Effects of High Heat Load Conditions on Blood Constituent Concentrations in Dorper, Katahdin, and St. Croix Sheep from Different Regions of the USA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
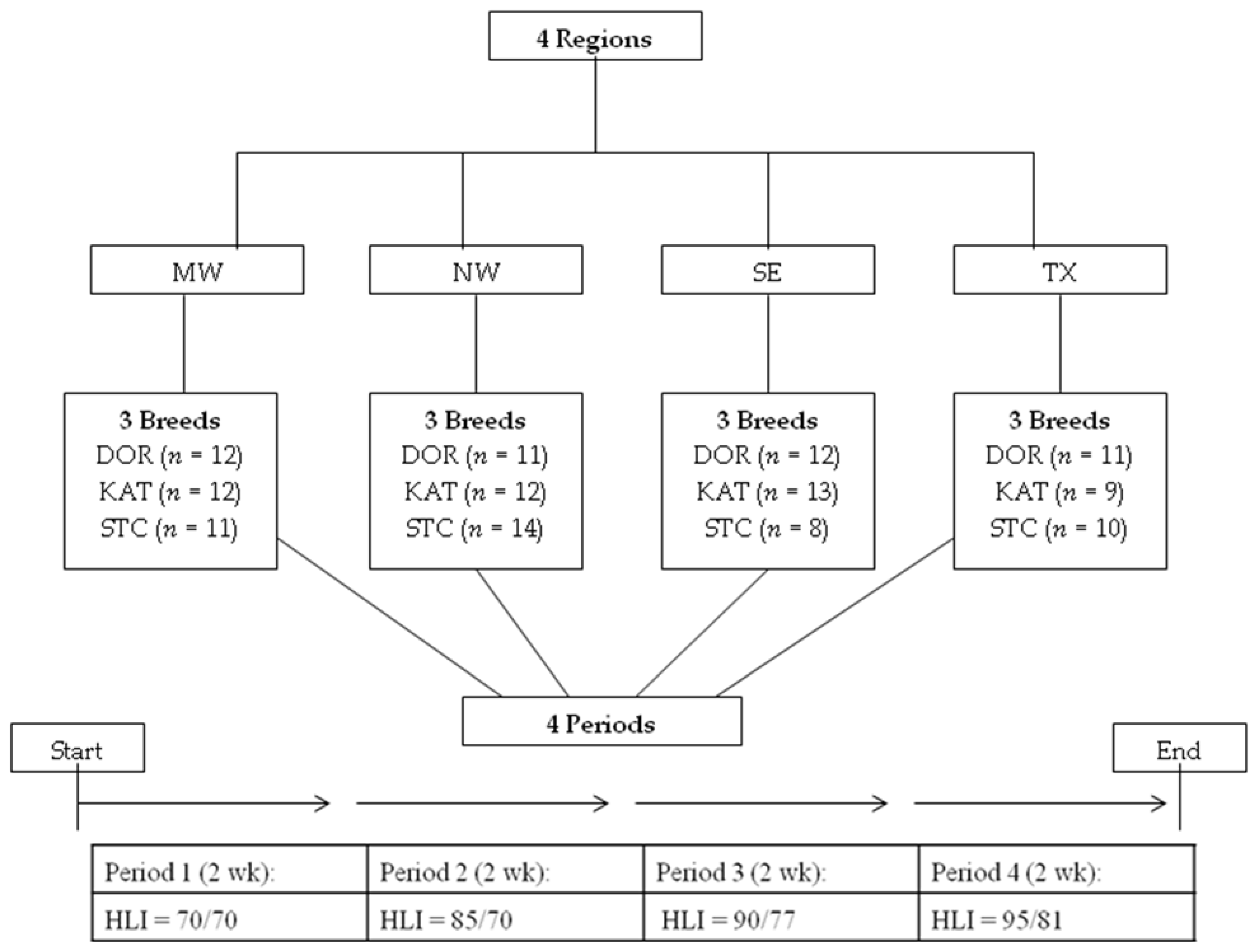
2.1. Animals, Housing, and Diet
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Hb, Oxygen, and PCV—All Periods
3.2. Blood Chemistry—All Periods
3.3. Blood Chemistry—Week of Period 4
4. Discussion
4.1. Hb, Oxygen, and PCV—All Periods
4.2. Blood Chemistry—All Periods
4.2.1. Period
4.2.2. Breed
4.3. Blood Chemistry—Week of Period 4
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Burke, J.M.; Apple, J.K.; Roberts, W.J.; Boger, C.B.; Kegley, E.B. Effect of Breed-Type on Performance and Carcass Traits of Intensively Managed Hair Sheep. Meat Sci. 2003, 63, 309–315. [Google Scholar] [CrossRef]
- Burke, J.M.; Apple, J.K. Growth Performance and Carcass Traits of Forage-Fed Hair Sheep Wethers. Small Rumin. Res. 2007, 67, 264–270. [Google Scholar] [CrossRef]
- Burke, J.M.; Miller, J.E. Relative Resistance to Gastrointestinal Nematode Parasites in Dorper, Katahdin, and St. Croix Lambs under Conditions Encountered in the Southeastern Region of the United States. Small Rumin. Res. 2004, 54, 43–51. [Google Scholar] [CrossRef]
- Wildeus, S. Hair Sheep Genetic Resources and Their Contribution to Diversified Small Ruminant Production in the United States. J. Anim. Sci. 1997, 75, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.W.; Murdoch, B.M.; Freking, B.A.; Redden, R.R.; Murphy, T.W.; Taylor, J.B.; Blackburn, H.D. Evolution of the Sheep Industry and Genetic Research in the United States: Opportunities for Convergence in the Twenty-First Century. Anim. Genet. 2021, 52, 395–408. [Google Scholar] [CrossRef]
- Blackburn, H.D.; Paiva, S.R.; Wildeus, S.; Getz, W.; Waldron, D.; Stobart, R.; Bixby, D.; Purdy, P.H.; Welsh, C.; Spiller, S. Genetic Structure and Diversity among Sheep Breeds in the United States: Identification of the Major Gene Pools. J. Anim. Sci. 2011, 89, 2336–2348. [Google Scholar] [CrossRef]
- Tadesse, D.; Puchala, R.; Goetsch, A.L. Effects of Hair Sheep Breed and Region of Origin on Feed Dry Matter Required for Maintenance without and with a Marked Feed Restriction. Livest. Sci. 2019, 226, 114–121. [Google Scholar] [CrossRef]
- Hussein, A.; Puchala, R.; Portugal, I.; Wilson, B.K.; Gipson, T.A.; Goetsch, A.L. Effects of Restricted Availability of Drinking Water on Body Weight and Feed Intake by Dorper, Katahdin, and St. Croix Sheep from Different Regions of the USA. J. Anim. Sci. 2020, 98, skz367. [Google Scholar] [CrossRef]
