Dietary Phytogenic Extracts Favorably Influence Productivity, Egg Quality, Blood Constituents, Antioxidant and Immunological Parameters of Laying Hens: A Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
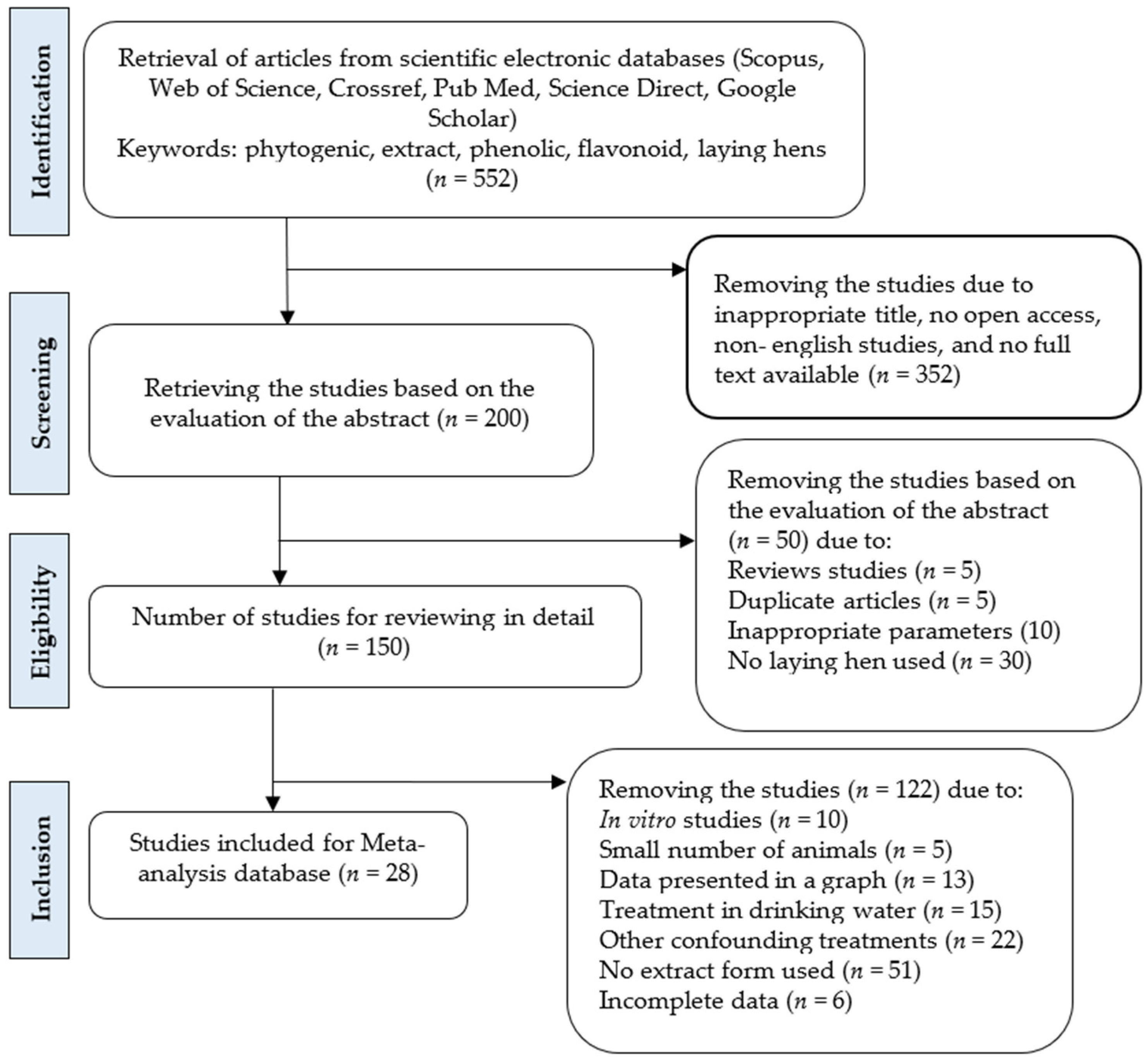
2.1. Database Development
2.2. Extraction and Description of Data
2.3. Statistical Analysis
| Author | Source | Main Bioactive Compound | Extract Level (mg/kg) | Chicken Breeds | Number of Birds | Age (Week) |
|---|---|---|---|---|---|---|
| Rahman et al. [8] | Mentha piperita | menthol, menthone, menthyl acetate | 0–200 | Babcock | 252 | 21–30 |
| Oh et al. [14] | Diospyros kaki L. | caffeic, p-coumaric, ferulic, gallic acids, tannins, terpenoids, naphthoquinones | 0–750 | Hy-lyne brown | 120 | 50–56 |
| Liu et al. [15] | commercial product | quercetin | 0–600 | Hessian | 240 | 28–36 |
| Ying et al. [16] | commercial product | quercetin | 0–600 | Hessian | 240 | 29–38 |
| Alagawany et al. [17] | Yucca schidigera | yuccaols, resveratrol | 0–150 | Hi-sex-brown | 96 | 36–52 |
| Ahmed et al. [18] | Olea europaea L. | hydroxytyrosol, vanillin, rutin, caffeic acid, catechin | 0–150 | Bandarah | 360 | 24–42 |
| Iskender et al. [19] | commercial product | hesperidin, naringin, quercetin | 0–500 | Lohmann white | 96 | 29–40 |
| Damaziak et al. [20] | Allium sativum L., Allium cepa L. | alicin, quercitin, gallic acid | 0–32 | ISA Brown | 216 | 22–32 |
| Sun et al. [21] | grape seed | procyanidins, proanthocyanidins | 0–150 | Hy-Line brown | 640 | 25–33 |
| Vakili and Heravi [22] | Thymus vulgaris L., Foeniculum vulgare | thymol, carvacrol (Thymus vulgaris); anethole, limonene, fenchone, estragole, safrole, camphene (Foeniculum vulgare) | 0–40 | Hy-Line | 200 | 26–38 |
| Park et al. [23] | Trigonella foenum-graecum L. | 4-hydroxy isoleucine, trigonelline, carotenoids, coumarins, saponins | 0–1000 | Hy-Line brown | 96 | 36–52 |
| Simitzis et al. [24] | commercial product | quercetin | 0–700 | Lohmann brown-classic | 192 | 70–74 |
| Damaziak et al. [25] | Zingiber officinale, Thymus vulgaris | gingerol, sholaol (Zingiber officinale); borneol, thymol, carvacrol (Thymus vulgaris) | 0–32 | ISA brown | 216 | 19–35 |
| Xie et al. [26] | Lonicera confusa, Astragali radix | luteolin, chlorogenic acid, caffeic acid (Lonicera confusa); astragaloside, formononetin, calycosin (Astragali radix) | 0–1000 | Lohmann pink-shell | 1440 | 52–64 |
| Song et al. [27] | Camelia oleifera | glucuronic acid, xylose, rhamnose, methyl pentose | 0–500 | Hy-Line brown | 180 | 26–38 |
| Huang et al. [28] | Camellia sinensis (L.) O. Ktze. | theanine, theobromine, caffeine, catechins | 0–300 | Lohmann brown | 240 | 30–38 |
| Huang et al. [29] | Rhizoma drynariae | naringin, neoeriocitrin, triterpenes, phenylpropanoids | 0–200 | Lohmann pink-shell | 216 | 54–67 |
| Dos Santos et al. [30] | Psidium cattleianum Sabine | ellagic acid, gallic acid, catechin, quercetin | 0–200 | ISA Brown | 75 | 45–49 |
