Estimation of Genetic Parameters of Heat Tolerance for Production Traits in Canadian Holsteins Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Data Availability
2.2. Milk Production and Weather Data
2.3. Statistical Analyses
2.4. Estimated Breeding Value and GxE Interaction
3. Results
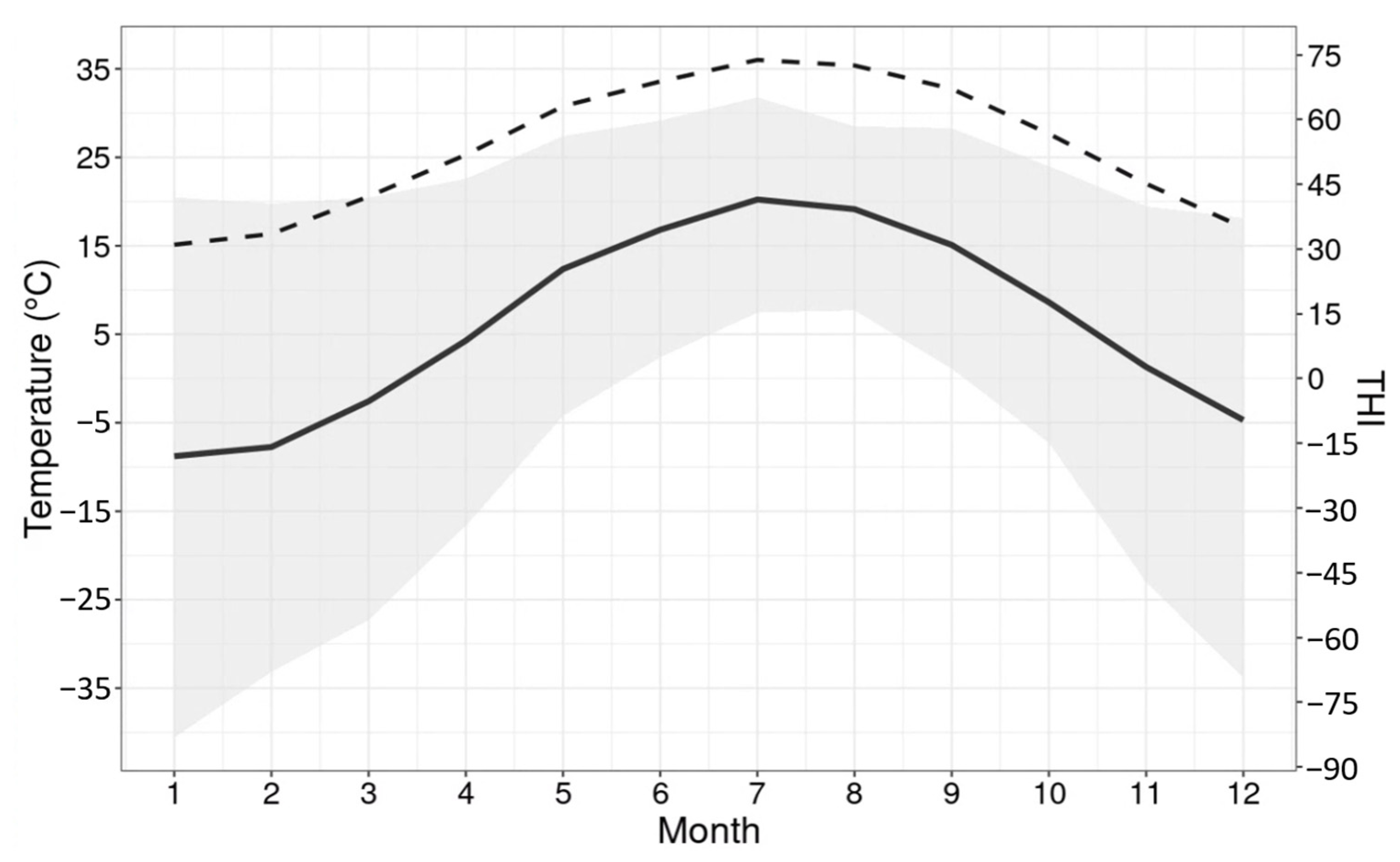
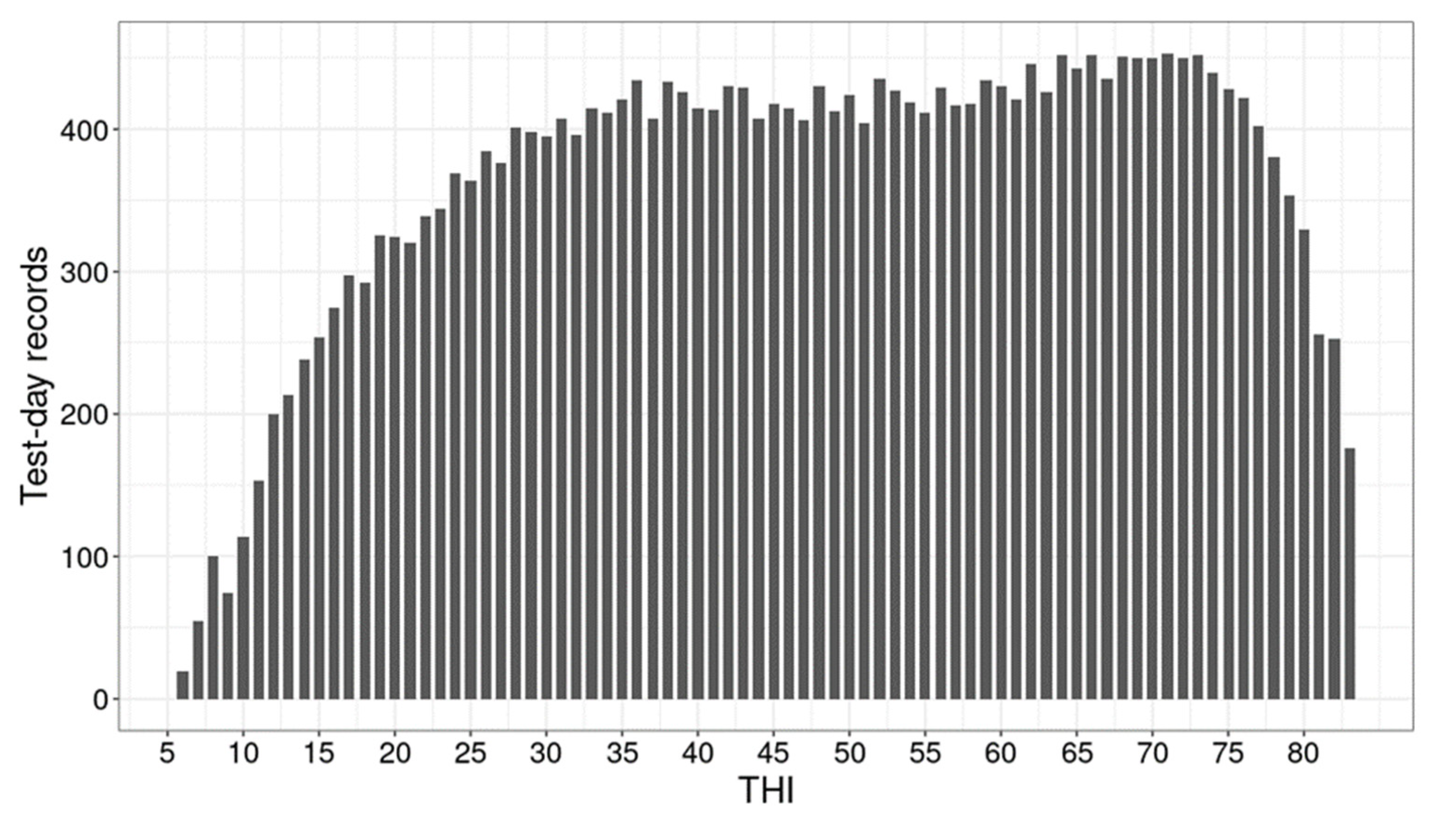