- Tadesse, D.; Puchala, R.; Gipson, T.A.; Goetsch, A.L. Effects of High Heat Load Conditions on Body Weight, Feed Intake, Temperature, and Respiration of Dorper, Katahdin, and St. Croix Sheep. J. Appl. Anim. Res. 2019, 47, 492–505. [Google Scholar] [CrossRef]
- De Rensis, F.; Garcia-Ispierto, I.; López-Gatius, F. Seasonal Heat Stress: Clinical Implications and Hormone Treatments for the Fertility of Dairy Cows. Theriogenology 2015, 84, 659–666. [Google Scholar] [CrossRef]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat Stress in Lactating Dairy Cows: A Review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Patra, A.K.; Kar, I. Heat Stress on Microbiota Composition, Barrier Integrity, and Nutrient Transport in Gut, Production Performance, and Its Amelioration in Farm Animals. J. Anim. Sci. Technol. 2021, 63, 211–247. [Google Scholar] [CrossRef] [PubMed]
- Podder, M.; Bera, S.; Naskar, S.; Sahu, D.; Mukherjee, J.; Patra, A.K. Physiological, Blood-Biochemical and Behavioural Changes of Ghoongroo Pigs in Seasonal Heat Stress of a Hot-Humid Tropical Environment. Int. J. Biometeorol. 2022, 66, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Pantoja, M.H.; Esteves, S.N.; Jacinto, M.A.C.; Pezzopane, J.R.M.; de Paz, C.C.P.; da Silva, J.A.R.; Junior, J.d.B.L.; Brandão, F.Z.; Moura, A.B.B.; Romanello, N. Thermoregulation of Male Sheep of Indigenous or Exotic Breeds in a Tropical Environment. J. Therm. Biol. 2017, 69, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.; von Keyserlingk, M.A.G. Invited Review: Effects of Heat Stress on Dairy Cattle Welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Amundson, J.L.; Mader, T.L.; Rasby, R.J.; Hu, Q.S. Environmental Effects on Pregnancy Rate in Beef Cattle. J. Anim. Sci. 2006, 84, 3415–3420. [Google Scholar] [CrossRef]
- Buffington, D.E.; Collazo-Arocho, A.; Canton, G.H.; Pitt, D.; Thatcher, W.W.; Collier, R.J. Black Globe-Humidity Index (BGHI) as Comfort Equation for Dairy Cows. Trans. ASAE 1981, 24, 711–0714. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Mader, T.L.; Holt, S.M.; Lisle, A. A New Heat Load Index for Feedlot Cattle. J. Anim. Sci. 2008, 86, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental Factors Influencing Heat Stress in Feedlot Cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Gaughan, J.B.; Mader, T.L.; Holt, S.M.; Sullivan, M.L.; Hahn, G.L. Assessing the Heat Tolerance of 17 Beef Cattle Genotypes. Int. J. Biometeorol. 2010, 54, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, J.; Yuan, C.; Jin, M.; Quan, K.; Chu, M.; Wei, C. M6 A MRNA Methylation Analysis Provides Novel Insights into Heat Stress Responses in the Liver Tissue of Sheep. Genomics 2021, 113, 484–492. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.M.; Faria, D.A.; Lucci, C.M.; Louvandini, H.; Pereira, S.A.; Paiva, S.R. Heat Stress Effects on Sheep: Are Hair Sheep More Heat Resistant? Theriogenology 2020, 155, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, U.L.; Puchala, R.; Sahlu, T.; Gipson, T.A.; Dawson, L.J.; Goetsch, A.L. Conditions to Evaluate Differences among Individual Sheep and Goats in Resilience to High Heat Load Index. Small Rumin. Res. 2017, 147, 89–95. [Google Scholar] [CrossRef]
- Hamzaoui, S.; Salama, A.A.K.; Albanell, E.; Such, X.; Caja, G. Physiological Responses and Lactational Performances of Late-Lactation Dairy Goats under Heat Stress Conditions. J. Dairy Sci. 2013, 96, 6355–6365. [Google Scholar] [CrossRef]
- Eisemann, J.H.; Nienaber, J.A. Tissue and Whole-Body Oxygen Uptake in Fed and Fasted Steers. Br. J. Nutr. 1990, 64, 399–411. [Google Scholar] [CrossRef]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical Analysis of Repeated Measures Data Using SAS Procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef]
- SAS SAS/STAT User’s Guide, Version 9.3; SAS Institute: Cary, NC, USA, 2011.
- Kumar, M.; Jindal, R.; Nayyar, S. Influence of Heat Stress on Antioxidant Status in Beetal Goats. Indian J. Small Rumin. 2011, 17, 178–181. [Google Scholar]
- Sivakumar, A.V.N.; Singh, G.; Varshney, V.P. Antioxidants Supplementation on Acid Base Balance during Heat Stress in Goats. Asian-Australas. J. Anim. Sci. 2010, 23, 1462–1468. [Google Scholar] [CrossRef]
- El-Nouty, F.D.; Al-Haidary, A.A.; Salah, M.S. Seasonal Variation in Hematological Values of High-and Average Yielding Holstein Cattle in Semi-Arid Environment. J. King Saudy Univ. Agric. Sci. 1990, 2, 173–182. [Google Scholar]
- Chaudhary, S.S.; Singh, V.K.; Upadhyay, R.C.; Puri, G.; Odedara, A.B.; Patel, P.A. Evaluation of Physiological and Biochemical Responses in Different Seasons in Surti Buffaloes. Vet. World 2015, 8, 727–731. [Google Scholar] [CrossRef]
- Okoruwa, M.I. Effect of Heat Stress on Thermoregulatory, Live Bodyweight and Physiological Responses of Dwarf Goats in Southern Nigeria. Eur. Sci. J. 2014, 10, 255–264. [Google Scholar]
- Giulivi, C.; Pacifici, R.E.; Davies, K.J. Exposure of Hydrophobic Moieties Promotes the Selective Degradation of Hydrogen Peroxide-Modified Hemoglobin by the Multicatalytic Proteinase Complex, Proteasome. Arch. Biochem. Biophys. 1994, 311, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.E.; Kono, Y.; Davies, K.J. Hydrophobicity as the Signal for Selective Degradation of Hydroxyl Radical-Modified Hemoglobin by the Multicatalytic Proteinase Complex, Proteasome. J. Biol. Chem. 1993, 268, 15405–15411. [Google Scholar] [CrossRef]