| Kılınç and Karaoğlu [31] | Hypericum perforatum L. | hypericin, hyperforin, flavonoids | 0–300 | Lohmann white | 336 | 40–52 |
| Liu et al. [32] | Curcuma longa | curcumin | 0–200 | Hy-Line brown | 240 | 40–46 |
| Mutlu and Yildirim [33] | Panax ginseng | saponin glycosides (ginsenosides), essential oils sterols, flavonoids | 0–150 | Atak-S brown | 80 | 28–32 |
| Widjastuti et al. [34] | Garcinia mangostana L. | xanthone, flavonoids, anthocyanins | 0–240 | Sentul | 40 | 20–32 |
| Wen et al. [35] | Zingiber officinale Roscoe | 6-gingerol, 8-gingerol, 10-gingerol | 0–100 | Hyline Brown | 288 | 40–48 |
| Abad et al. [36] | Allium spp | alicin, quercitin, gallic acid | 0–700 | Lohmann Brown | 180 | 36–40 |
| Zhu et al. [37] | Neohesperidin | neohesperidin | 0–400 | Lohmann | 240 | 66–74 |
| Guo et al. [38] | Macleaya cordata | sanguinarine, chelerythrine | 0–200 | Xuefeng black-bone | 576 | 47–59 |
| Peng et al. [39] | Eucommia ulmoides | chlorogenic acid, aucubin, geniposidic acid | 0–500 | Spotted-brown | 120 | 56–67 |
| Guo et al. [40] | Pinusmassoniana Lamb | flavonoids, shikimic acid | 0–400 | Peking pink | 60 | 50–58 |
3. Results
3.1. Productive Performances and Egg Quality
3.2. Blood Constituents and Egg Yolk Cholesterol
3.3. Immunological and Antioxidant Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batiha, G.E.S.; Beshbishy, A.M.; Wasef, L.; Elewa, Y.; El-Hack, M.E.A.; Taha, A.E.; Al-Sagheer, A.A.; Devkota, H.P.; Tufarelli, V. Uncaria tomentosa (Willd. ex Schult.) DC.: A review on chemical constituents and biological activities. Appl. Sci. 2020, 10, 2668. [Google Scholar] [CrossRef]
- Sumiati; Darmawan, A.; Hermana, W. Performances and egg quality of laying ducks fed diets containing cassava (Manihot esculenta Crantz) leaf meal and golden snail (Pomacea canaliculata). Trop. Anim. Sci. J. 2020, 43, 227–232. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Phurpa, W. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotech. 2021, 12, 48. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Abd El-Hack, M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef]
- Sharma, M.K.; Dinh, T.; Adhikari, P.A. Production performance, egg quality, and small intestine histomorphology of the laying hens supplemented with phytogenic feed additive. J. Appl. Poult. Res. 2020, 29, 362–371. [Google Scholar] [CrossRef]
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Bayram, I.; Gultepe, E.E. Effect of mentha on performance, haematological and biochemical parameters in laying hens. S. Afr. J. Anim. Sci. 2021, 51, 2. [Google Scholar] [CrossRef]
- Naumann, S.; Haller, D.; Eisner, P.; Schweiggert-Weisz, U. Mechanisms of interactions between bile acids and plant compounds—A Review. Int. J. Mol. Sci. 2020, 21, 6495. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific opinion on the safety and efficacy of tannic acid when used as feed flavouring for all animal species. EFSA J. 2014, 12, 3828. [Google Scholar] [CrossRef]
