Pathological Similarities in the Development of Papillomavirus-Associated Cancer in Humans, Dogs, and Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. HPV Viral Life Cycle
3. Pathophysiology in Human, Canis familiaris (Cf), and Felis catus (Fc) Papillomavirus-Associated Cancer Development
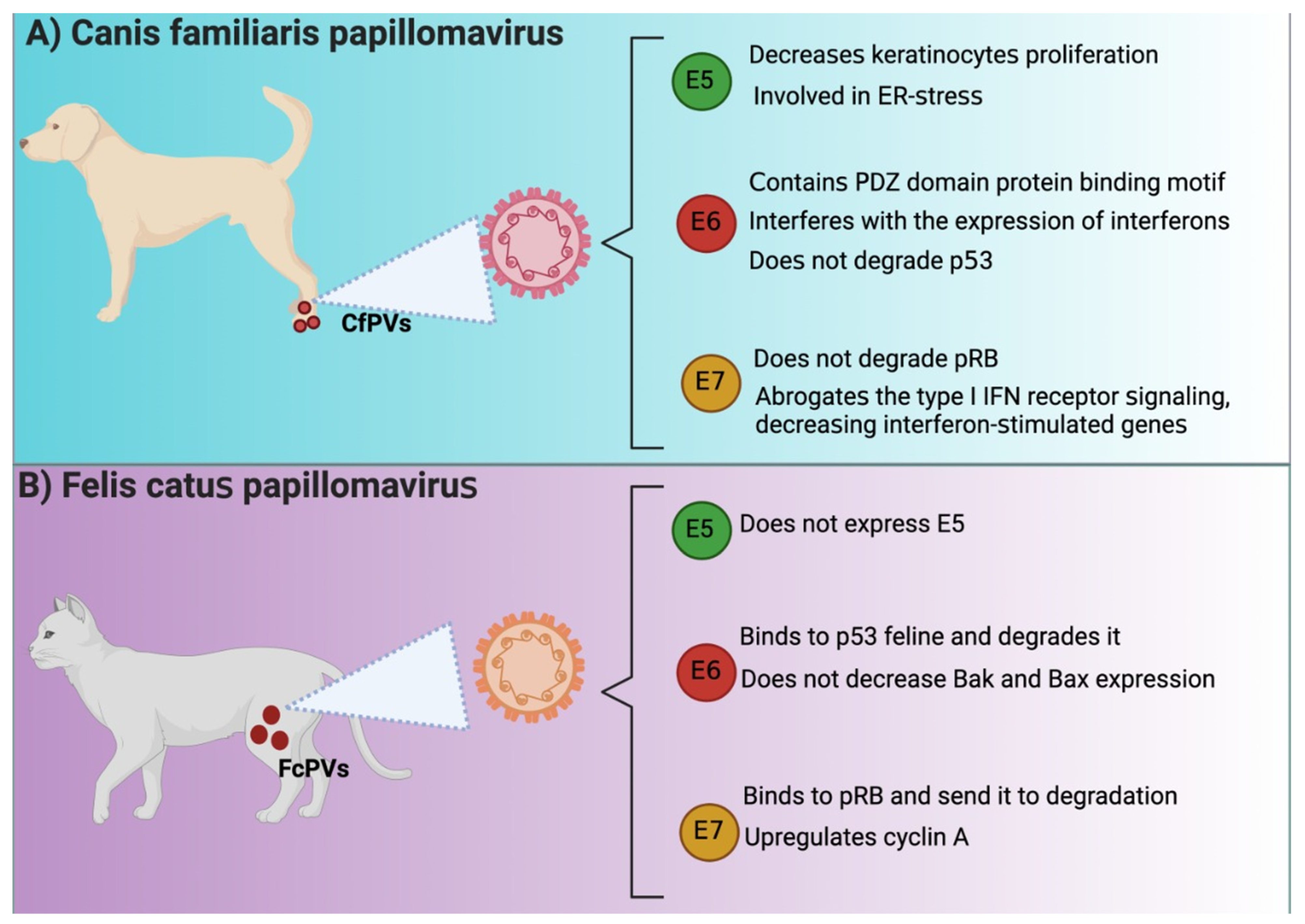
4. PV E6 Oncoprotein as a Biomarker of Carcinogenesis in HPV, CfPV, and FcPV
5. PV E7 Oncoprotein as a Biomarker of Carcinogenesis in HPV, CfPV, and FcPV
6. PV E5 Oncoprotein as a Biomarker of Carcinogenesis in HPV, CfPV, and FcPV
7. Conclusions and Conclusions Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munday, J.S.; Thomson, N.A.; Luff, J.A. Papillomaviruses in Dogs and Cats. Vet. J. 2017, 225, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rector, A.; Van Ranst, M. Animal Papillomaviruses. Virology 2013, 445, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Thomson, N.A. Papillomaviruses in Domestic Cats. Viruses 2021, 13, 1664. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. The Papillomavirus E2 Proteins. Virology 2013, 445, 57–79. [Google Scholar] [CrossRef]
- Doorbar, J. The E4 Protein; Structure, Function and Patterns of Expression. Virology 2013, 445, 80–98. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human Papillomavirus Molecular Biology and Disease Association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef]
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef]
- Aksoy, P.; Gottschalk, E.Y.; Meneses, P.I. HPV Entry into Cells. Mutat. Res. Rev. Mutat. Res. 2017, 772, 13–22. [Google Scholar] [CrossRef]
- Woodham, A.W.; Silva, D.M.D.; Skeate, J.G.; Raff, A.B.; Ambroso, M.R.; Brand, H.E.; Isas, J.M.; Langen, R.; Kast, W.M. The S100A10 Subunit of the Annexin A2 Heterotetramer Facilitates L2-Mediated Human Papillomavirus Infection. PLoS ONE 2012, 7, e43519. [Google Scholar] [CrossRef]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Guion, L.G.M.; Keiffer, T.R.; Sapp, M. Human Papillomavirus Major Capsid Protein L1 Remains Associated with the Incoming Viral Genome throughout the Entry Process. J. Virol. 2017, 91, e00537-17. [Google Scholar] [CrossRef]
- Bergant Marušič, M.; Ozbun, M.A.; Campos, S.K.; Myers, M.P.; Banks, L. Human Papillomavirus L2 Facilitates Viral Escape from Late Endosomes via Sorting Nexin 17. Traffic 2012, 13, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Caodaglio, A.S.; Sichero, L. Regulation of HPV Transcription. Clinics 2018, 73, e486. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Pim, D.; Banks, L. The Role of the E6-P53 Interaction in the Molecular Pathogenesis of HPV. Oncogene 1999, 18, 7690–7700. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, W.; Roman, A. The E7 Proteins of Low- and High-Risk Human Papillomaviruses Share the Ability to Target the PRB Family Member P130 for Degradation. Proc. Natl. Acad. Sci. USA 2006, 103, 437–442. [Google Scholar] [CrossRef]