- Srikandakumar, A.; Johnson, E.H.; Mahgoub, O. Effect of Heat Stress on Respiratory Rate, Rectal Temperature and Blood Chemistry in Omani and Australian Merino Sheep. Small Rumin. Res. 2003, 49, 193–198. [Google Scholar] [CrossRef]
- Tadesse, D.; Puchala, R.; Goetsch, A.L. Effects of Restricted Feed Intake on Blood Constituent Concentrations in Dorper, Katahdin, and St. Croix Sheep from Different Regions of the USA. Vet. Anim. Sci. 2021, 14, 100211. [Google Scholar] [CrossRef]
- Stallknecht, B.; Vissing, J.; Galbo, H. Lactate Production and Clearance in Exercise. Effects of Training. A Mini-Review. Scand. J. Med. Sci. Sports 1998, 8, 127–131. [Google Scholar] [CrossRef]
- Slimen, I.B.; Chniter, M.; Najar, T.; Ghram, A. Meta-Analysis of Some Physiologic, Metabolic and Oxidative Responses of Sheep Exposed to Environmental Heat Stress. Livest. Sci. 2019, 229, 179–187. [Google Scholar] [CrossRef]
- Koluman, N.; Daskiran, I. Effects of Ventilation of the Sheep House on Heat Stress, Growth and Thyroid Hormones of Lambs. Trop. Anim. Health Prod. 2011, 43, 1123–1127. [Google Scholar] [CrossRef]
- Todini, L.; Malfatti, A.; Valbonesi, A.; Trabalza-Marinucci, M.; Debenedetti, A. Plasma Total T3 and T4 Concentrations in Goats at Different Physiological Stages, as Affected by the Energy Intake. Small Rumin. Res. 2007, 68, 285–290. [Google Scholar] [CrossRef]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological Traits as Affected by Heat Stress in Sheep—a Review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Helal, A.; Hashem, A.L.S.; Abdel-Fattah, M.S.; El-Shaer, H.M. Effect of Heat Stress on Coat Characteristics and Physiological Responses of Balady and Damascus Goats in Sinai, Egypt. Am.-Eurasian J. Agric. Environ. Sci. 2010, 7, 60–69. [Google Scholar]
- Rathwa, S.D.; Vasava, A.A.; Pathan, M.M.; Madhira, S.P.; Patel, Y.G.; Pande, A.M. Effect of Season on Physiological, Biochemical, Hormonal, and Oxidative Stress Parameters of Indigenous Sheep. Vet. World 2017, 10, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Mehaba, N.; Coloma-Garcia, W.; Such, X.; Caja, G.; Salama, A.A. Heat Stress Affects Some Physiological and Productive Variables and Alters Metabolism in Dairy Ewes. J. Dairy Sci. 2021, 104, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Mehaba, N.; Salama, A.A.; Such, X.; Albanell, E.; Caja, G. Lactational Responses of Heat-Stressed Dairy Goats to Dietary L-Carnitine Supplementation. Animals 2019, 9, 567. [Google Scholar] [CrossRef]
- Joo, S.S.; Lee, S.J.; Park, D.S.; Kim, D.H.; Gu, B.-H.; Park, Y.J.; Rim, C.Y.; Kim, M.; Kim, E.T. Changes in Blood Metabolites and Immune Cells in Holstein and Jersey Dairy Cows by Heat Stress. Animals 2021, 11, 974. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Lu, M.; Yang, X.; Xue, Y.; Yao, W. A Controlled Heat Stress during Late Gestation Affects Thermoregulation, Productive Performance, and Metabolite Profiles of Primiparous Sow. J. Therm. Biol. 2019, 81, 33–40. [Google Scholar] [CrossRef]