3.1. Milk Production and Weather Conditions
3.2. Variance Component Estimates
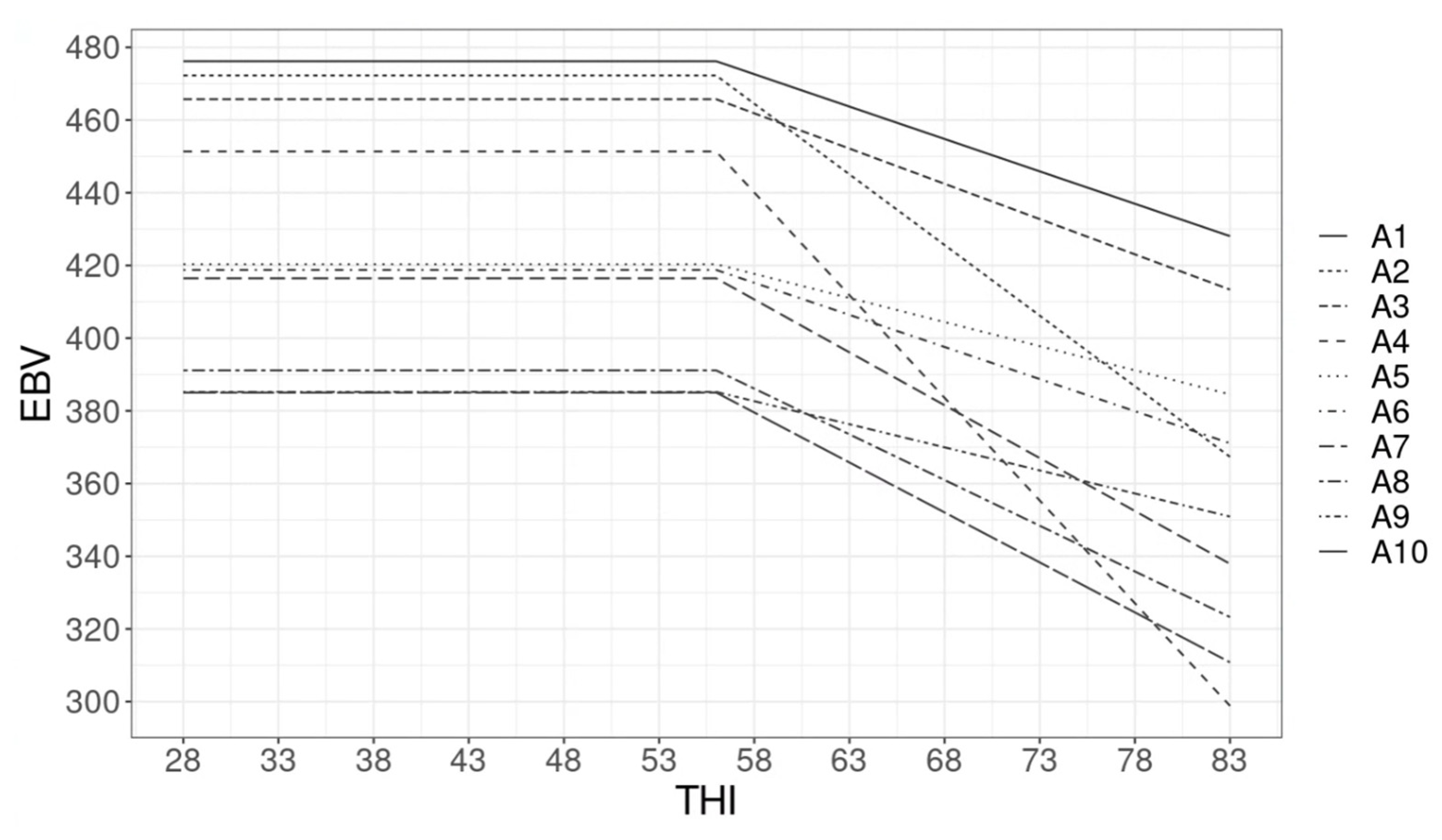
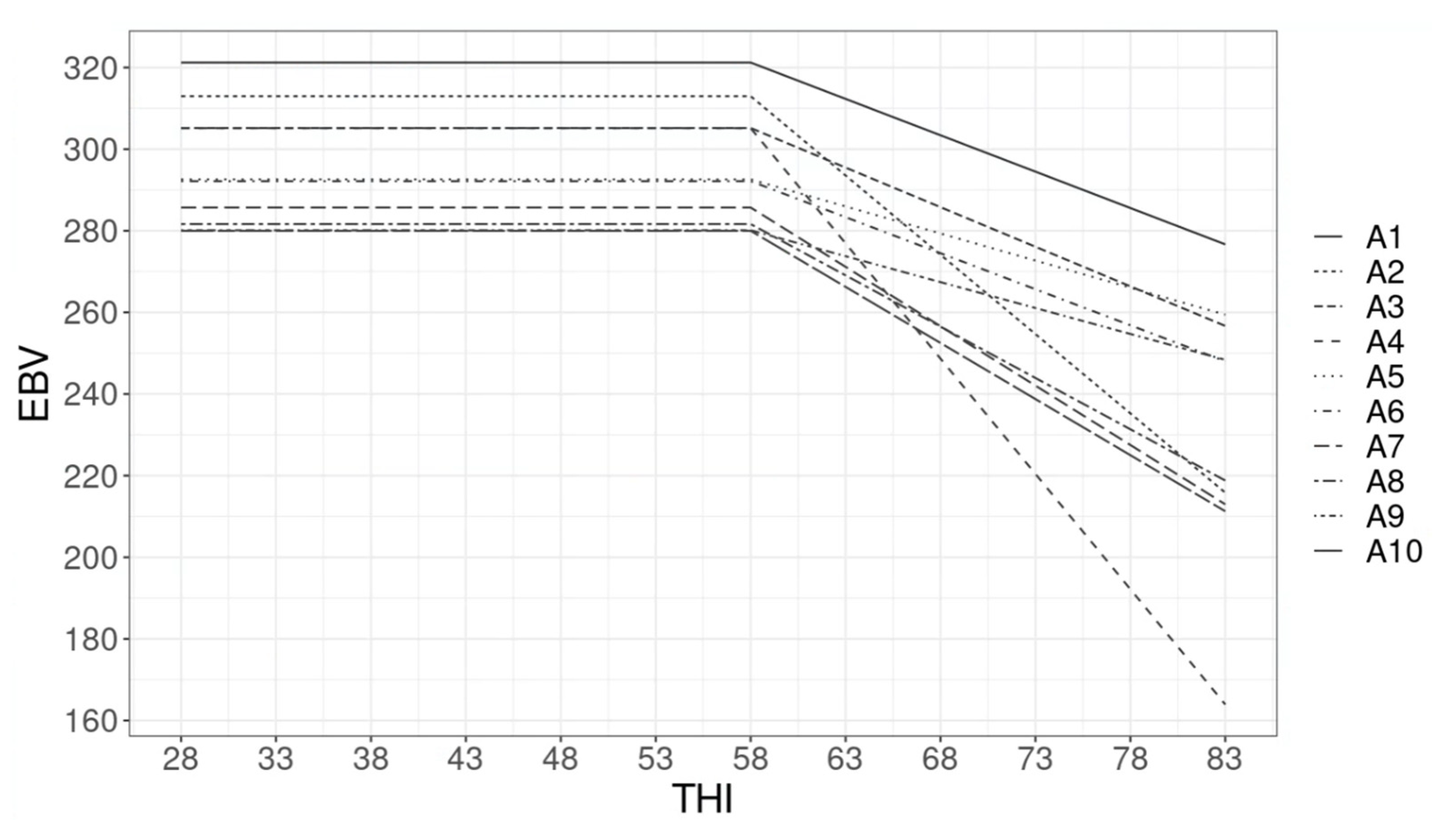
3.3. Estimated Breeding Value and GxE
4. Discussion
4.1. Milk Production and Weather Conditions
4.2. Variance Component Estimates
4.3. Estimated Breeding Value and GxE
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.; Martin, P.; Baes, C. A 100-Year Review: Identification and Genetic Selection of Economically Important Traits in Dairy Cattle. J. Dairy Sci. 2017, 100, 10251–10271. [Google Scholar] [CrossRef] [PubMed]
- Weigel, K.A.; VanRaden, P.M.; Norman, H.D.; Grosu, H. A 100-Year Review: Methods and Impact of Genetic Selection in Dairy Cattle—From Daughter–Dam Comparisons to Deep Learning Algorithms. J. Dairy Sci. 2017, 100, 10234–10250. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada. Canadian farms. Statistics on farms in Canada. Available online: https://agriculture.canada.ca/en/sector/animal-industry/canadian-dairy-information-centre/dairy-statistics-and-market-information/farm-statistics (accessed on 17 December 2022).
- Statistics Canada. Table 32-10-0130-01 Number of Cattle, by Class and Farm Type. Available online: https://doi.org/10.25318/3210013001-eng (accessed on 17 December 2022).
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited Review: Physiological and Behavioral Effects of Heat Stress in Dairy Cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef] [PubMed]
- Carabaño, M.J.; Ramón, M.; Menéndez-Buxadera, A.; Molina, A.; Díaz, C. Selecting for Heat Tolerance. Anim. Front. 2019, 9, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.; Misztal, I.; Tsuruta, S. Short Communication: Genetic Trends of Milk Yield under Heat Stress for US Holsteins. J. Dairy Sci. 2010, 93, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Global Warming of 1.5 C—Summary for Policymakers; IPCC: Geneva, Switzerland, 2018; Available online: https://www.ipcc.ch/sr15/chapter/spm/ (accessed on 17 December 2022).
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat Stress: Physiology of Acclimation and Adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Pinedo, P.J.; de Vries, A. Season of Conception Is Associated with Future Survival, Fertility, and Milk Yield of Holstein Cows. J. Dairy Sci. 2017, 100, 6631–6639. [Google Scholar] [CrossRef]
- Laporta, J.; Ferreira, F.C.; Ouellet, V.; Dado-Senn, B.; Almeida, A.K.; de Vries, A.; Dahl, G.E. Late-Gestation Heat Stress Impairs Daughter and Granddaughter Lifetime Performance. J. Dairy Sci. 2020, 103, 7555–7568. [Google Scholar] [CrossRef]
- Hahn, G.L.; Gaughan John, B.; Mader Terry, L.; Eigenberg, R.A. Chapter 5: Thermal Indices and Their Applications for Livestock Environments. In Livestock Energetics and Thermal Environment Management; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2009; pp. 113–130. [Google Scholar]
- Dikmen, S.; Hansen, P.J. Is the Temperature-Humidity Index the Best Indicator of Heat Stress in Lactating Dairy Cows in a Subtropical Environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ravagnolo, O.; Misztal, I.; Hoogenboom, G. Genetic Component of Heat Stress in Dairy Cattle, Development of Heat Index Function. J. Dairy Sci. 2000, 83, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, R.; Lahmar, M.; Majdoub, A.; Djemali, M.; Belyea, R. The Relationship of Temperature-Humidity Index with Milk Production of Dairy Cows in a Mediterranean Climate. Anim. Res. 2002, 51, 479–491. [Google Scholar] [CrossRef]
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The Effects of Heat Stress in Italian Holstein Dairy Cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Williams, K.E.; Berke, O.; Pearl, D.L.; Hand, K.; Kelton, D.F. Heat Stress Related Dairy Cow Mortality during Heat Waves and Control Periods in Rural Southern Ontario from 2010–2012. BMC Vet Res. 2015, 11, 291. [Google Scholar] [CrossRef]
- Ouellet, V.; Cabrera, V.E.; Charbonneau, É. The Relationship between the Number of Consecutive Days with Heat Stress and Milk Production of Holstein Dairy Cows Raised in a Humid Continental Climate. J. Dairy Sci. 2019, 102, 8537–8545. [Google Scholar] [CrossRef] [PubMed]
- Campos, I.L.; Chud, T.C.S.; Oliveira, H.R.; Baes, C.F.; Cánovas, A.; Schenkel, F.S. Using Publicly Available Weather Station Data to Investigate the Effects of Heat Stress on Milk Production Traits in Canadian Holstein Cattle. Can. J. Anim. Sci. 2022, 102, 368–381. [Google Scholar] [CrossRef]
- Ravagnolo, O.; Misztal, I. Genetic Component of Heat Stress in Dairy Cattle, Parameter Estimation. J. Dairy Sci. 2000, 83, 2126–2130. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Bowman, P.J.; Haile-Mariam, M.; Pryce, J.E.; Hayes, B.J. Genomic Selection for Tolerance to Heat Stress in Australian Dairy Cattle. J. Dairy Sci. 2016, 99, 2849–2862. [Google Scholar] [CrossRef]
- 23. Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman Group Ltd.: London, UK, 1996; 464p. [Google Scholar]
- de Jong, G. Quantitative Genetics of Reaction Norms. J. Evol. Biol. 1990, 3, 447–468. [Google Scholar] [CrossRef]
- Schaeffer, L.R. Application of Random Regression Models in Animal Breeding. Livest. Prod. Sci. 2004, 86, 35–45. [Google Scholar] [CrossRef]
- Misztal, I. Breeding and Genetics Symposium: Resilience and Lessons from Studies in Genetics of Heat Stress. J. Anim. Sci. 2017, 95, 1780. [Google Scholar] [CrossRef] [PubMed]
- LaZerte, S.E.; Albers, S. Weathercan: Download and Format Weather Data from Environment and Climate Change Canada. J. Open Source Softw. 2018, 3, 571. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org/ (accessed on 17 December 2022).