- Cullen, A.P.; Reid, R.; Campion, M.; Lörincz, A.T. Analysis of the Physical State of Different Human Papillomavirus DNAs in Intraepithelial and Invasive Cervical Neoplasm. J. Virol. 1991, 65, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Vinokurova, S.; Wentzensen, N.; Kraus, I.; Klaes, R.; Driesch, C.; Melsheimer, P.; Kisseljov, F.; Dürst, M.; Schneider, A.; von Knebel Doeberitz, M. Type-Dependent Integration Frequency of Human Papillomavirus Genomes in Cervical Lesions. Cancer Res. 2008, 68, 307–313. [Google Scholar] [CrossRef]
- McBride, A.A.; Warburton, A. The Role of Integration in Oncogenic Progression of HPV-Associated Cancers. PLoS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef]
- Gray, E.; Pett, M.R.; Ward, D.; Winder, D.M.; Stanley, M.A.; Roberts, I.; Scarpini, C.G.; Coleman, N. In Vitro Progression of Human Papillomavirus 16 Episome-Associated Cervical Neoplasia Displays Fundamental Similarities to Integrant-Associated Carcinogenesis. Cancer Res. 2010, 70, 4081–4091. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Pedraza-Chaverri, J. Nuclear Factor Erythroid 2-Related Factor 2 in Human Papillomavirus-Related Cancers. Rev. Med. Virol. 2021, 32, e2308. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Manzo-Merino, J.; Lizano, M. Cellular Redox, Cancer and Human Papillomavirus. Virus Res. 2018, 246, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Chen Wongworawat, Y.; Filippova, M.; Williams, V.M.; Filippov, V.; Duerksen-Hughes, P.J. Chronic Oxidative Stress Increases the Integration Frequency of Foreign DNA and Human Papillomavirus 16 in Human Keratinocytes. Am. J. Cancer Res. 2016, 6, 764–780. [Google Scholar] [PubMed]
- zur Hausen, H. Papillomavirus Infections—A Major Cause of Human Cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Fakhry, C.; Gillison, M.L. Clinical Implications of Human Papillomavirus in Head and Neck Cancers. J. Clin. Oncol. 2006, 24, 2606–2611. [Google Scholar] [CrossRef]
- Yuan, H.; Ghim, S.; Newsome, J.; Apolinario, T.; Olcese, V.; Martin, M.; Delius, H.; Felsburg, P.; Jenson, B.; Schlegel, R. An Epidermotropic Canine Papillomavirus with Malignant Potential Contains an E5 Gene and Establishes a Unique Genus. Virology 2007, 359, 28–36. [Google Scholar] [CrossRef]
- Tobler, K.; Favrot, C.; Nespeca, G.; Ackermann, M. Detection of the Prototype of a Potential Novel Genus in the Family Papillomaviridae in Association with Canine Epidermodysplasia Verruciformis. J. Gen. Virol. 2006, 87, 3551–3557. [Google Scholar] [CrossRef]
- Lange, C.E.; Tobler, K.; Ackermann, M.; Panakova, L.; Thoday, K.L.; Favrot, C. Three Novel Canine Papillomaviruses Support Taxonomic Clade Formation. J. Gen. Virol. 2009, 90, 2615–2621. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Stanley, M.A. The Immunology of Animal Papillomaviruses. Vet. Immunol. Immunopathol. 2000, 73, 101–127. [Google Scholar] [CrossRef]