- Rasooli, A.; Jalali, M.T.; Nouri, M.; Mohammadian, B.; Barati, F. Effects of Chronic Heat Stress on Testicular Structures, Serum Testosterone and Cortisol Concentrations in Developing Lambs. Anim. Reprod. Sci. 2010, 117, 55–59. [Google Scholar] [CrossRef]
- Christison, G.I.; Johnson, H.D. Cortisol Turnover in Heat-Stressed Cows. J. Anim. Sci. 1972, 35, 1005–1010. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat Stress Effects on Livestock: Molecular, Cellular and Metabolic Aspects, a Review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef]
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals 2020, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Raup, T.J.; Bottje, W.G. Effect of Carbonated Water on Arterial PH, p Co2 and Plasma Lactate in Heat-Stressed Broilers. Br. Poult. Sci. 1990, 31, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Koelkebeck, K.W.; Odom, T.W. Laying Hen Responses to Acute Heat Stress and Carbon Dioxide Supplementation: I. Blood Gas Changes and Plasma Lactate Accumulation. Comp. Biochem. Physiol. A Physiol. 1994, 107, 603–606. [Google Scholar] [CrossRef]
| Variable 2 | ||||
|---|---|---|---|---|
| Source of Variation | Hb (g/dL) | HbO2 (%) | O2 (mmol/L) | PCV (%) |
| Set | 0.060 | <0.001 | <0.001 | 0.010 |
| Age | 0.627 | 0.246 | 0.214 | 0.611 |
| Breed | 0.840 | 0.001 | 0.013 | 0.350 |
| Region | 0.140 | 0.633 | 0.279 | 0.085 |
| Breed × region | 0.297 | 0.204 | 0.122 | 0.435 |
| Period | <0.001 | <0.001 | <0.001 | <0.001 |
| Breed × period | 0.452 | 0.824 | 0.785 | 0.604 |
| Region × period | 0.917 | 0.273 | 0.681 | 0.901 |
| Breed × region × period | 0.539 | 0.687 | 0.454 | 0.587 |
| Week | 0.004 | 0.007 | 0.064 | 0.020 |
| Breed × week | 0.541 | 0.918 | 0.716 | 0.239 |
| Region × week | 0.501 | 0.510 | 0.732 | 0.070 |
| Period × week | <0.001 | <0.001 | 0.023 | <0.001 |
| Breed × region × week | 0.527 | 0.593 | 0.770 | 0.467 |
| Breed × period × week | 0.018 | 0.913 | 0.889 | 0.026 |
| Region × period × week | 0.770 | 0.734 | 0.381 | 0.665 |
| Breed × region × period × week | 0.278 | 0.974 | 0.924 | 0.536 |
| Variable 2 | ||||
|---|---|---|---|---|
| Source of Variation | Hb (g/dL) | Hb O2 (%) | O2 (mmol/L) | PCV (%) |
| Set | 0.012 | <0.001 | <0.001 | 0.003 |
| Age | 0.714 | 0.374 | 0.335 | 0.472 |
| Breed | 0.702 | 0.004 | 0.021 | 0.177 |
| Region | 0.103 | 0.338 | 0.192 | 0.035 |
| Breed × region | 0.255 | 0.223 | 0.136 | 0.422 |
| Period | <0.001 | <0.001 | <0.001 | <0.001 |
