Monitoring Means and Results of Biosecurity in Pig Fattening Farms: Systematic Assessment of Measures in Place and Exploration of Biomarkers of Interest
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of a Biosecurity Risk Analysis Tool
2.2. Biomarkers
2.2.1. Clinical Scores and Mortality
2.2.2. Slaughter Check
2.2.3. Antimicrobial Use
dosage (mg/kg/d) × n animals × expected weight at treatment (kg)
2.3. Protocol for Data Collection and the Tailor-Made Biosecurity Plan
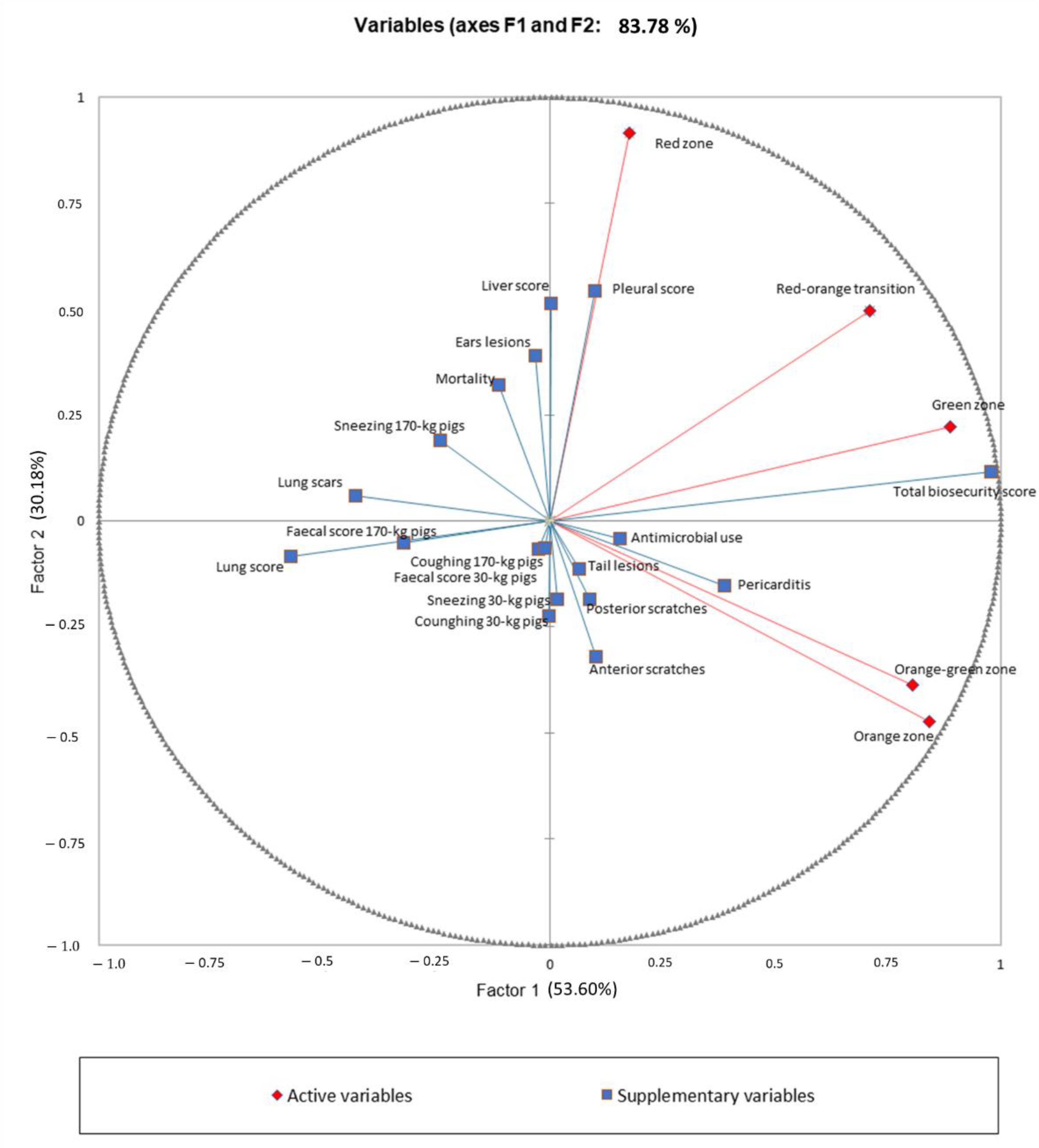
2.4. Data Analysis
| Lesions | Scale | Description |
|---|---|---|
| Lungs | ||
| Lung score (Madec score) | 0–24 | Pneumonic lesions (enzootic pneumonia-like, often due to Mycoplasma hyopneumoniae: purple to grey rubbery consolidation, increased firmness, failure to collapse and marked edema) were scored according to Madec’s grid [29]. Each lobe, except the accessory lobe, was scored from 0 to 4, to give a maximum possible total score of 24. |
| Absence of lesions | 0–1 | Lungs in which all the lobes, except the accessory one, received score 0. |
| Severe lesions | 0–1 | Lungs with a Madec score ≥5/24. |
| Scars | 0–1 | Presence of recovered enzootic pneumonia-like lesions, with thickened interlobular purple to grey (depending on the age) connective tissue which appears as retracted tissue. |
| Abscesses | 0–1 | Presence of at least one abscess in the lungs. |
| Consolidations | 0–1 | Pneumonic lesions complicated by secondary bacterial pathogens (e.g., Pasteurella spp., Bordetella spp.), firmer and heavier than enzootic pneumonia-like lesions. In the case of a cut surface, lesion was mottled by arborized clusters of gray-to-white exudate-distended alveoli, and mucopurulent exudate could be expressed from the airways [30]. |
| Lobular/chessboard pattern lesions | 0–1 | Presence of scattered multifocal spots of purple to grey discoloration indicative of probable co-existence of viruses (Porcine Reproductive and Respiratory Virus, Porcine Circovirus, Influenza Virus) and/or Mycoplasma spp. or foreign body (e.g., dust/particulate matter) [31]. |
| Pleura | ||
| Pleura score(SPES score) | 0–4 | SPES grid [32]. 0: Absence of pleural lesions; 1: Cranioventral pleuritis and/or pleural adherence between lobes or at ventral border of lobes; 2: Dorsocaudal unilateral focal pleuritis; 3: Bilateral pleuritis of type 2 or extended unilateral pleuritis (at least 1/3 of one diaphragmatic lobe); 4: Severely extended bilateral pleuritis (at least 1/3 of both diaphragmatic lobes). Most probable etiology: Actinobacillus pleuropneumoniae, Heamophilus parasuis, Pasteurella spp., Bordetella spp., Mycoplasma hyorhinis. |
| Severe lesions | 0–1 | Pleura with a SPES score ≥3. |
| Sequestra | 0–1 | Presence of at least one sequestra in the lungs (acute: firm, rubbery and mottled dark red purple to lighter white areas with abundant fibrin, and hemorrhagic, necrotic parenchyma; or chronic: resolution of non-necrotic areas from acute infections results in remaining cavitated necrotic foci that are surrounded by scar tissue). Often associated with Actinobacillus pleuropneumoniae infection [33]. |
| Actinobacillus pleuropneumoniae index (APP index) | 0–4 | Frequency of pleuritis lesions with a SPES score ≥2 in a batch mean pleuritis lesion score of animals with SPES ≥2. The APP index ranges from 0 (no animal in the batch showing dorsocaudal pleuritis) to 4 (all animals with severely extended bilateral dorsocaudal pleuritis) [20]. |
| Liver | ||
| Liver score | 1–3 | Scoring based on the number of milk spot lesions due to Ascaris suum presence and their migration. 1: no lesions or less than 4 lesions; 2: from 4 to 10 lesions; 3: more than 10 lesions. |
| Severe lesions | 0–1 | Livers with a score 3. |
| Total lesions | 0–1 | Livers with a score ≥2. |
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Union. Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). Off. J. 2016, 84, 1–208. [Google Scholar]
- Bellini, S.; Casadei, G.; De Lorenzi, G.; Tamba, M. A Review of Risk Factors of African Swine Fever Incursion in Pig Farming within the European Union Scenario. Pathogens 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.; Van Immerseel, F. Biosecurity in Animal Production and Veterinary Medicine; CABI: Wallingford, UK, 2019. [Google Scholar]
- Alarcón, L.V.; Allepuz, A.; Mateu, E. Biosecurity in pig farms: A review. Porc. Health Manag. 2021, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- OIE. Compartmentalisation Guidelines—African Swine Fever; World Organisation for Animal Health (OIE): Paris, France, 2021. [Google Scholar]
- Burton, R.J.; Kuczera, C.; Schwarz, G. Exploring farmers’ cultural resistance to voluntary agri-environmental schemes. Sociol. Rural. 2008, 48, 16–37. [Google Scholar] [CrossRef]
- Kristensen, E.; Jakobsen, E.B. Danish dairy farmers’ perception of biosecurity. Prev. Vet. Med. 2011, 99, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, F.; Donovan, G.A. Biosecurity and risk management for dairy replacements. Vet. Clin. North Am. Food Anim. Pract. 2008, 24, 155–190. [Google Scholar] [CrossRef] [PubMed]
- Collineau, L.; Rojo-Gimeno, C.; Léger, A.; Backhans, A.; Loesken, S.; Nielsen, E.O.; Postma, M.; Emanuelson, U.; Beilage, E.; Sjölund, M.; et al. Herd-specific interventions to reduce antimicrobial usage in pig production without jeopardising technical and economic performance. Prev. Vet. Med. 2017, 144, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Raasch, S.; Collineau, L.; Postma, M.; Backhans, A.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Stärk, K.; Dewulf, J.; et al. Effectiveness of alternative measures to reduce antimicrobial usage in pig production in four European countries. Porc. Health Manag. 2020, 6, 6. [Google Scholar] [CrossRef]
- Postma, M.; Backhans, A.; Collineau, L.; Loesken, S.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Beilage, E.G.; Nielsen, E.O.; Stärk, K.; et al. Evaluation of the relationship between the biosecurity status, production parameters, herd characteristics and antimicrobial usage in farrow-to-finish pig production in four EU countries. Porc. Health Manag. 2016, 2, 9. [Google Scholar] [CrossRef]
- Moore, R.E.; Kirwan, J.; Doherty, M.K.; Whitfield, P.D. Biomarker discovery in animal health and disease: The application of post-genomic technologies. Biomark. Insights 2007, 2, 185–196. [Google Scholar] [CrossRef]
- De Oliveira Sidinei, M.E.A.; Marcato, S.M.; Perez, H.L.; Bánkuti, F.I. Biosecurity, environmental sustainability, and typological characteristics of broiler farms in Paraná State, Brazil. Prev. Vet. Med. 2021, 194, 105426. [Google Scholar] [CrossRef]
- VetInfo, Italian National Zootechnical Registry. Available online: https://www.vetinfo.it/j6_statistiche/#/report-pbi/31 (accessed on 2 October 2021).
- FAO. Available online: https://www.fao.org/ag/againfo/programmes/en/empres/news_060815b.html (accessed on 31 August 2022).
- Indrawan, D.; Cahyadi, E.R.; Daryanto, A.; Hogeveen, H. The role of farm business type on biosecurity practices in West Java broiler farms. Prev. Vet. Med. 2020, 176, 104910. [Google Scholar] [CrossRef]
- Nathues, H.; Spergser, J.; Rosengarten, R.; Kreienbrock, L.; Grosse Beilage, E. Value of the clinical examination in diagnosing enzootic pneumonia in fattening pigs. Vet. J. 2012, 193, 443–447. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Toft, N. Intra- and inter-observer agreement when using a descriptive classification scale for clinical assessment of faecal consistency in growing pigs. Prev. Vet. Med. 2011, 98, 288–291. [Google Scholar] [CrossRef]
- Scollo, A.; Gottardo, F.; Contiero, B.; Mazzoni, C.; Leneveu, P.; Edwards, S.A. Benchmarking of pluck lesions at slaughter as a health monitoring tool for pigs slaughtered at 170 kg (heavy pigs). Prev. Vet. Med. 2017, 144, 20–28. [Google Scholar] [CrossRef]
- Merialdi, G.; Dottori, M.; Bonilauri, P.; Luppi, A.; Gozio, S.; Pozzi, P.S.; Spaggiari, B.; Martelli, P. Survey of pleuritis and pulmonary lesions in pigs at abattoir with a focus on the extent of the condition and herd risk factors. Vet. J. 2012, 193, 234–239. [Google Scholar] [CrossRef]
- Bottacini, M.; Scollo, A.; Edwards, S.A.; Contiero, B.; Veloci, M.; Pace, V.; Gottardo, F. Skin lesion monitoring at slaughter on heavy pigs (170 kg): Welfare indicators and ham defects. PLoS ONE 2018, 13, e0207115. [Google Scholar] [CrossRef]
- EMA. Principles on Assignment of Defined Daily Dose for Animals (DDDvet) and Defined Course Dose for Animals (DCDvet). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/principles-assignment-defined-daily-dose-animals-dddvet-defined-course-dose-animals-dcdvet_en.pdf (accessed on 24 May 2022).
- Donaldson, A. Biosecurity after the event: Risk politics and animal disease. Environ. Plan. A 2008, 40, 1552–1567. [Google Scholar] [CrossRef]
- Diana, A.; Lorenzi, V.; Penasa, M.; Magni, E.; Alborali, G.L.; Bertocchi, L.; De Marchi, M. Effect of welfare standards and biosecurity practices on antimicrobial use in beef cattle. Sci. Rep. 2020, 10, 20939. [Google Scholar] [CrossRef]
- Cliff, N. The eigenvalues-greater-than-one rule and the reliability of components. Psychol. Bull. 1988, 103, 276. [Google Scholar] [CrossRef]
- Delpont, M.; Guinat, C.; Guérin, J.L.; Le Leu, E.; Vaillancourt, J.P.; Paul, M.C. Biosecurity measures in French poultry farms are associated with farm type and location. Prev. Vet. Med. 2021, 195, 105466. [Google Scholar] [CrossRef]
- Karl, C.A.; Andres, D.; Carlos, M.; Peña, M.; Juan, H.O.; Jorge, O. Farm Biosecurity and Influenza A virus detection in Swine Farms: A Comprehensive Study in Colombia. BMC Porc. Health Manag. 2022; under review. [Google Scholar] [CrossRef]
- Siengsanan-Lamont, J.; Kamolsiripichaiporn, S.; Ruanchaimun, S.; Patchimasiri, T.; Jongrakwattana, B.; Blacksell, S.D. Biosafety and Biosecurity Challenges Facing Veterinary Diagnostic Laboratories in Lower-Middle Income Countries in Southeast Asia: A Case Study of Thailand. Appl. Biosaf. 2019, 24, 220–230. [Google Scholar] [CrossRef]
- Madec, F.; Derrien, H. Fréquence, intensité et localisation des lésions pulmonaires chez le porc charcutier. J. Rech. Porc. Fr. 1981, 13, 231–236. [Google Scholar]
- VanAlstine, W.G. Respiratory system. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwarts, K.J., Stevenson, G.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 348–362. [Google Scholar]
- Leneveu, P.; Pommier, P.; Pagot, E.; Morvan, H.; Lewandowski, E. Slaughterhouse Evaluation of Respiratory Tract Lesions in Pigs; RoudennGrafik Inc.: Guingamp/Plérin, France, 2016; 110p. [Google Scholar]
- Dottori, M.; Nigrelli, A.D.; Bonilauri, P.; Merialdi, G.; Gozio, S.; Cominotti, F. Proposta per un nuovo sistema di punteggiatura delle pleuriti suine in sede di macellazione: La griglia SPES (Slaughterhouse Pleurisy Evaluation System). Large Anim. Rev. 2007, 13, 161–165. [Google Scholar]
- Gottschalk, M. Actinobacillosis. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwarts, K.J., Stevenson, G.W., Eds.; John Wiley & Sons Gottschalk Inc.: Hoboken, NJ, USA, 2012; pp. 653–669. [Google Scholar]
- Shannon, C.; Stebbing, P.D.; Dunn, A.M.; Quinn, C.H. Getting on board with biosecurity: Evaluating the effectiveness of marine invasive alien species biosecurity policy for England and Wales. Mar. Policy 2020, 122, 104275. [Google Scholar] [CrossRef]
- Casal, J.; De Manuel, A.; Mateu, E.; Martín, M. Biosecurity measures on swine farms in Spain: Perceptions by farmers and their relationship to current on-farm measures. Prev. Vet. Med. 2007, 82, 138–150. [Google Scholar] [CrossRef]
- Valeeva, N.I.; van Asseldonk, M.A.; Backus, G.B. Perceived risk and strategy efficacy as motivators of risk management strategy adoption to prevent animal diseases in pig farming. Prev. Vet. Med. 2011, 102, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Laanen, M.; Maes, D.; Hendriksen, C.; Gelaude, P.; De Vliegher, S.; Rosseel, Y.; Dewulf, J. Pig, cattle and poultry farmers with a known interest in research have comparable perspectives on disease prevention and on-farm biosecurity. Prev. Vet. Med. 2014, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amass, S.F.; Halbur, P.G.; Byrne, B.A.; Schneider, J.L.; Koons, C.W.; Cornick, N.; Ragland, D. Mechanical transmission of enterotoxigenic Escherichia coli to weaned pigs by people, and biosecurity procedures that prevented such transmission. J. Swine Health Prod. 2003, 11, 61–68. [Google Scholar]
- Moore, C. Biosecurity and minimal disease herds. Vet. Clin. N. Am. Food Anim. Pract. 1992, 8, 461–474. [Google Scholar] [CrossRef]
- Pritchard, G.; Dennis, I.; Waddilove, J. Biosecurity: Reducing disease risks to pig breeding herds. Practice 2005, 27, 230–237. [Google Scholar] [CrossRef]
- Andres, V.M.; Davies, R.H. Biosecurity measures to control Salmonella and other infectious agents in pig farms: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 317–335. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, M.; Goyal, S.M.; Cheeran, M.C.-J.; Torremorell, M. Evaluation of biosecurity measures to prevent indirect transmission of porcine epidemic diarrhea virus. BMC Vet. Res. 2017, 13, 89. [Google Scholar] [CrossRef]
- Arabi, S.A.M.; Guma’a, M.A.A. Biosecurity practices in commercial poultry farms located in ElFashir Locality-Sudan. J. Biol. Pharm. 2021, 1, 33–43. [Google Scholar]
- Da Costa, R.M.; Gasa, J.; Calderón Díaz, J.A. Using the Biocheck.UGentTM scoring tool in Irish farrow-to-finish pig farms: Assessing biosecurity and its relation to productive performance. Porc. Health Manag. 2019, 5, 4. [Google Scholar] [CrossRef]
- Mannion, C.; Egan, J.; Lynch, P.B.; Leonard, F.C. An investigation into the efficacy of washing trucks following the transportation of pigs—A Salmonella perspective. Foodborne Pathog. Dis. 2008, 5, 261–271. [Google Scholar] [CrossRef]
- Caswell, J.L.; Williams, K.J. Respiratory system. In Pathology of Domestic Animals, 6th ed.; Kennedy, J., Ed.; Palmer’s, Saunders Elsevier: St. Louis, MO, USA, 2007; pp. 591–593. [Google Scholar]
- Maes, D.; Segales, J.; Meyns, T.; Sibila, M.; Pieters, M.; Haesebrouck, F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2008, 126, 297–309. [Google Scholar] [CrossRef]
- Gunn, G.J.; Heffernan, C.; Hall, M.; McLeod, A.; Hovi, M. Measuring and comparing constraints to improved biosecurity amongst GB farmers, veterinarians and the auxiliary industries. Prev. Vet. Med. 2008, 84, 310–323. [Google Scholar] [CrossRef]
- Cox, R.; Revie, C.W.; Hurnik, D.; Sanchez, J. Use of Bayesian belief network techniques to explore the interaction of biosecurity practices on the probability of porcine disease occurrence in Canada. Prev. Vet. Med. 2016, 131, 20–30. [Google Scholar] [CrossRef]
- Simon-Grifé, M.; Martín-Valls, G.E.; Vilar-Ares, M.J.; García-Bocanegra, I.; Martín, M.; Mateu, E.; Casal, J. Biosecurity practices in Spanish pig herds: Perceptions of farmers and veterinarians of the most important biosecurity measures. Prev. Vet. Med. 2013, 110, 223–231. [Google Scholar] [CrossRef]
- Roche, S.M.; Kelton, D.F.; Meehan, M.; Von Massow, M.; Jones-Bitton, A. Exploring dairy producer and veterinarian perceptions of barriers and motivators to adopting on-farm management practices for Johne’s disease control in Ontario, Canada. J. Dairy Sci. 2019, 102, 4476–4488. [Google Scholar] [CrossRef]
- Alarcon, P.; Wieland, B.; Mateus, A.L.; Dewberry, C. Pig farmers’ perceptions, attitudes, influences and management of information in the decision-making process for disease control. Prev. Vet. Med. 2014, 116, 223–242. [Google Scholar] [CrossRef]
- Racicot, M.; Venne, D.; Durivage, A.; Vaillancourt, J.P. Evaluation of strategies to enhance biosecurity compliance on poultry farms in Québec: Effect of audits and cameras. Prev. Vet. Med. 2012, 103, 208–218. [Google Scholar] [CrossRef]
- Hernández-Jover, M.; Gilmour, J.; Schembri, N.; Sysak, T.; Holyoake, P.K.; Beilin, R.; Toribio, J.A. Use of stakeholder analysis to inform risk communication and extension strategies for improved biosecurity amongst small-scale pig producers. Prev. Vet. Med. 2012, 104, 258–270. [Google Scholar] [CrossRef]

| Zone | Risk Factor | Objective | Items (n) |
|---|---|---|---|
| Public | Neighbourhood activities | Awareness of at-risk situation due to neighbourhood | 5 |
| External vehicles | Maintain in the public zone vehicles and persons with no necessary access to the professional zone | 4 | |
| Dead animals | Reduce the load of pathogens associated with elimination of dead animals | 3 | |
| Public/professional transition | Contamination from truck and visitors | Prevent contamination of the professional zone by trucks and visitors | 7 |
| Contamination by wildlife | Prevent contamination of the professional zone by wildlife | 1 | |
| Contamination by staff in charge of elimination of dead animals | Prevent contamination by staff in charge of elimination of dead animals in the public zone | 4 | |
| Staff and visitors | Prevent introduction of diseases by staff and visitors entering the farm | 8 | |
| Unnecessary access | No unnecessary access to the professional zone | 4 | |
| Professional | Contamination by wildlife | Prevent contamination of the professional zone by wildlife | 2 |
| Contamination by manure | Prevent contamination by the manure | 2 | |
| Pathogen persistence | Prevent persistence of pathogens in the professional zone by washing procedures and debris removal | 2 | |
| Contamination by staff storing dead animals | Prevent contamination by staff in charge of storing dead animals in the professional zone | 6 | |
| Professional/herd transition | Pathogens from animals | Prevent of pathogens from animals introduced into the herd | 3 |
| Pathogens from other purchases | Prevent introduction of pathogens by other purchases | 2 | |
| Pathogens from shared equipment | Prevent introduction of pathogens by shared equipment entering the farm | 2 | |
| Pathogens from staff/visitors | Prevent introduction of pathogens by staff/visitors | 8 | |
| Unnecessary access | No unnecessary access to the livestock zone | 4 | |
