c [48], as well as controlling plasma triglyceride levels at postprandial states [49]. On the other hand, hydroxytyrosol has been demonstrated to increase plasma levels of HDL-c while decreasing total cholesterol and LDL-c both in humans [50] and pigs [6]. However, it is important to note that, in the present trial, the correlation in metabolic parameters between mothers and piglets is low. One of the limitations of the study is the relatively low number of sows used, so further research is needed to fully understand the processes implicated in differences between Group C and Group T fetuses. Another possible explanation is the capability of the fetus to achieve a certain level of metabolic autonomy, especially in situations of maternal nutritional changes and challenges (as reviewed by [51]).
5. Conclusions
The present study shows that maternal supplementation with hydroxytyrosol and n-3 PUFA has differential effects on the growth and metabolism of the offspring at prenatal stages depending on the litter size. Overall, fetuses from small litters were heavier and bigger, with different plasma metabolites than those from large litters. However, Groups C and T behaved differently in small and large litters, especially at the brain level, but also at the glucose metabolism, probably preventing newborn piglets from death due to the higher brain development during fetal stages, as well as for better thermoregulation. Nevertheless, even with the positive outcomes in the percentage of IUGR and metabolites, more research is needed previously on mechanistic issues and prenatal and postnatal outcomes previously to any advice of use in pregnant females. Also, more studies would be needed to ascertain the beneficial results in swine production.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11061699/s1, Table S1: Mean (±S.E.M.) of body weight, subcutaneous fat, pro-/antioxidant and metabolic status in control sows (Group C) and treated with hydroxytyrosol and n-3-PUFA from Day 35 to Day 100 of gestation (Group T) at the end of the experiment; Table S2: Detailed information (total number and percentage) on sex (total, male, and female fetuses) and weight distribution (presence of IUGR animal) in the pregnancies studied in the current trial; Table S3: Mean (±S.E.M.) of body measures and weights of fetuses born from control sows (Group C) or sows treated with hydroxytyrosol and n-3 PUFA from Day 35 to Day 100 of gestation (Group T) by treatment and sex (Table A) and by litter size and treatment (Table B); Table S4: Mean (±S.E.M.) of weights ratios of fetuses born from control sows (Group C) or sows treated with hydroxytyrosol and n-3 PUFA from Day 35 to Day 100 of gestation (Group T) by treatment and sex (Table A) and by litter size and treatment (Table B); Table S5: Mean (±S.E.M.) of plasma concentrations of indexes for glucids and lipids metabolism of fetuses born from control sows (Group C) or sows treated with hydroxytyrosol and n-3 PUFA from Day 35 to Day 100 of gestation (Group T) by treatment and sex (Table A) and by litter size and treatment (Table B).
Author Contributions
Conceptualization: A.H.-M., C.Ó., B.I. and A.G.-B.; Methodology, A.H.-M., J.L.P.-P., C.G.-C., M.V.-G., A.L., R.B., Y.N., S.A., C.Ó., B.I. and A.G.-B.; Formal analysis, A.H.-M., S.A., B.I. and A.G.-B.; Investigation, A.H.-M., J.L.P.-P., C.G.-C., M.V.-G., A.L., R.B., Y.N., S.A., C.Ó., B.I. and A.G.-B.; Writing—original draft preparation, A.H.-M. and A.G.-B.; Writing—review and editing; J.L.P.-P., C.G.-C., M.V.-G., A.L., R.B., Y.N., S.A., C.Ó. and B.I; Project administration, A.G.-B.; Funding acquisition, A.G.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The experimental work was supported by funds from the Ministry of Economy and Competitiveness (project AGL2013–48121-C3-2-R and AGL2016–79321-C2–1-R), co-funded by FEDER. AHM, CGC, and MVG were backed by the Spanish Government (AHM: FPI National Program Grant BES-2017-080541; CGC: FPI National Program Grant BES-2014-070464; MVG: FPU National Program Grant FPU014/01285).
Institutional Review Board Statement
The experiment was performed according to the Spanish Policy for Animal Protection (RD 53/2013), which meets the European Union Directive 2010/63/UE on the protection of research animals. The INIA Committee of Ethics in Animal Research assessed and approved the experimental procedures (report CEEA 2013/036, 19 February 2014).
Data Availability Statement
All data are contained in the article or supplementary material.