- Prihambodo, T.R.; Sholikin, M.M.; Qomariyah, N.; Jayanegara, A.; Batubara, I.; Utomo, D.B.; Nahrowi. Effects of dietary flavonoids on performance, blood constituents, carcass composition and small intestinal morphology of broilers: A meta-analysis. Anim. Biosci. 2021, 34, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, C.; Irawan, A.; Jayanegara, A.; Sholikin, M.M.; Prihambodo, T.R.; Yanza, Y.Z.; Wina, E.; Sadarman, S.; Krisnan, R.; Isbandi, I. Effect of dietary tannins on the performance, lymphoid organ weight, and amino acid ileal digestibility of broiler chickens: A meta-analysis. Vet. World 2021, 14, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, N. Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 2001, 84, 741–755. [Google Scholar] [CrossRef]
- Oh, T.; Zheng, L.; Shin, Y.K.; An, B.K.; Kang, C.W. Effects of dietary persimmon peel and its ethanol extract on the production performance and liver lipids in the late stage of egg production in laying hens. Asian-Aust. J. Anim. Sci. 2013, 26, 260–265. [Google Scholar] [CrossRef][Green Version]
- Liu, H.N.; Liu, Y.; Hu, L.L.; Suo, Y.L.; Zhang, L.; Jin, F.; Li, Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef]
- Ying, Y.; Chun-yan, H.; Tabassum, C.M.; Ling, L.; Jia-ying, Y.; Sheng-nan, W.; Yao, L. Effect of quercetin on egg quality and components in laying hens of different weeks. J. North. Agric. Univ. 2015, 22, 23–32. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; El-Kholy, M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. Pollut. Res. 2015, 23, 6774–6782. [Google Scholar] [CrossRef]
- Ahmed, M.M.; El-Saadany, A.S.; Shreif, E.Y.; El-Barbary, A.M. Effect of dietary olive leaves extract (oleuropein) supplementation on productive, physiological and immunological parameters in bandarah chickens 2-during production period. Egypt. Poult. Sci. J. 2017, 37, 277–292. [Google Scholar]
- Iskender, H.; Yenice, G.; Dokumacioglu, E.; Kaynar, O.; Hayirli, A.; Kaya, A. The effects of dietary flavonoid supplementation on the antioxidant status of laying hens. Braz. J. Poult. Sci. 2016, 18, 663–668. [Google Scholar] [CrossRef]
- Damaziak, K.; Riedel, J.; Gozdowski, D.; Niemiec, J.; Siennicka, A.; Róg, D. Productive performance and egg quality of laying hens fed diets supplemented with garlic and onion extracts. J. Appl. Poult. Res. 2017, 26, 337–349. [Google Scholar] [CrossRef]
- Sun, P.; Lu, Y.; Cheng, H.; Song, D. The effect of grape seed extract and yeast culture on both cholesterol content of egg yolk and performance of laying hens. J. Appl. Poult. Res. 2018, 27, 564–569. [Google Scholar] [CrossRef]
- Vakili, R.; Heravi, M.R. Performance and egg quality of laying hens fed diets supplemented with herbal extracts and flaxseed. Poult. Sci. J. 2016, 4, 107–116. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.M.; Kim, I. Egg production, egg quality, blood profiles, cecal microflora, and excreta noxious gas emission in laying hens fed with fenugreek (Trigonella foenum-graecum L.) seed extract. J. Poult. Sci. 2018, 55, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Simitzis, P.; Spanou, D.; Glastra, N.; Goliomytis, M. Impact of dietary quercetin on laying hen performance, egg quality and yolk oxidative stability. Anim. Feed Sci. Tech. 2018, 239, 27–42. [Google Scholar] [CrossRef]