| Breed × period | 0.019 | 0.703 | 0.771 | 0.062 |
| Region × period | 0.991 | 0.047 | 0.085 | 0.967 |
| Breed × region × period | 0.154 | 0.941 | 0.654 | 0.167 |
| Breed 2 | Region 3 | Period 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item 5 | Period | DOR | KAT | STC | SEM | MW | NW | SE | TX | SEM | 1 | 2 | 3 | 4 | SEM |
| Hb (g/dL) | 12.5 | 12.0 | 12.0 | 12.2 | 0.19 | ||||||||||
| 1 | 12.8 efg | 13.0 g | 12.9 fg | 0.20 | |||||||||||
| 2 | 12.1 bcd | 12.1 bcd | 12.2 cde | ||||||||||||
| 3 | 12.3 def | 12.0 bcd | 12.0 bcd | ||||||||||||
| 4 | 11.9 bc | 11.2 a | 11.8 ab | ||||||||||||
| Hb O2 (%) | 69.3 a | 72.3 ab | 75.7 b | 1.30 | |||||||||||
| 1 | 64.5 ab | 59.9 a | 67.1 bc | 64.7 ab | 2.30 | ||||||||||
| 2 | 69.6 bcd | 72.1 cd | 73.6 def | 74.9 defg | |||||||||||
| 3 | 72.8 cde | 72.8 cde | 69.8 bcd | 80.2 fg | |||||||||||
| 4 | 78.3 efg | 79.3 fg | 80.8 g | 78.4 efg | |||||||||||
| O2 (mmol/L) | 5.07 a | 5.20 a | 5.53 b | 0.114 | 5.32 | 5.08 | 5.19 | 5.47 | 0.130 | 4.92 a | 5.26 b | 5.36 bc | 5.52 c | 0.093 | |
| PCV (%) | 31.8 | 31.6 | 32.7 | 0.44 | 33.1 b | 31.4 a | 31.3a | 32.4 ab | 0.51 | 33.9 b | 31.6 a | 31.6 a | 31.1 a | 0.32 | |
| Source of Variation 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item 3 | Set | Age | BRD | REG | BR×REG | PRD | BRD×PRD | REG×PRD | BRD×REG×PRD |
| GLC (mg/dL) | <0.001 | 0.001 | 0.031 | 0.214 | 0.295 | 0.853 | 0.970 | 0.844 | 0.799 |
| LAC (mg/dL) | <0.001 | 0.213 | 0.646 | 0.044 | 0.934 | 0.005 | 0.721 | 0.911 | 0.241 |
| UN (mg/dL) | <0.001 | 0.256 | <0.001 | 0.106 | 0.021 | 0.204 | 0.084 | 0.741 | 0.902 |
| CRT (mg/dL) | <0.001 | 0.820 | <0.001 | 0.479 | 0.092 | <0.001 | 0.950 | 0.609 | 0.602 |
| TP (g/l) | <0.001 | 0.257 | 0.016 | 0.292 | 0.492 | 0.016 | 0.526 | 0.122 | 0.918 |
| ALB (g/l) | <0.001 | 0.040 | 0.061 | 0.945 | 0.175 | 0.067 | 0.257 | 0.410 | 0.909 |
| TG (mg/dL) | <0.001 | 0.172 | <0.001 | 0.993 | 0.006 | 0.455 | 0.347 | 0.725 | 0.872 |
| CHL (mg/dL) | <0.001 | 0.347 | 0.090 | 0.138 | 0.922 | 0.032 | 0.877 | 0.303 | 0.636 |
| COR (ng/mL) | 0.032 | 0.311 | 0.003 | 0.781 | 0.384 | 0.020 | 0.854 | 0.448 | 0.516 |
| THY (μg/dL) | 0.168 | 0.186 | 0.399 | 0.942 | 0.134 | <0.001 | 0.063 | 0.643 | 0.060 |
| HSP (ng/mL) | 0.001 | 0.698 | 0.341 | 0.608 | 0.548 | 0.030 | 0.792 | 0.658 | 0.938 |
| Breed 2 | Region 3 | Period 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item 5 | Breed | DOR | KAT | STC | SEM | MW | NW | SE | TX | SEM | 1 | 2 | 3 | 4 | SEM |
| GLC | 50.0 a | 52.6 b | 52.1 b | 0.76 | 50.4 | 52.8 | 51.2 | 51.8 | 0.87 | 52.0 | 51.4 | 51.1 | 51.6 | 0.75 | |
| LAC | 26.8 | 25.6 | 26.4 | 1.01 | 28.4 a | 27.3 a | 25.3 ab | 24.0 b | 1.15 | 27.9 b | 25.3 ab | 27.8 b | 24.0 a | 0.99 | |