Acknowledgments
The authors thank the INIA animal staff for the assistance with animal care and Pedro Cuesta and Iagoba Cano (Department of Research Support, Universidad Complutense de Madrid) for statistical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal Nutrition and Fetal Development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.; Menichini, F.F.; Statti, G.; Menichini, F.F. Biological and Pharmacological Activities of Iridoids: Recent Developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Pardo, Z.; Heras-Molina, A.; Pesantez, J.L.; Encinas, T.; Torres-Rovira, L.; Astiz, S.; Nieto, R.; Ovilo, C.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on hepatic fat accretion and energy and fatty acids profile of fetal tissues. Nutrients 2019, 11, 1534. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Barbero, A.; Pesantez, J.; Zinellu, A.; Berlinguer, F.; Gonzalez-Añover, P.; Gonzalez, J.; Encinas, T.; Torres-Rovira, L.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Placental Gene Expression and Fetal Antioxidant Status, DNA-Methylation and Phenotype. Int. J. Mol. Sci. 2019, 20, 1187. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Gomez, M.; Garcia-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; Gonzalez-Añover, P.; Gomez-Fidalgo, E.; Sanchez-Sanchez, R.; Ovilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Gomez, M.; Heras-Molina, A.; Garcia-Contreras, C.; Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Martinez-Fernandez, B.; Gonzalez, J.; Encinas, T.; Astiz, S.; Ovilo, C.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Postnatal Growth, Metabolism and Body Composition of the Offspring. Antioxidants 2019, 8, 535. [Google Scholar] [CrossRef]
- Shrestha, N.; Sleep, S.L.; Cuffe, J.S.M.; Holland, O.J.; Perkins, A.V.; Yau, S.Y.; McAinch, A.J.; Hryciw, D.H. Role of omega-6 and omega-3 fatty acids in fetal programming. Clin. Exp. Pharm. Physiol. 2020, 47, 907–915. [Google Scholar] [CrossRef]
- Leskanich, C.O.; Noble, R.C. The comparative roles of polyunsaturated fatty acids in pig neonatal development. Br. J. Nutr. 1999, 81, 87–106. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Bell, S.J.; Ausdal, W.V. Omega-3 Fatty Acid supplementation during pregnancy. Rev. Obstet. Gynecol. 2008, 1, 162–169. [Google Scholar]
- Haggarty, P. Fatty Acid Supply to the Human Fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Hachey, D.L. Benefits and risks of modifying maternal fat intake in pregnancy and lactation. Am. J. Clin. Nutr. 1994, 59, 454S–464S. [Google Scholar] [CrossRef] [PubMed]
- Amusquivar, E.; Rupérez, F.J.; Barbas, C.; Herrera, E. Low Arachidonic Acid Rather than α-Tocopherol Is Responsible for the Delayed Postnatal Development in Offspring of Rats Fed Fish Oil Instead of Olive Oil during Pregnancy and Lactation. J. Nutr. 2000, 130, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Thorsdottir, I.; Birgisdottir, B.E.; Halldorsdottir, S.; Geirsson, R.T. Association of Fish and Fish Liver Oil Intake in Pregnancy with Infant Size at Birth among Women of Normal Weight before Pregnancy in a Fishing Community. Am. J. Epidemiol. 2004, 160, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002, 277, 8755–8758. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K. Fatty Acids in Foods and Their Health Implications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Prostek, A.; Gajewska, M.; Kamola, D.; Bałasińska, B. The influence of EPA and DHA on markers of inflammation in 3T3-L1 cells at different stages of cellular maturation. Lipids Health Dis. 2014, 13, 3. [Google Scholar] [CrossRef]
- Heras-Molina, A.; Pesantez-Pacheco, J.L.; Astiz, S.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Encinas, T.; Óvilo, C.; Isabel, B.; Gonzalez-Bulnes, A. Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Effects on Growth, Metabolism, and Body Composition of the Offspring. Animals 2020, 10, 1946. [Google Scholar] [CrossRef]
- Committee on Nutrient Requirements of Swine; Board on Agriculture and Natural Resources; Division on Earth and Life Studies; National Research Council of the National Academies. Nutrient Requirements of Swine: Eleventh Revised Edition; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Torres-Rovira, L.; Barbero, A.; Ayuso, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Developmental Origins of Health and Disease in swine: Implications for animal production and biomedical research. Theriogenology 2016, 86, 110–119. [Google Scholar] [CrossRef]
- Lopez-Bote, C.; Rey, A.; Ruiz, J.; Isabel, B.; Sanz Arias, R. Effect of feeding diets high in monounsaturated fatty acids and α-tocopheryl acetate to rabbits on resulting carcass fatty acid profile and lipid oxidation. Anim. Sci. 1997, 64, 177–186. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Astiz, S.; Torres-Rovira, L.; Sanchez-Sanchez, R.; Gomez-Fidalgo, E.; Gonzalez, J.; Isabel, B.; Rey, A.; Ovilo, C.; et al. Ontogeny of Sex-Related Differences in Foetal Developmental Features, Lipid Availability and Fatty Acid Composition. Int. J. Mol. Sci. 2017, 18, 1171. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Konelab 20—Thermo Scientific—PDF Catalogs | Technical Documentation. Available online: https://pdf.medicalexpo.com/pdf/thermo-scientific/konelab-20/78678-85059.html (accessed on 21 January 2021).
- Rudolph, A.M. The fetal circulation and its response to stress. J. Dev. Physiol. 1984, 6, 11–19. [Google Scholar]
- Webel, S.K.; Otto, E.R.; Webel, D.M.; Moser, R.L.; Spencer, J.D.; Orr, D.E. Effect of protected n-3 polyunsaturated fatty acids (FertiliumTM) on litter size in sows. J. Anim. Sci. 2003, 81, 15000403. [Google Scholar]
- Rooke, J.A.; Sinclair, A.G.; Edwards, S.A.; Cordoba, R.; Pkiyach, S.; Penny, P.C.; Penny, P.; Finch, A.M.; Horgan, G.W. The effect of feeding salmon oil to sows throughout pregnancy on pre-weaning mortality of piglets. Anim. Sci. 2001, 73, 489–500. [Google Scholar] [CrossRef]
- Gonzalez-Añover, P.; Encinas, T.; Torres-Rovira, L.; Pallares, P.; Muñoz-Frutos, J.; Gomez-Izquierdo, E.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Ovulation rate, embryo mortality and intrauterine growth retardation in obese swine with gene polymorphisms for leptin and melanocortin receptors. Theriogenology 2011, 75, 34–41. [Google Scholar] [CrossRef]
- van der Lende, T.; de Jager, D. Death risk and preweaning growth rate of piglets in relation to the within-litter weight distribution at birth. Livest. Prod. Sci. 1991, 28, 73–84. [Google Scholar] [CrossRef]
- Lavery, A.; Lawlor, P.G.; Miller, H.M.; Magowan, E. The Effect of Dietary Oil Type and Energy Intake in Lactating Sows on the Fatty Acid Profile of Colostrum and Milk, and Piglet Growth to Weaning. Animals 2019, 9, 1092. [Google Scholar] [CrossRef]
- Luo, J.; Huang, F.; Xiao, C.; Fang, Z.; Peng, J.; Jiang, S. Responses of growth performance and proinflammatory cytokines expression to fish oil supplementation in lactation sows’ and/or weaned piglets’ diets. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Smit, M.N.; Spencer, J.D.; Patterson, J.L.; Dyck, M.K.; Dixon, W.T.; Foxcroft, G.R. Effects of dietary enrichment with a marine oil-based n-3 LCPUFA supplement in sows with predicted birth weight phenotypes on birth litter quality and growth performance to weaning. Animal 2015, 9, 471–480. [Google Scholar] [CrossRef]
- Grieger, J.A.; Clifton, V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients 2014, 7, 153–178. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Navas, M.D.; Lopez-Villodres, J.A.; Trujillo, M.; Espartero, J.L.; De La Cruz, J.P. Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia–reoxygenation. Neurosci. Lett. 2008, 446, 143–146. [Google Scholar] [CrossRef]
- López de las Hazas, M.-C.; Godinho-Pereira, J.; Macià, A.; Almeida, A.F.; Ventura, M.R.; Motilva, M.-J.; Santos, C.N. Brain uptake of hydroxytyrosol and its main circulating metabolites: Protective potential in neuronal cells. J. Funct. Foods 2018, 46, 110–117. [Google Scholar] [CrossRef]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Müller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Hay, W.W., Jr.; Ehrhardt, R.A. Placental transport of nutrients and its implications for fetal growth. J. Reprod Fertil. Suppl. 1999, 54, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W., Jr. Placental transport of nutrients to the fetus. Horm. Res. 1994, 42, 215–222. [Google Scholar] [CrossRef]
- Hay, W.W., Jr. Recent observations on the regulation of fetal metabolism by glucose. J. Physiol. 2006, 572, 17–24. [Google Scholar] [CrossRef]
- Saintonge, J.; Côté, R. Brain development in relation to fetal weight and maternal glucose tolerance during normal gestation. Brain Dev. 1987, 9, 26–32. [Google Scholar] [CrossRef]
- Girard, J.; Ferre, P.; Pegorier, J.; Duee, P. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol. Rev. 1992, 72, 507–562. [Google Scholar] [CrossRef]
- Johnson, R.N.; Metcalf, P.A.; Baker, J.R. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin. Chim. Acta 1983, 127, 87–95. [Google Scholar] [CrossRef]
- Segovia, S.A.; Vickers, M.H.; Zhang, X.D.; Gray, C.; Reynolds, C.M. Maternal supplementation with conjugated linoleic acid in the setting of diet-induced obesity normalises the inflammatory phenotype in mothers and reverses metabolic dysfunction and impaired insulin sensitivity in offspring. J. Nutr. Biochem. 2015, 26, 1448–1457. [Google Scholar] [CrossRef]
- Flachs, P.; Rossmeisl, M.; Bryhn, M.; Kopecky, J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin. Sci. 2009, 116, 1–16. [Google Scholar] [CrossRef]
- Yang, L.G.; Song, Z.X.; Yin, H.; Wang, Y.Y.; Shu, G.F.; Lu, H.X.; Wang, S.K.; Sun, G.J. Low n-6/n-3 PUFA Ratio Improves Lipid Metabolism, Inflammation, Oxidative Stress and Endothelial Function in Rats Using Plant Oils as n-3 Fatty Acid Source. Lipids 2016, 51, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.M.; Gibney, M.J. Long-chain n-3 polyunsaturated fatty acids and triacylglycerol metabolism in the postprandial state. Lipids 1999, 34, S259–S265. [Google Scholar] [CrossRef]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.T. Fetal metabolism and fetal growth. J. Reprod. Fertil. 1976, 47, 189–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).