- Ouellet, V.; Bellavance, A.L.; Fournel, S. Charbonneau Short Communication: Summer on-Farm Environmental Condition Assessments in Québec Tiestall Farms and Adaptation of Temperature-Humidity Index Calculated with Local Meteorological Data. J. Dairy Sci. 2019, 102, 7503–7508. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J.; Williams, E.; Vennes, C. Geosphere: Spherical Trigonometry. R Package Version 1.5-10. Package Geosphere 2019. Available online: https://mran.microsoft.com/package/geosphere (accessed on 17 December 2022).
- West, J.W. Effects of Heat-Stress on Production in Dairy Cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Spiers, D.E.; Spain, J.N.; Sampson, J.D.; Rhoads, R.P. Use of Physiological Parameters to Predict Milk Yield and Feed Intake in Heat-Stressed Dairy Cows. J. Therm. Biol. 2004, 29, 759–764. [Google Scholar] [CrossRef]
- Bohmanova, J.; Misztal, I.; Tsuruta, S.; Norman, H.D.; Lawlor, T.J. Short Communication: Genotype by Environment Interaction Due to Heat Stress. J. Dairy Sci. 2008, 91, 840–846. [Google Scholar] [CrossRef]
- Misztal, I.; Tsuruta, S.; Strabel, T.; Auvray, B.; Druet, T.; Lee, D.H. BLUPF90 and Related Programs (BGF90). Commun. No. 28-07. In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002; Volume 33. [Google Scholar]
- Interbull National Genetic Evaluation Form Provided by Countries. Available online: https://interbull.org/ib/geforms (accessed on 19 July 2020).
- Bisson, G.; Séguin, M.; Roy, R. Producing cost-effective milk in 2021. 2021. Available online: https://lactanet.ca/en/producing-cost-effective-milk-in-2021/ (accessed on 17 December 2022).
- Aguilar, I.; Misztal, I.; Tsuruta, S. Genetic Components of Heat Stress for Dairy Cattle with Multiple Lactations. J. Dairy Sci. 2009, 92, 5702–5711. [Google Scholar] [CrossRef]
- Sigdel, A.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Whole Genome Mapping Reveals Novel Genes and Pathways Involved in Milk Production Under Heat Stress in US Holstein Cows. Front. Genet. 2019, 10, 928. [Google Scholar] [CrossRef]
- Hammami, H.; Vandenplas, J.; Vanrobays, M.L.; Rekik, B.; Bastin, C.; Gengler, N. Genetic Analysis of Heat Stress Effects on Yield Traits, Udder Health, and Fatty Acids of Walloon Holstein Cows. J. Dairy Sci. 2015, 98, 4956–4968. [Google Scholar] [CrossRef]
- Cheruiyot, E.K.; Nguyen, T.T.T.; Haile-Mariam, M.; Cocks, B.G.; Abdelsayed, M.; Pryce, J.E. Genotype-by-Environment (Temperature-Humidity) Interaction of Milk Production Traits in Australian Holstein Cattle. J. Dairy Sci. 2020, 103, 2460–2476. [Google Scholar] [CrossRef]
- Santana, M.L.; Bignardi, A.B.; Pereira, R.J.; Stefani, G.; el Faro, L. Genetics of Heat Tolerance for Milk Yield and Quality in Holsteins. Animal 2017, 11, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Bohlouli, M.; Alijani, S.; Naderi, S.; Yin, T.; König, S. