Evaluation of the Fecal Proteome in Healthy and Diseased Cheetahs (Acinonyx jubatus) Suffering from Gastrointestinal Disorders
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Sample Preparation and Two-Dimensional Electrophoresis (2DE)
2.3. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durant, S.M.; Mitchell, N.; Groom, R.; Pettorelli, N.; Ipavec, A.; Jacobson, A.P.; Woodroffe, R.; Böhm, M.; Hunter, L.T.B.; Becker, M.S.; et al. The global decline of cheetah Acinonyx jubatus and what it means for conservation. Proc. Natl. Acad. Sci. USA 2017, 114, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Munson, L. Diseases of captive cheetahs (Acinonyx jubatus): Results of the cheetah research council pathology survey, 1989–1992. Zoo Biol. 1993, 12, 105–124. [Google Scholar] [CrossRef]
- Terio, K.A.; Munson, L.; Marker, L.; Aldridge, B.M.; Solnick, J.V. Comparison of Helicobacter spp. in Cheetahs (Acinonyx jubatus) with and without Gastritis. J. Clin. Microbiol. 2005, 43, 229–234. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Pasquali, C.; Appel, R.D.; Ou, K.; Golaz, O.; Sanchez, J.-C.; Yan, J.X.; Gooley, A.A.; Hughes, G.; Humphery-Smith, I.; et al. From Proteins to Proteomes: Large Scale Protein Identification by Two-Dimensional Electrophoresis and Arnino Acid Analysis. Nat. Biotechnol. 1996, 14, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, K.; Huang, C.; Nice, E.C. Mining the fecal proteome: From biomarkers to personalised medicine. Expert Rev. Proteom. 2017, 14, 445–459. [Google Scholar] [CrossRef]
- Cerquetella, M.; Marchegiani, A.; Mangiaterra, S.; Rossi, G.; Gavazza, A.; Tesei, B.; Spaterna, A.; Sagratini, G.; Ricciutelli, M.; Polzonetti, V.; et al. Faecal proteome in clinically healthy dogs and cats: Findings in pooled faeces from 10 cats and 10 dogs. Vet. Rec. Open 2021, 8, e9. [Google Scholar] [CrossRef]
- Cerquetella, M.; Rossi, G.; Spaterna, A.; Tesei, B.; Gavazza, A.; Pengo, G.; Pucciarelli, S.; Scortichini, L.; Sagratini, G.; Ricciutelli, M.; et al. Fecal Proteomic Analysis in Healthy Dogs and in Dogs Suffering from Food Responsive Diarrhea. Sci. World J. 2019, 2019, 2742401. [Google Scholar] [CrossRef]
- Rossi, G.; Gavazza, A.; Vincenzetti, S.; Mangiaterra, S.; Galosi, L.; Marchegiani, A.; Pengo, G.; Sagratini, G.; Ricciutelli, M.; Cerquetella, M. Clinicopathological and Fecal Proteome Evaluations in 16 Dogs Presenting Chronic Diarrhea Associated with Lymphangiectasia. Vet. Sci. 2021, 8, 242. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Henze, A.; Homann, T.; Serteser, M.; Can, O.; Sezgin, O.; Coskun, A.; Unsal, I.; Schweigert, F.; Ozpinar, A. Post-translational modifications of transthyretin affect the triiodonine-binding potential. J. Cell. Mol. Med. 2015, 19, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.; Bahl, J.M.; Tanassi, J.T.; Simonsen, A.H.; Heegaard, N.H. Characterization and stability of transthyretin isoforms in cerebrospinal fluid examined by immunoprecipitation and high-resolution mass spectrometry of intact protein. Methods 2012, 56, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Khan, S.; Rahman, S.; Singh, L.R. The Extracellular Protein, Transthyretin Is an Oxidative Stress Biomarker. Front. Physiol. 2019, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Obici, L.; Suhr, O.B. Diagnosis and treatment of gastrointestinal dysfunction in hereditary TTR amyloidosis. Clin. Auton. Res. 2019, 29 (Suppl. S1), 55–63. [Google Scholar] [CrossRef] [PubMed]
- Martin-Vaquero, P.; DA Costa, R.C.; Allen, M.J.; Moore, S.; Keirsey, J.K.; Green, K.B. Proteomic Analysis of Cerebrospinal Fluid in Canine Cervical Spondylomyelopathy. Spine 2015, 40, 601–612. [Google Scholar] [CrossRef]
- Kuleš, J.; Horvatić, A.; Guillemin, N.; Ferreira, R.F.; Mischke, R.; Mrljak, V.; Chadwick, C.C.; Eckersall, P.D. The plasma proteome and the acute phase protein response in canine pyometra. J. Proteom. 2020, 223, 103817. [Google Scholar] [CrossRef]
- Koester, D.C.; Wildt, D.E.; Maly, M.; Comizzoli, P.; Crosier, A.E. Non-invasive identification of protein biomarkers for early pregnancy diagnosis in the cheetah (Acinonyx jubatus). PLoS ONE 2017, 12, e0188575. [Google Scholar] [CrossRef]
- Curry, E.; Stoops, M.; Roth, T. Non-invasive detection of candidate pregnancy protein biomarkers in the feces of captive polar bears (Ursus maritimus). Theriogenology 2012, 78, 308–314. [Google Scholar] [CrossRef]
- O’Reilly, E.L.; Horvatić, A.; Kuleš, J.; Gelemanović, A.; Mrljak, V.; Huang, Y.; Brady, N.; Chadwick, C.C.; Eckersall, P.D.; Ridyard, A. Faecal proteomics in the identification of biomarkers to differentiate canine chronic enteropathies. J. Proteom. 2021, 254, 104452. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, Z.; Hu, H.; Zhu, Y.; Zhang, W.; Tang, Q.; Huang, R.; Gao, F.; Zou, C.; Wang, G.; et al. IgG Fc Binding Protein (FCGBP) is Down-Regulated in Metastatic Lesions and Predicts Survival in Metastatic Colorectal Cancer Patients. OncoTargets Ther. 2021, 14, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ogata, H.; Morikawa, M.; Iijima, S.; Harada, N.; Yoshida, T.; Brown, W.R.; Inoue, N.; Hamada, Y.; Ishii, H.; et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002, 51, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Thomsson, K.A.; Hansson, G.C. Proteomic Analyses of the Two Mucus Layers of the Colon Barrier Reveal That Their Main Component, the Muc2 Mucin, Is Strongly Bound to the Fcgbp Protein. J. Proteome Res. 2009, 8, 3549–3557. [Google Scholar] [CrossRef]
- Harada, N.; Iijima, S.; Kobayashi, K.; Yoshida, T.; Brown, W.R.; Hibi, T.; Oshima, A.; Morikawa, M. Human IgGFc Binding Protein (FcγBP) in Colonic Epithelial Cells Exhibits Mucin-like Structure. J. Biol. Chem. 1997, 272, 15232–15241. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Blaser, M.J.; Brown, W.R. Identification of a unique IgG Fc binding site in human intestinal epithelium. J. Immunol. 1989, 143, 2567–2574. [Google Scholar] [PubMed]
- Granzier, H.L.; Labeit, S. The Giant Protein Titin. A Major Player in Myocardial Mechanics, Signaling, and Disease. Circ. Res. 2004, 94, 284–295. [Google Scholar] [CrossRef]
- Guevel, L.; Lavoie, J.R.; Perez-Iratxeta, C.; Rouger, K.; Dubreil, L.; Feron, M.; Talon, S.; Brand, M.; Megeney, L.A. Quantitative Proteomic Analysis of Dystrophic Dog Muscle. J. Proteome Res. 2011, 10, 2465–2478. [Google Scholar] [CrossRef]
- Dalpe, G.; Leclerc, N.; Vallée, A.; Messer, A.; Mathieu, M.; De Repentigny, Y.; Kothary, R. Dystonin Is Essential for Maintaining Neuronal Cytoskeleton Organization. Mol. Cell. Neurosci. 1998, 10, 243–257. [Google Scholar] [CrossRef]
- Ali, A.; Hu, L.; Zhao, F.; Qiu, W.; Wang, P.; Ma, X.; Zhang, Y.; Chen, L.; Qian, A. BPAG1, a distinctive role in skin and neurological diseases. Semin. Cell Dev. Biol. 2017, 69, 34–39. [Google Scholar] [CrossRef]
- No, J.H.; Das Nishu, S.; Hong, J.-K.; Lyou, E.S.; Kim, M.S.; Wee, G.N.; Lee, T.K. Raman-Deuterium Isotope Probing and Metagenomics Reveal the Drought Tolerance of the Soil Microbiome and Its Promotion of Plant Growth. mSystems 2022, 7, e0124921. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chou, H.-C.; Lin, S.-T.; Chen, Y.-H.; Chang, Y.-J.; Chen, L.; Chan, H.-L. Cardioprotective Effects of Quercetin in Cardiomyocyte under Ischemia/Reperfusion Injury. Evid. Based Complement. Altern. Med. 2013, 2013, 364519. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.; Siwy, J.; Wendt, R.; Lipphardt, M.; Koziolek, M.J.; Maixnerova, D.; Peters, B.; Kerschbaum, J.; Leierer, J.; Neprasova, M.; et al. Urine proteomics for prediction of disease progression in patients with IgA nephropathy. Nephrol. Dial. Transplant. 2020, 37, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Song, X.; Wang, S.; Wang, J.; Li, L. Knockdown of PDIA6 Inhibits Cell Proliferation and Enhances the Chemosensitivity in Gastric Cancer Cells. Cancer Manag. Res. 2020, 12, 11051–11062. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Su, X.; Chen, Y.; Xu, Y.; Wang, Z.; Mo, X. Proteomics analysis of plasma protein changes in patent ductus arteriosus patients. Ital. J. Pediatr. 2020, 46, 64. [Google Scholar] [CrossRef]
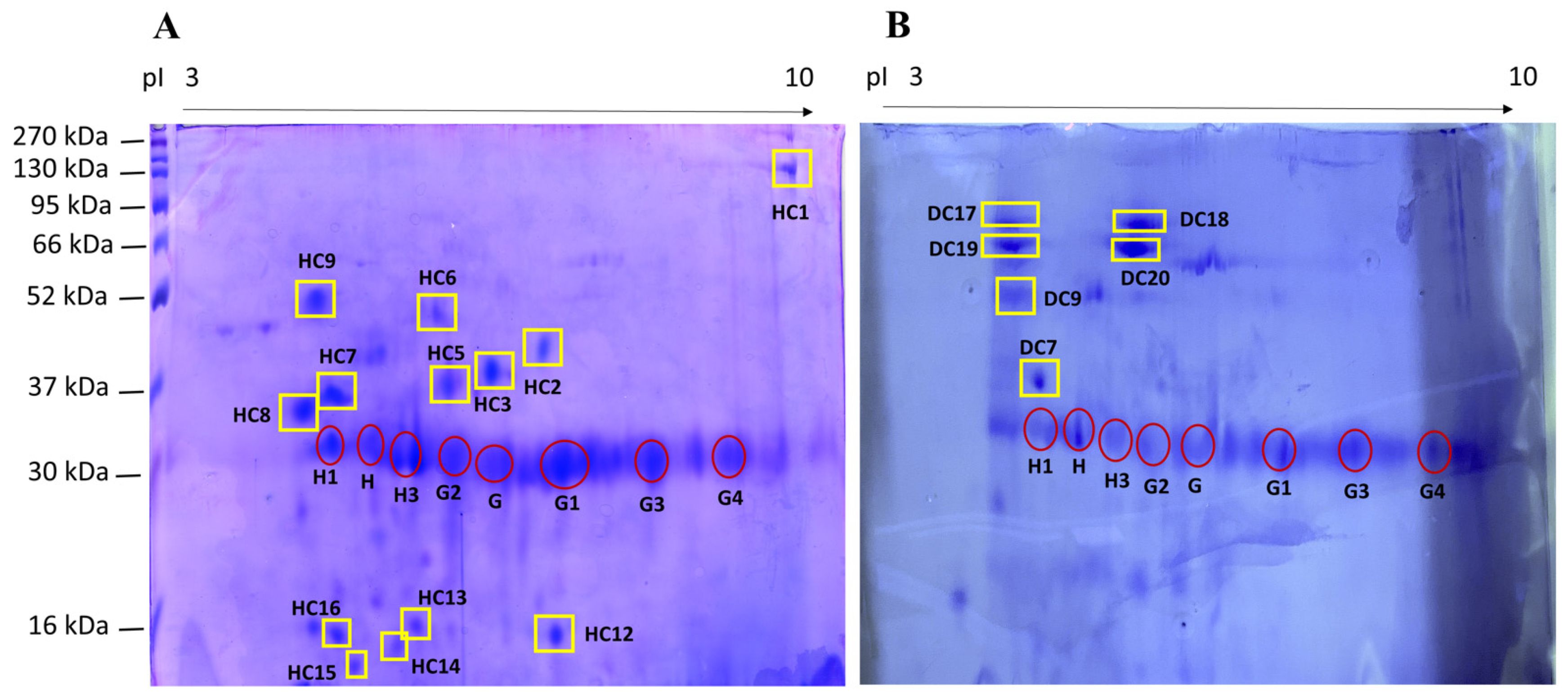
| Spot ID HC a | Spot ID DC a | Mr (kDa)/pI b | Normalized Quantity (×103) b | Protein Name c | Mr (kDa)/pI c | Score c | Sequence c |
|---|---|---|---|---|---|---|---|
| HC1 | - | 135.5/9.3 | 175 ± 147 | Collagen alpha-1 (II) chain | 141.8/6.6 | 40 | GIAGPQGPR |
| HC2 | - | 44.2/6.9 | 128 ± 50 | Transthyretin | 15.7/5.9 | 109 | GSAPAANVGVK |
| HC3 | - | 40.7/6.4 | 307 ± 2 | Transthyretin | 15.7/5.9 | 144 | GSAPAANVGVK |
| HC5 | - | 39.1/5.9 | 324 ± 48 | Transthyretin | 15.2/5.9 | 59 | KAADDTWEPFASG |
| HC6 | - | 48.1/5.7 | 149 ± 27 | IgG Fc-binding protein | 571.6/5.1 | 128 | VLVENEHRG |
| HC7 (1) | DC7 (2) | 36.9/4.7 (1) 39.8/4.7 (2) | 401 ± 25 (1) 3298 ± 518 (2) | n.d. | n.d. | n.d. | n.d. |
| HC8 | - | 35.7/4.4 | 460 ± 15 | IgG Fc-binding protein | 571.6/5.1 | 50 | LDSLVAQQLQSK |
| HC9 (3) | DC9 (4) | 51.8/4.5 (3) 50.2/4.4 (4) | 288 ± 130 (1) 7634 (2) | Titin | 3813.0/6.0 | 85 | APTPSPVR |
| HC12 | - | 18.6/7.1 | 207 ± 40 | Dystonin | 833.7/5.2 | 73 | AVTTALK |
| HC13 | - | 18.7/5.5 | 163 ± 36 | Isopentenyl-diphosphate Delta-isomerase 1 | 26.4/5.6 | 55 | AANGEIK |
| HC14 | - | 17.8/5.3 | 144 ± 43 | Sodium/potassium-transporting ATPase subunit alpha-1 | 112.6/5.6 | 81 | CRGAGIKV |
| HC15 | - | 16.4/4.8 | 125 ± 10 | Protein disulfide-isomerase A6 | 48.1/4.9 | 51 | KAATALKD |
| HC16 | - | 18.1/4.7 | 192 ± 6 | Dystonin | 833.7/5.2 | 83 | AVTTALK |
| - | DC17 | 61.7/4.6 | 6477 ± 3214 | Albumin | 68.6/5.5 | 190 | KAPVSTPTLVEV |
| - | DC18 | 62.6/5,5 | 6290 ± 864 | n.d. | n.d. | n.d. | n.d. |
| - | DC19 | 57.3/4.7 | 5880 ± 1240 | n.d. | n.d. | n.d. | n.d. |
| - | DC 20 | 58.9/5.7 | 7922 ± 922 | Albumin | 68.6/5.5 | 421 | KAPVSTPTLVEV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangiaterra, S.; Vincenzetti, S.; Rossi, G.; Marchegiani, A.; Gavazza, A.; Petit, T.; Sagratini, G.; Ricciutelli, M.; Cerquetella, M. Evaluation of the Fecal Proteome in Healthy and Diseased Cheetahs (Acinonyx jubatus) Suffering from Gastrointestinal Disorders. Animals 2022, 12, 2392. https://doi.org/10.3390/ani12182392
Mangiaterra S, Vincenzetti S, Rossi G, Marchegiani A, Gavazza A, Petit T, Sagratini G, Ricciutelli M, Cerquetella M. Evaluation of the Fecal Proteome in Healthy and Diseased Cheetahs (Acinonyx jubatus) Suffering from Gastrointestinal Disorders. Animals. 2022; 12(18):2392. https://doi.org/10.3390/ani12182392
Chicago/Turabian StyleMangiaterra, Sara, Silvia Vincenzetti, Giacomo Rossi, Andrea Marchegiani, Alessandra Gavazza, Thierry Petit, Gianni Sagratini, Massimo Ricciutelli, and Matteo Cerquetella. 2022. "Evaluation of the Fecal Proteome in Healthy and Diseased Cheetahs (Acinonyx jubatus) Suffering from Gastrointestinal Disorders" Animals 12, no. 18: 2392. https://doi.org/10.3390/ani12182392
APA StyleMangiaterra, S., Vincenzetti, S., Rossi, G., Marchegiani, A., Gavazza, A., Petit, T., Sagratini, G., Ricciutelli, M., & Cerquetella, M. (2022). Evaluation of the Fecal Proteome in Healthy and Diseased Cheetahs (Acinonyx jubatus) Suffering from Gastrointestinal Disorders. Animals, 12(18), 2392. https://doi.org/10.3390/ani12182392