- Harwood, C.A.; Surentheran, T.; McGregor, J.M.; Spink, P.J.; Leigh, I.M.; Breuer, J.; Proby, C.M. Human Papillomavirus Infection and Non-Melanoma Skin Cancer in Immunosuppressed and Immunocompetent Individuals. J. Med. Virol. 2000, 61, 289–297. [Google Scholar] [CrossRef]
- Keresztes, G.; Mutai, H.; Heller, S. TMC and EVER Genes Belong to a Larger Novel Family, the TMC Gene Family Encoding Transmembrane Proteins. BMC Genom. 2003, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, F.; Banks, L. The Human Papillomavirus E6 Protein and Its Contribution to Malignant Progression. Oncogene 2001, 20, 7874–7887. [Google Scholar] [CrossRef] [PubMed]
- Vande Pol, S.B.; Klingelhutz, A.J. Papillomavirus E6 Oncoproteins. Virology 2013, 445, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Denk, C.; Butz, K.; Schneider, A.; Dürst, M.; Hoppe-Seyler, F. P53 Mutations Are Rare Events in Recurrent Cervical Cancer. J. Mol. Med. 2001, 79, 283–288. [Google Scholar] [CrossRef]
- Tomaić, V.; Pim, D.; Banks, L. The Stability of the Human Papillomavirus E6 Oncoprotein Is E6AP Dependent. Virology 2009, 393, 7–10. [Google Scholar] [CrossRef]
- Baleja, J.D.; Cherry, J.J.; Liu, Z.; Gao, H.; Nicklaus, M.C.; Voigt, J.H.; Chen, J.J.; Androphy, E.J. Identification of Inhibitors to Papillomavirus Type 16 E6 Protein Based on Three-Dimensional Structures of Interacting Proteins. Antivir. Res. 2006, 72, 49–59. [Google Scholar] [CrossRef]
- Sterlinko Grm, H.; Weber, M.; Elston, R.; McIntosh, P.; Griffin, H.; Banks, L.; Doorbar, J. Inhibition of E6-Induced Degradation of Its Cellular Substrates by Novel Blocking Peptides. J. Mol. Biol. 2004, 335, 971–985. [Google Scholar] [CrossRef]
- Pim, D.; Bergant, M.; Boon, S.S.; Ganti, K.; Kranjec, C.; Massimi, P.; Subbaiah, V.K.; Thomas, M.; Tomaić, V.; Banks, L. Human Papillomaviruses and the Specificity of PDZ Domain Targeting. FEBS J. 2012, 279, 3530–3537. [Google Scholar] [CrossRef]
- Lee, H.-J.; Zheng, J.J. PDZ Domains and Their Binding Partners: Structure, Specificity, and Modification. Cell Commun. Signal. 2010, 8, 8. [Google Scholar] [CrossRef]
- Thomas, M.; Narayan, N.; Pim, D.; Tomaić, V.; Massimi, P.; Nagasaka, K.; Kranjec, C.; Gammoh, N.; Banks, L. Human Papillomaviruses, Cervical Cancer and Cell Polarity. Oncogene 2008, 27, 7018–7030. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Weiss, R.S.; Javier, R.T. Binding of Human Virus Oncoproteins to HDlg/SAP97, a Mammalian Homolog of the Drosophila Discs Large Tumor Suppressor Protein. Proc. Natl. Acad. Sci. USA 1997, 94, 6670–6675. [Google Scholar] [CrossRef] [PubMed]
- Banks, L.; Pim, D.; Thomas, M. Human Tumour Viruses and the Deregulation of Cell Polarity in Cancer. Nat. Rev. Cancer 2012, 12, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Laimins, L.A. Role of the PDZ Domain-Binding Motif of the Oncoprotein E6 in the Pathogenesis of Human Papillomavirus Type 31. J. Virol. 2004, 78, 12366–12377. [Google Scholar] [CrossRef]
- Spanos, W.C.; Hoover, A.; Harris, G.F.; Wu, S.; Strand, G.L.; Anderson, M.E.; Klingelhutz, A.J.; Hendriks, W.; Bossler, A.D.; Lee, J.H. The PDZ Binding Motif of Human Papillomavirus Type 16 E6 Induces PTPN13 Loss, Which Allows Anchorage-Independent Growth and Synergizes with Ras for Invasive Growth. J. Virol. 2008, 82, 2493–2500. [Google Scholar] [CrossRef]
- Song, S.; Liem, A.; Miller, J.A.; Lambert, P.F. Human Papillomavirus Types 16 E6 and E7 Contribute Differently to Carcinogenesis. Virology 2000, 267, 141–150. [Google Scholar] [CrossRef]
- Quinlan, S.; May, S.; Weeks, R.; Yuan, H.; Luff, J.A. Abrogation of Constitutive and Induced Type I and Type III Interferons and Interferon-Stimulated Genes in Keratinocytes by Canine Papillomavirus 2 E6 and E7. Viruses 2020, 12, E677. [Google Scholar] [CrossRef]
- Gutiérrez-González, L.H.; Santos-Mendoza, T. Viral Targeting of PDZ Polarity Proteins in the Immune System as a Potential Evasion Mechanism. FASEB J. 2019, 33, 10607–10617. [Google Scholar] [CrossRef]
- Quinlan, S.; May, S.; Weeks, R.; Yuan, H.; Luff, J. Canine Papillomavirus 2 E6 Does Not Interfere With UVB-Induced Upregulation of P53 and P53-Regulated Genes. Front. Vet. Sci. 2021, 8, 570982. [Google Scholar] [CrossRef]
- Altamura, G.; Corteggio, A.; Pacini, L.; Conte, A.; Pierantoni, G.M.; Tommasino, M.; Accardi, R.; Borzacchiello, G. Transforming Properties of Felis Catus Papillomavirus Type 2 E6 and E7 Putative Oncogenes in Vitro and Their Transcriptional Activity in Feline Squamous Cell Carcinoma in Vivo. Virology 2016, 496, 1–8. [Google Scholar] [CrossRef]
- Boyer, S.N.; Wazer, D.E.; Band, V. E7 Protein of Human Papilloma Virus-16 Induces Degradation of Retinoblastoma Protein through the Ubiquitin-Proteasome Pathway. Cancer Res. 1996, 56, 4620–4624. [Google Scholar] [PubMed]
- Giacinti, C.; Giordano, A. RB and Cell Cycle Progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Münger, K.; Basile, J.R.; Duensing, S.; Eichten, A.; Gonzalez, S.L.; Grace, M.; Zacny, V.L. Biological Activities and Molecular Targets of the Human Papillomavirus E7 Oncoprotein. Oncogene 2001, 20, 7888–7898. [Google Scholar] [CrossRef]
- Roman, A.; Munger, K. The Papillomavirus E7 Proteins. Virology 2013, 445, 138–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, D.; Prabhu, A.; Schlegel, R.; Yuan, H. The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein. PLoS Pathog. 2010, 6, e1001089. [Google Scholar] [CrossRef]
- Cornet, I.; Bouvard, V.; Campo, M.S.; Thomas, M.; Banks, L.; Gissmann, L.; Lamartine, J.; Sylla, B.S.; Accardi, R.; Tommasino, M. Comparative Analysis of Transforming Properties of E6 and E7 from Different Beta Human Papillomavirus Types. J. Virol. 2012, 86, 2366–2370. [Google Scholar] [CrossRef]
- DiMaio, D.; Petti, L.M. The E5 Proteins. Virology 2013, 445, 99–114. [Google Scholar] [CrossRef]