- Damaziak, K.; Riedel, J.; Gozdowski, D.; Niemiec, J.; Siennicka, A.; Róg, D. Effects of ginger or ginger and thyme extract in laying hens feeding on productive results and eggs quality. Anim. Sci. 2018, 57, 5–18. [Google Scholar] [CrossRef]
- Xie, T.; Bai, S.P.; Zhang, K.Y.; Ding, X.M.; Wang, J.P.; Zeng, Q.F.; Peng, H.W.; Lu, H.Y.; Bai, J.; Xuan, Y.; et al. Effects of Lonicera confusa and Astragali Radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult. Sci. 2019, 98, 4838–4847. [Google Scholar] [CrossRef]
- Song, D.; Wang, Y.W.; Lu, Z.X.; Wang, W.W.; Miao, H.J.; Zhou, H.; Wang, L.; Li, A.K. Effects of dietary supplementation of microencapsulated Enterococcus fecalis and the extract of Camellia oleifera seed on laying performance, egg quality, serum biochemical parameters, and cecal microflora diversity in laying hens. Poult. Sci. 2019, 98, 2880–2887. [Google Scholar] [CrossRef]
- Huang, J.; Hao, Q.; Wang, Q.; Wang, Y.; Wan, X.; Zhou, Y. Supplementation with green tea extract affects lipid metabolism and egg yolk lipid composition in laying hens. J. Appl. Poult. Res. 2019, 28, 881–891. [Google Scholar] [CrossRef]
- Huang, J.; Tong, X.F.; Yu, Z.; Hu, Y.P.; Zhang, L.; Liu, Y.; Zhou, Z.X. Dietary supplementation of total flavonoids from Rhizoma drynariae improves bone health in older caged laying hens. Poult. Sci. 2020, 99, 5047–5054. [Google Scholar] [CrossRef]
- Dos Santos, A.F.A.; Da Silva, A.S.; Galli, G.M. Addition of yellow strawberry guava leaf extract in the diet of laying hens had antimicrobial and antioxidant effect capable of improving egg quality. Bio. Agric. Biotech. 2020, 29, 101788. [Google Scholar] [CrossRef]
- Kılınç, G.; Karaoğlu, M. Effects of grape (Vitis vinifera L.) seed oil and St John’s wort (Hypericum perforatum L.) extract supplementation into laying hens diets on performance, egg quality, and some blood parameters. Int. J. Sci. Let. 2020, 2, 26–38. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Gao, P.; Xie, X.; Li, D.; Yu, D.; Yu, M. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens. Poult. Sci. 2020, 99, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, M.I.S.; Yildirim, A. Effect of dietary supplementation of Panax ginseng leaf extract on production performance and egg quality of hens at the beginning of their laying period. Large Anim. Rev. 2020, 26, 341–448. [Google Scholar]
- Widjastuti, T.; Adriani, L.; Asmara, I.Y.; Setiawan, I.; Abun; Nurlaeni, L. Effect of mangosteen peel extract (Garcinia mangostana l.) with supplemental zinc and copper on performance and egg quality of sentul laying chicken. Jordan J. Biol. Sci. 2021, 5, 1015–1020. [Google Scholar] [CrossRef]
- Wen, C.; Gu, Y.; Tao, Z.; Cheng, Z.; Wang, T.; Zhou, Y. Effects of ginger extract on laying performance, egg quality, and antioxidant status of laying hens. Animals 2019, 9, 857. [Google Scholar] [CrossRef]
- Abad, P.; Manzanares, A.N.; Ariza, J.J.; Baños, A.; García-Campaña, A.M. Effect of allium extract supplementation on egg quality, productivity, and ıntestinal microbiota of laying hens. Animals 2021, 11, 41. [Google Scholar] [CrossRef]
- Zhu, A.N.; Zhang, K.Y.; Wang, J.P.; Bai, S.P.; Zeng, Q.F.; Peng, H.W.; Ding, X.M. Effect of different concentrations of neohesperidin dihydrochalcone on performance, egg quality, serum biochemistry and intestinal morphology in laying hens. Poult. Sci. 2021, 100, 101097. [Google Scholar] [CrossRef]
- Guo, S.; Lei, J.; Liu, L.; Qu, X.; Li, P.; Liu, X.; Guo, Y.; Gao, Q.; Lan, F.; Xiao, B.; et al. Effects of Macleaya cordata extract on laying performance, egg quality, and serum indices in Xuefeng black-bone chicken. Poult. Sci. 2021, 100, 101031. [Google Scholar] [CrossRef]
- Peng, M.; Huang, T.; Yang, Q.; Peng, S.; Jin, Y.; Wang, X. Dietary supplementation Eucommia ulmoides extract at high content served as a feed additive in the hens industry. Poult. Sci. 2022, 101, 101650. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, S.; Zhao, L.; Zhang, J.; Ji, C.; Ma, Q. Pine (Pinus massoniana Lamb.) needle extract supplementation ımproves performance, egg quality, serum parameters, and the gut microbiome in laying hens. Front. Nutr. 2022, 9, 810462. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic feed additives in poultry: Achievements, perspective and challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Dilawar, M.A.; Mun, H.S.; Rathnayake, D.; Yang, E.J.; Seo, Y.S.; Park, H.S.; Yang, C.J. Egg quality parameters, production performance and immunity of laying hens supplemented with plant extracts. Animals 2021, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, S.; Eser, H.; Onbaşilar, I.; Yalçin, S. Effects of dried thyme (Thymus vulgaris L.) leaves on performance, some egg quality traits and immunity in laying hens. Ank. Üniv. Vet. Fak. Derg. 2020, 67, 303–311. [Google Scholar] [CrossRef]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Gut microbiota-polyphenol interactions in chicken: A review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef]
- Tellez-Isaias, G.; Vuong, C.N.; Graham, B.D.; Selby, C.M.; Graham, L.E.; Senas-Cuesta, R.; Barros, T.L.; Beer, L.C.; Coles, M.E.; Forga, A.J.; et al. Developing probiotics, prebiotics, and organic acids to control Salmonella spp. in commercial turkeys at the University of Arkansas, USA. Ger. J. Vet. Res. 2021, 1, 7–12. [Google Scholar] [CrossRef]
- Yang, J.X.; Chaudhry, M.T.; Yao, J.Y. Effects of phyto-estrogen quercetin on productive performance, hormones, reproductive organs and apoptotic genes in laying hens. J. Anim. Phys. Anim. Nutr. 2018, 102, 505–513. [Google Scholar] [CrossRef]
- Mutlu, S.I.; Seven, I.; Birben, N.; Arslan, A.S.; Seven, P.T. Alleviation potential of quercetin in laying quails exposed to lead: Effects on productive performance, egg quality, cecal microflora, and nutrient digestibility. J. Environ. Agric Sci. 2021, 10, 20–27. [Google Scholar] [CrossRef]
- Groundwater, P.W.; Narlawar, R.; Liao, V.W. A carbocyclic curcumin inhibits proliferation of gram-positive bacteria by targeting FtsZ. Biochemistry 2017, 56, 514–524. [Google Scholar] [CrossRef]
- Kamboh, A.A.; Zhu, W.Y. Individual and combined effects of genistein and hesperidin on immunity and intestinal morphometry in lipopolysacharide-challenged broiler chickens. Poult. Sci. 2014, 93, 2175–2183. [Google Scholar] [CrossRef]
- Zainuddin; Darmawan, A.; Wiryawan, K.G.; Nahrowi. Effects of dietary Bacillus coagulans D3372 supplementation as probiotics on broiler performance, ileal microflora, meat quality, nutrient retention, and metabolizable energy. Adv. Anim. Vet. Sci. 2020, 8, 115–223. [Google Scholar] [CrossRef]
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Ozturk, E. The Effect of fresh and aged garlic extract-enriched diets on the growth performance of broilers and the oxidative rancidity and customer acceptance of chicken meat. Turk. J. Agric. 2019, 7, 2267–2274. [Google Scholar] [CrossRef]
- Koçyiğit, A.; Selek, Ş. Exogenous antioxidants are double-edged swords. Bezmialem Sci. 2016, 2, 70–85. [Google Scholar] [CrossRef]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as antioxidants for extending food shelf-life and in the prevention of health diseases: Encapsulation and interfacial phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Hager-Theodorides, A.L.; Massouras, T.; Simitzis, P.E.; Moschou, K.; Zoidis, E.; Sfakianaki, E.; Politi, K.; Charismiadou, M.; Goliomytis, M.; Deligeorgis, S. Hesperidin and naringin improve broiler meat fatty acid profile and modulate the expression of genes involved in fatty acid β-oxidation and antioxidant defense in a dose-dependent manner. Foods 2021, 10, 739. [Google Scholar] [CrossRef]
- Kamboh, A.A.; Hang, S.Q.; Khan, M.A.; Zhu, W.Y. In vivo immunomodulatory effects of plant flavonoids in lipopolysaccharide-challenged broilers. Animal 2016, 10, 1619–1625. [Google Scholar] [CrossRef]
- Hassan, F.; Roushdy, E.; Kishawy, A.; Zaglool, A.; Tukur, H.; Saadeldin, I. Growth performance, antioxidant capacity, lipid-related transcript expression and the economics of broiler chickens fed different levels of rutin. Animals 2018, 9, 7. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Rev. Int. 2020, 37, 759–811. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.C.; Li, S.; Zhan, J.; Ho, C.T. Immunomodulatory effects of green tea polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Yuandani; Jantan, I.; Rohani, A.S.; Sumantri, I.B. Immunomodulatory effects and mechanisms of curcuma species and their bioactive compounds: A Review. Front. Pharmacol. 2021, 12, 643119. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.L.; Latif, S.; Toson, A.; Elwan, H.; Helpawy, E.S. Inclusion of phytogenic feed additives in diet of growing rabbits: Effects on antioxidant enzymes and ımmunoglobulins. Biomed. J. Sci. Tech. Res. 2021, 33, 25499–25503. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition—A review. Ann. Anim. Sci. 2017, 17, 41–48. [Google Scholar] [CrossRef]
- Zhou, Y.; Mao, S.; Zhou, M. Effect of the flavonoid baicalein as a feed additive on the growth performance, immunity, and antioxidant capacity of broiler chickens. Poult. Sci. 2019, 98, 2790–2799. [Google Scholar] [CrossRef]
- Yu, Z.; Mao, C.; Fu, X.; Ma, M. High density lipoprotein from egg yolk (EYHDL) improves dyslipidemia by mediating fatty acids metabolism in high fat diet-induced obese mice. Food. Sci. Anim. Res. 2019, 39, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, E.; Ma, L.; Zhai, P. Dietary resveratrol increases the expression of hepatic 7α-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis. 2012, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Karadağoğlu, Ö.; Şahin, T.; Ölmez, M.; Yakan, A.; Özsoy, B. Changes in serum biochemical and lipid profile, and fatty acid composition of breast meat of broiler chickens fed supplemental grape seed extract. Turk. J. Vet. Anim. Sci. 2020, 44, 182–190. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Wang, J.P.; Pietro, C.; Zhang, K.Y.; Ding, X.M.; Bai, S.P. Effect of epigallocatechin-3-gallate on lipid metabolism related gene expression and yolk fatty acid profiles of laying hens exposed to vanadium. Biol. Trace Elem. Res. 2019, 190, 501–508. [Google Scholar] [CrossRef]
| Parameter | n | Intercept | SE Intercept | Slope | SE Slope | p-Value | RMSE | AIC | Model | Trend |
|---|---|---|---|---|---|---|---|---|---|---|
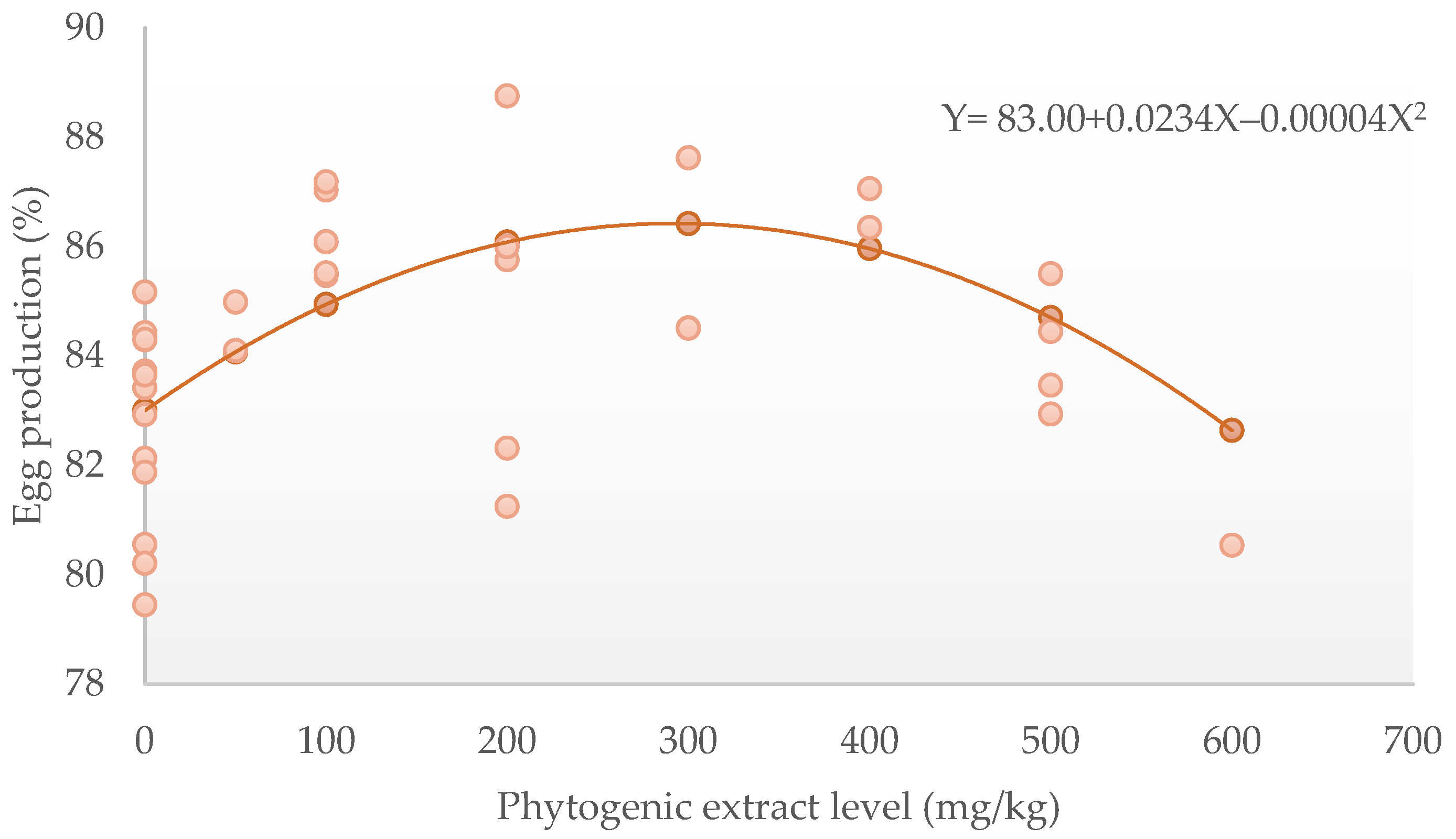
| Egg production (%) | 72 | 83 | 1.77 | 0.0234 | 0.007055 | |||||
| −0.00004 | 0.000014 | 0.02 | 7.25 | 457 | Q | Positive | ||||
| Feed intake (g/hen/day) | 97 | 112 | 2.32 | 0.00315 | 0.00177 | 0.08 | 6.16 | 576 | L | Positive |
| FCR | 94 | 2.1 | 0.049 | −0.00027 | 0.000185 | |||||
| 0.000000431 | 0.00000012 | <0.001 | 0.21 | −36 | Q | Negative | ||||
| Egg weight (g/egg) | 102 | 61.2 | 0.78 | 0.000948 | 0.000639 | 0.14 | 2.21 | 349 | L | - |
| Egg mass (g/hen/day) | 101 | 49.6 | 2.47 | 0.0119 | 0.00388 | |||||
| −0.00002 | 0.0000074 | 0.03 | 4.38 | 450 | Q | Positive |
| Parameter | n | Intercept | SE Intercept | Slope | SE Slope | p-Value | RMSE | AIC | Model | Trend |
|---|---|---|---|---|---|---|---|---|---|---|
| Eggshell thickness (mm) | 99 | 0.36 | 0.0056 | 0.000011 | 0.00000777 | 0.16 | 0.04 | 422 | L | - |
| Eggshell strength (Newton) | 92 | 37.4 | 0.91 | 0.00119 | 0.00118 | 0.32 | 5.86 | 514 | L | - |
| Albumen weight (%) | 21 | 60.8 | 1.88 | −0.00022 | 0.00155 | 0.89 | 2.41 | 93.3 | L | - |
| Egg yolk weight (%) | 42 | 27.2 | 1.21 | 0.000672 | 0.000367 | 0.08 | 1.31 | 155 | L | Positive |
| Eggshell weight (%) | 33 | 12.7 | 0.66 | 0.00102 | 0.001104 | 0.37 | 1.32 | 102 | L | - |
| Haugh unit | 119 | 85 | 1.41 | 0.00167 | 0.00157 | 0.29 | 7.34 | 559 | L | - |
| Parameter | n | Intercept | SE Intercept | Slope | SE Slope | p-Value | RMSE | AIC | Model | Trend |