| Herd | Animal contact between age groups | Prevent transmission of pathogens between age groups by animal contacts | 2 |
| Animal contact with contaminated premises | Prevent transmission of pathogens between age groups by premises | 2 | |
| Animal contact with contaminated staff | Prevent transmission of pathogens between age groups by staff | 4 | |
| Animal contact with contaminated materials | Prevent transmission of pathogens between animals by materials and intervention | 5 | |
| High load of pathogens | Reduce risk of exposure to high loads of pathogens | 4 | |
| Heterogeneous herd immunity | Reduce at-risk situations due to heterogeneous herd immunity | 5 | |
| Contaminated feed or water or enrichment material | Prevent contaminated feed or water or enrichment material | 8 |
| First VisitMean ± SD (Minimum-Maximum) | Third VisitMean ± SD (Minimum-Maximum) | p-Value | |
|---|---|---|---|
| Biosecurity scores | |||
| Public zone (%) | 61.0 ± 11.2 (43.7–87.5) | 60.5 ± 10.2 (47.9–87.5) | ns |
| Public/professional transition (%) | 54.2 ± 16.6 (25.0–80.2) | 54.9 ±17.7 (25.0–84.4) | ns |
| Professional zone (%) | 56.2 ± 13.3 (35.0–85.0) | 61.0 ± 14.8 (37.5–92.5) | 0.012 |
| Professional/herd transition (%) | 37.6 ± 7.6 (27.6–48.7) | 38.0 ± 7.7 (27.6–48.7) | ns |
| Herd zone (%) | 69.6 ± 7.0 (58.3–81.5) | 69.7 ± 7.5 (58.3–81.5) | ns |
| Total biosecurity score (%) | 55.7 ± 8.7 (38.7–70.9) | 56.8 ± 9.4 (39.5–77.5) | 0.047 |
| Clinical scores | |||
| Coughing (%) | |||
| 30-kg pigs | 1.1 ± 1.7 (0.0–6.7) | 1.4 ± 1.0 (0.0–3.1) | ns |
| 170-kg pigs | 0.7 ± 0.8 (0.1–3.1) | 0.5 ± 0.5 (0.0–1.2) | ns |
| Sneezing (%) | |||
| 30-kg pigs | 1.5 ± 1.3 (0.2–4.9) | 1.0 ± 0.8 (0.0–2.7) | ns |
| 170-kg pigs | 1.0 ± 1.1 (0.0–3.8) | 0.5 ± 0.5 (0.0–0.4) | ns 1 |
| Faeces score (1–4) | |||
| 30-kg pigs | 1.6 ± 0.4 (1.1–2.4) | 1.6 ± 0.0 (1.1–2.4) | ns |
| 170-kg pigs | 1.0 ± 0.0 (1.0–1.1) | 1.0 ± 0.0 (1.0–1.1) | ns |
| Mortality (%) | 4.8 ± 1.7 (2.7–8.8) | 4.9 ± 1.5 (2.5–7.8) | ns |
| Slaughter checks | |||
| Pluck lesions | |||
| Lung score | 0.5 ± 0.3 (0.0–1.0) | 0.6 ± 0.4 (0.1–1.3) | ns |
| Lung scars (%) | 8.7 ± 5.3 (0.0–18.7) | 6.6 ± 3.1 (3.1–13.6) | ns |
| Pleural score | 0.9 ± 0.3 (0.3–1.5) | 0.7 ± 0.5 (0.2–1.5) | ns |
| Liver score | 1.2 ± 0.1 (1.0–1.4) | 1.3 ± 0.2 (1.1–1.6) | ns |
| Pericarditis (%) | 7.4 ± 5.6 (0.0 16.7) | 4.0 ± 3.6 (0.0–13.8) | ns |
| Skin lesions | |||
| Ear lesions (%) | 2.5 ± 2.9 (0.0–7.8) | 1.0 ± 2.5 (0.0–6.7) | ns |
| Tail lesions (%) | 6.3 ± 10.0 (0.0–27.4) | 1.0 ± 1.1 (0.0–2.8) | ns |
| Posterior scratches (%) | 16.5 ± 10.9 (3.9–40.3) | 2.0 ± 1.9 (0.0–5.0) | 0.027 |
| Anterior scratches (%) | 19.6 ± 15.2 (0.0–58.8) | 11.7 ± 7.1 (0.2–20.4) | ns |
| AMU (DDDvet/PCU) | 22.7 ± 11.0 (2.8–40.8) | 15.2 ± 13.6 (1.1–41.2) | ns 2 |
| Item | Cluster A | Cluster B | Cluster C | Cluster D |
|---|---|---|---|---|
| N. farms | 4 | 8 | 2 | 1 |
| Active variables | ||||
| Biosecurity scores | ||||
| Public zone (%) | 0.0 ± 0.0 | 1.3 ± 2.5 | −8.3 ± 2.9 | 0.0 ± 0.0 |
| Public/professional transition (%) | 2.8 ± 1.6 | 0.0 ± 0.0 | −2.6 ± 0.7 | 4.1 ± 0.0 |
| Professional zone (%) | 7.5 ± 5.4 | 0.3 ± 0.9 | 8.7 ± 5.3 | 22.5 ± 0.0 |
| Professional/herd transition (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.9 | 3.9 ± 0.0 |
| Herd zone (%) | 0.7 ± 1.3 | −0.1 ± 0.3 | −1.4 ± 2.0 | 2.8 ± 0.0 |
| Supplementary Variables | ||||
| Biosecurity scores | ||||
| Total biosecurity score (%) | 2.2 ± 1.2 | 0.3 ± 0.4 | −0.6 ± 2.3 | 6.7 ± 0.0 |
| Clinical scores | ||||
| Coughing (%) | ||||
| 30-kg pigs | 0.0 ± 0.6 | −0.3 ± 2.0 | 0.9 ± 0.5 | 0.7 ± 0.0 |
| 170-kg pigs | −0.4 ± 0.7 | −0.1 ± 0.6 | 0.2 ± 0.6 | 0.0 ± 0.0 |
| Sneezing (%) | ||||
| 30-kg pigs | −0.7 ± 1.4 | −0.4 ± 1.4 | 0.0 ± 0.1 | −0.5 ± 0.0 |
| 170-kg pigs | −0.4 ± 1.3 | −0.4 ± 0.6 | −0.2 ± 0.8 | −1.7 ± 0.0 |
| Faeces score (1–4) | ||||
| 30-kg pigs | 0.0 ± 0.3 | 0.0 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 170-kg pigs | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 | −0.1 ± 0.0 |
| Mortality (%) | −1.1 ± 1.7 | 0.5 ± 2.5 | −1.2 ± 1.7 | −0.1 ± 0.0 |
| Slaughter checks | ||||
| Pluck lesions | ||||
| Lung score | −0.2 ± 0.4 | 0.2 ± 4.5 | 0.3 ± 1.4 | −0.5 ± 0.0 |
| Lung scars (%) | −7.0 ± 3.2 | 0.2 ± 4.7 | −3.7 ± 15.1 | −5.4 ± 0.0 |
| Pleural score | −0.2 ± 0.4 | 0.1 ± 0.3 | −0.8 ± 0.5 | 0.2 ± 0.0 |
| Liver score | 0.3 ± 0.2 | 0.1 ± 0.0 | −0.1 ± 0.0 | 0.0 ± 0.0 |
| Pericarditis (%) | −5.0 ± 6.4 | −6.1 ± 7.1 | −2.8 ±9.7 | 11.7 ± 0.0 |
| Skin lesions | ||||
| Ear lesions (%) | 0.0 ± 0.0 | −1.7 ± 3.8 | −6.2 ± 3.6 | −2.2 ± 0.0 |
| Tail lesions (%) | 2.1 ± 0.5 | −7.4 ± 13.4 | 0.6 ± 0.4 | −4.5 ± 0.0 |
| Posterior scratches (%) | −2.8 ± 8.3 | −13.9 ± 8.6 | −6.2 ± 8.3 | −10.8 ± 0.0 |
| Anterior scratches (%) | −0.9 ± 22.6 | −21.4 ± 22.3 | 12.5 ± 22.4 | −12.3 ± 0.0 |
| AMU (DDDvet/PCU) | 3.0 ± 20.3 | −12.8 ± 10.8 | −3.7 ± 13.8 | −13.9 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scollo, A.; Levallois, P.; Fourichon, C.; Motta, A.; Mannelli, A.; Lombardo, F.; Ferrari, P. Monitoring Means and Results of Biosecurity in Pig Fattening Farms: Systematic Assessment of Measures in Place and Exploration of Biomarkers of Interest. Animals 2022, 12, 2655. https://doi.org/10.3390/ani12192655
Scollo A, Levallois P, Fourichon C, Motta A, Mannelli A, Lombardo F, Ferrari P. Monitoring Means and Results of Biosecurity in Pig Fattening Farms: Systematic Assessment of Measures in Place and Exploration of Biomarkers of Interest. Animals. 2022; 12(19):2655. https://doi.org/10.3390/ani12192655
Chicago/Turabian StyleScollo, Annalisa, Pierre Levallois, Christine Fourichon, Ambra Motta, Alessandro Mannelli, Francesco Lombardo, and Paolo Ferrari. 2022. "Monitoring Means and Results of Biosecurity in Pig Fattening Farms: Systematic Assessment of Measures in Place and Exploration of Biomarkers of Interest" Animals 12, no. 19: 2655. https://doi.org/10.3390/ani12192655
APA StyleScollo, A., Levallois, P., Fourichon, C., Motta, A., Mannelli, A., Lombardo, F., & Ferrari, P. (2022). Monitoring Means and Results of Biosecurity in Pig Fattening Farms: Systematic Assessment of Measures in Place and Exploration of Biomarkers of Interest. Animals, 12(19), 2655. https://doi.org/10.3390/ani12192655