- Crusius, K.; Auvinen, E.; Steuer, B.; Gaissert, H.; Alonso, A. The Human Papillomavirus Type 16 E5-Protein Modulates Ligand-Dependent Activation of the EGF Receptor Family in the Human Epithelial Cell Line HaCaT. Exp. Cell Res. 1998, 241, 76–83. [Google Scholar] [CrossRef]
- Di Domenico, F.; Foppoli, C.; Blarzino, C.; Perluigi, M.; Paolini, F.; Morici, S.; Coccia, R.; Cini, C.; De Marco, F. Expression of Human Papilloma Virus Type 16 E5 Protein in Amelanotic Melanoma Cells Regulates Endo-Cellular PH and Restores Tyrosinase Activity. J. Exp. Clin. Cancer Res. 2009, 28, 4. [Google Scholar] [CrossRef][Green Version]
- Kim, S.-H.; Oh, J.-M.; No, J.-H.; Bang, Y.-J.; Juhnn, Y.-S.; Song, Y.-S. Involvement of NF-KappaB and AP-1 in COX-2 Upregulation by Human Papillomavirus 16 E5 Oncoprotein. Carcinogenesis 2009, 30, 753–757. [Google Scholar] [CrossRef]
- Oh, J.-M.; Kim, S.-H.; Cho, E.-A.; Song, Y.-S.; Kim, W.-H.; Juhnn, Y.-S. Human Papillomavirus Type 16 E5 Protein Inhibits Hydrogen-Peroxide-Induced Apoptosis by Stimulating Ubiquitin-Proteasome-Mediated Degradation of Bax in Human Cervical Cancer Cells. Carcinogenesis 2010, 31, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Condjella, R.; Liu, X.; Suprynowicz, F.; Yuan, H.; Sudarshan, S.; Dai, Y.; Schlegel, R. The Canine Papillomavirus E5 Protein Signals from the Endoplasmic Reticulum. J. Virol. 2009, 83, 12833–12841. [Google Scholar] [CrossRef]
- Momoi, T. Caspases Involved in ER Stress-Mediated Cell Death. J. Chem. Neuroanat. 2004, 28, 101–105. [Google Scholar] [CrossRef]
- Urbano, A.C.; Nascimento, C.; Soares, M.; Correia, J.; Ferreira, F. Clinical Relevance of the Serum CTLA-4 in Cats with Mammary Carcinoma. Sci. Rep. 2020, 10, 3822. [Google Scholar] [CrossRef]
- Gameiro, A.; Urbano, A.C.; Ferreira, F. Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma. Vet. Sci. 2021, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; De Zan, G.; Cardazzo, B.; Bargelloni, L.; Castagnaro, M. Feline Mammary Tumours in Comparative Oncology. J. Dairy Res. 2005, 72, 98–106. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Pedraza-Chaverri, J. Pathological Similarities in the Development of Papillomavirus-Associated Cancer in Humans, Dogs, and Cats. Animals 2022, 12, 2390. https://doi.org/10.3390/ani12182390
Cruz-Gregorio A, Aranda-Rivera AK, Pedraza-Chaverri J. Pathological Similarities in the Development of Papillomavirus-Associated Cancer in Humans, Dogs, and Cats. Animals. 2022; 12(18):2390. https://doi.org/10.3390/ani12182390
Chicago/Turabian StyleCruz-Gregorio, Alfredo, Ana Karina Aranda-Rivera, and José Pedraza-Chaverri. 2022. "Pathological Similarities in the Development of Papillomavirus-Associated Cancer in Humans, Dogs, and Cats" Animals 12, no. 18: 2390. https://doi.org/10.3390/ani12182390
APA StyleCruz-Gregorio, A., Aranda-Rivera, A. K., & Pedraza-Chaverri, J. (2022). Pathological Similarities in the Development of Papillomavirus-Associated Cancer in Humans, Dogs, and Cats. Animals, 12(18), 2390. https://doi.org/10.3390/ani12182390