|---|---|---|---|---|---|---|---|---|---|---|
| Egg yolk cholesterol (mg/g) | 20 | 13.4 | 0.9 | −0.0132 | 0.00676 | 0.08 | 4.04 | 118 | L | Negative |
| Serum cholesterol (mg/dL) | 54 | 151 | 7.73 | −0.168 | 0.0318 | |||||
| 0.000239 | 0.000048 | <0.001 | 44.6 | 488 | Q | Negative | ||||
| LDL (mg/dL) | 36 | 50.7 | 8.49 | −0.0473 | 0.0128 | |||||
| 0.000042 | 0.000015 | 0.01 | 19.2 | 304 | Q | Negative | ||||
| HDL (mg/dL) | 37 | 34 | 7.38 | 0.00657 | 0.0028 | 0.03 | 12.6 | 284 | L | Positive |
| Total protein (g/L) | 42 | 54 | 2.44 | −0.00396 | 0.00515 | 0.45 | 11.9 | 288 | L | - |
| Glucose (mg/dL) | 25 | 204 | 22 | −0.0195 | 0.0302 | 0.53 | 22.2 | 216 | L | - |
| Albumin (g/dL) | 21 | 2.33 | 0.13 | −0.00028 | 0.000328 | 0.4 | 0.31 | 9 | L | - |
| AST (U/L) | 26 | 205 | 21.1 | −0.0292 | 0.0279 | 0.31 | 41.1 | 246 | L | - |
| ALT (U/L) | 23 | 2.64 | 0.69 | −0.00028 | 0.00103 | 0.8 | 0.97 | 66.1 | L | - |
| Parameter | n | Intercept | SE Intercept | Slope | SE Slope | p-Value | RMSE | AIC | Model | Trend |
|---|---|---|---|---|---|---|---|---|---|---|
| IgG (mg/dL) | 22 | 3.56 | 0.75 | 0.00176 | 0.000593 | 0.01 | 0.74 | 38.7 | L | Positive |
| IgM (mg/dL) | 20 | 33.2 | 10.92 | 0.01073 | 0.01103 | 0.36 | 13.6 | 106.4 | Q | - |
| IgA (mg/dL) | 21 | 38.6 | 15.9 | 0.0158 | 0.00388 | 0.002 | 4.84 | 94.6 | L | Positive |
| TSOD (U/mL) | 33 | 194 | 18.8 | 0.0491 | 0.018 | 0.01 | 32.7 | 310 | L | Positive |
| GSH-Px (U/mL) | 28 | 7.56 | 0.86 | 0.0029 | 0.00122 | 0.03 | 7.4 | 160 | L | Positive |
| MDA (nmol/mL) | 21 | 4.21 | 0.16 | −0.00093 | 0.00024 | 0.002 | 1.44 | 59.3 | L | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darmawan, A.; Hermana, W.; Suci, D.M.; Mutia, R.; Sumiati; Jayanegara, A.; Ozturk, E. Dietary Phytogenic Extracts Favorably Influence Productivity, Egg Quality, Blood Constituents, Antioxidant and Immunological Parameters of Laying Hens: A Meta-Analysis. Animals 2022, 12, 2278. https://doi.org/10.3390/ani12172278
Darmawan A, Hermana W, Suci DM, Mutia R, Sumiati, Jayanegara A, Ozturk E. Dietary Phytogenic Extracts Favorably Influence Productivity, Egg Quality, Blood Constituents, Antioxidant and Immunological Parameters of Laying Hens: A Meta-Analysis. Animals. 2022; 12(17):2278. https://doi.org/10.3390/ani12172278
Chicago/Turabian StyleDarmawan, Arif, Widya Hermana, Dwi Margi Suci, Rita Mutia, Sumiati, Anuraga Jayanegara, and Ergin Ozturk. 2022. "Dietary Phytogenic Extracts Favorably Influence Productivity, Egg Quality, Blood Constituents, Antioxidant and Immunological Parameters of Laying Hens: A Meta-Analysis" Animals 12, no. 17: 2278. https://doi.org/10.3390/ani12172278
APA StyleDarmawan, A., Hermana, W., Suci, D. M., Mutia, R., Sumiati, Jayanegara, A., & Ozturk, E. (2022). Dietary Phytogenic Extracts Favorably Influence Productivity, Egg Quality, Blood Constituents, Antioxidant and Immunological Parameters of Laying Hens: A Meta-Analysis. Animals, 12(17), 2278. https://doi.org/10.3390/ani12172278