| UN | 17.9 | 18.5 | 18.1 | 17.8 | 0.30 | ||||||||||
| DOR | 17.4 abc | 18.0 bcd | 16.7ab | 17.2 abc | 0.69 | ||||||||||
| KAT | 17.7 abc | 15.9 a | 18.9cd | 17.4 abc | |||||||||||
| STC | 19.1 cde | 19.9 de | 21.1e | 17.9 bc | |||||||||||
| CRT | 0.99 c | 0.86 b | 0.80 a | 0.015 | 0.88 | 0.91 | 0.88 | 0.87 | 0.017 | 0.84 a | 0.91 c | 0.87 b | 0.92 c | 0.013 | |
| TP | 6.50 a | 6.68 ab | 6.95 b | 0.017 | 6.63 | 6.91 | 6.70 | 6.60 | 0.122 | 6.94 b | 6.66 a | 6.60 a | 6.65 a | 0.094 | |
| ALB | 2.46 | 2.55 | 2.56 | 0.032 | 2.51 | 2.54 | 2.53 | 2.52 | 0.038 | 2.60 | 2.50 | 2.50 | 2.51 | 0.033 | |
| TG | 26.8 | 26.4 | 27.1 | 27.5 | 0.68 | ||||||||||
| DOR | 28.6 cde | 25.4 abc | 27.3 bcd | 31.9 de | 1.78 | ||||||||||
| KAT | 27.8 bcd | 33.5 e | 29.2 cde | 25.3 abc | |||||||||||
| STC | 24.5 abc | 22.6 a | 23.7 abc | 23.4 ab | |||||||||||
| CHL | 55.9 | 61.1 | 57.5 | 1.74 | 58.2 | 54.3 | 59.9 | 60.1 | 1.99 | 60.2 b | 56.5 a | 58.3 ab | 57.6 a | 1.26 | |
| COR 6 | 6.30 a | 8.79 b | 6.22 a | 0.596 | 7.08 | 7.10 | 6.60 | 7.64 | 0.684 | 7.62 b | 6.59 a | 0.404 | |||
| THY 6 | 5.29 | 5.65 | 5.31 | 0.215 | 5.52 | 5.39 | 5.44 | 5.31 | 0.246 | 5.83 b | 5.00 a | 0.140 | |||
| HSP 6 | 147 | 136 | 138 | 5.6 | 138 | 137 | 142 | 148 | 6.5 | 136 a | 146 b | 4.0 | |||
| Source of Variation 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item 3 | Set | Age | BRD | REG | BRD × REG | WK | BRD × WK | REG × WK | BRD×REG × WK |
| Hb (g/dL) | <0.001 | 0.720 | 0.350 | 0.394 | 0.316 | <0.001 | 0.007 | 0.580 | 0.568 |
| Hb O2 (%) | <0.001 | 0.135 | 0.046 | 0.968 | 0.304 | 0.062 | 0.851 | 0.959 | 0.597 |
| O2 (mmol/L) | <0.001 | 0.153 | 0.362 | 0.685 | 0.097 | 0.632 | 0.424 | 0.807 | 0.473 |
| PCV (%) | <0.001 | 0.619 | 0.326 | 0.123 | 0.461 | <0.001 | 0.021 | 0.584 | 0.936 |
| GLC (mg/dL) | <0.001 | 0.022 | 0.067 | 0.126 | 0.695 | 0.261 | 0.687 | 0.231 | 0.675 |
| LAC (mg/dL) | <0.001 | 0.733 | 0.972 | 0.111 | 0.601 | 0.013 | 0.803 | 0.816 | 0.154 |
| UN (mg/dL) | <0.001 | 0.315 | <0.001 | 0.502 | 0.018 | 0.172 | 0.762 | 0.144 | 0.167 |
| CRT (mg/dL) | <0.001 | 0.723 | <0.001 | 0.710 | 0.125 | 0.176 | 0.702 | 0.024 | 0.116 |
| TP (g/l) | <0.001 | 0.354 | 0.005 | 0.700 | 0.633 | 0.298 | 0.967 | 0.094 | 0.246 |
| ALB (g/l) | <0.001 | 0.128 | 0.013 | 0.970 | 0.240 | 0.304 | 0.975 | 0.072 | 0.169 |
| TG (mg/dL) | <0.001 | 0.542 | <0.001 | 0.719 | 0.021 | 0.630 | 0.445 | 0.866 | 0.814 |
| CHL (mg/dL) | <0.001 | 0.560 | 0.139 | 0.207 | 0.907 | 0.797 | 0.810 | 0.456 | 0.456 |
| Breed 2 | Region 3 | Week 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item 5 | Breed | Week | DOR | KAT | STC | SEM | MW | NW | SE | TX | SEM | 7 | 8 | SEM |
| Hb | 7 | 12.3 b | 12.2 b | 12.1 b | 0.21 | 12.1 | 11.7 | 11.8 | 12.1 | 0.23 | ||||
| 8 | 11.9 b | 11.2 a | 11.9 b | |||||||||||
| Hb O2 | 74.9 a | 79.1 b | 79.4 b | 1.45 | 78.0 | 77.5 | 78.4 | 77.3 | 1.66 | 76.4 | 79.2 | 1.10 | ||
| O2 | 5.42 | 5.53 | 5.68 | 0.126 | 5.64 | 5.42 | 5.50 | 5.60 | 0.145 | 5.57 | 5.52 | 0.089 | ||
| PCV | 7 | 32.5 c | 32.3 bc | 32.4 bc | 0.55 | 32.5 | 31.2 | 30.9 | 32.3 | 0.57 | ||||
| 8 | 31.0 ab | 30.1 a | 32.1 bc | |||||||||||
| GLC | 49.2 | 51.3 | 52.6 | 1.01 | 50.9 | 53.1 | 51.0 | 49.1 | 1.15 | 50.5 | 51.5 | 0.73 | ||
| LAC | 25.6 | 25.7 | 26.0 | 1.32 | 28.0 | 27.2 | 24.2 | 23.6 | 1.50 | 27.5b | 24.0a | 1.02 | ||
| UN | DOR | 16.5 ab | 17.4 abc | 15.7 ab | 17.1 ab | 0.86 | 17.4 | 17.8 | 0.28 | |||||
| KAT | 16.9 ab | 15.2 a | 17.5 bc | 17.2 abc | ||||||||||
| STC | 19.6 cd | 20.0 cd | 21.3 d | 17.1 ab | ||||||||||
| CRT | 1.01 b | 0.88 a | 0.83 a | 0.020 | ||||||||||
| 7 | 0.86 a | 0.94 c | 0.92 abc | 0.88 ab | 0.026 | |||||||||
| 8 | 0.92 bc | 0.91 abc | 0.90 abc | 0.93 c | ||||||||||
| TP | 6.34 a | 6.49 a | 6.96 b | 0.131 | 6.58 | 6.74 | 6.56 | 6.50 | 0.150 | 6.54 | 6.65 | 0.092 | ||
| ALB | 2.41 a | 2.48 ab | 2.58 b | 0.039 | 2.47 | 2.49 | 2.50 | 2.49 | 0.045 | 2.47 | 2.51 | 0.030 | ||
| TG | DOR | 29.1 bcd | 26.7 abc | 26.3 abc | 33.2 de | 2.24 | 27.2 | 27.6 | 0.73 | |||||
| KAT | 31.1 cd | 35.1 e | 28.9 bcd | 24.7 abc | ||||||||||
| STC | 24.1 ab | 22.7 a | 23.8 ab | 23.4 ab | ||||||||||
| CHL | 55.4 | 60.8 | 56.6 | 2.05 | 58.1 | 53.5 | 59.4 | 59.6 | 2.35 | 57.5 | 57.8 | 1.29 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadesse, D.; Patra, A.K.; Puchala, R.; Goetsch, A.L. Effects of High Heat Load Conditions on Blood Constituent Concentrations in Dorper, Katahdin, and St. Croix Sheep from Different Regions of the USA. Animals 2022, 12, 2273. https://doi.org/10.3390/ani12172273
Tadesse D, Patra AK, Puchala R, Goetsch AL. Effects of High Heat Load Conditions on Blood Constituent Concentrations in Dorper, Katahdin, and St. Croix Sheep from Different Regions of the USA. Animals. 2022; 12(17):2273. https://doi.org/10.3390/ani12172273
Chicago/Turabian StyleTadesse, Dereje, Amlan Kumar Patra, Ryszard Puchala, and Arthur Louis Goetsch. 2022. "Effects of High Heat Load Conditions on Blood Constituent Concentrations in Dorper, Katahdin, and St. Croix Sheep from Different Regions of the USA" Animals 12, no. 17: 2273. https://doi.org/10.3390/ani12172273
APA StyleTadesse, D., Patra, A. K., Puchala, R., & Goetsch, A. L. (2022). Effects of High Heat Load Conditions on Blood Constituent Concentrations in Dorper, Katahdin, and St. Croix Sheep from Different Regions of the USA. Animals, 12(17), 2273. https://doi.org/10.3390/ani12172273






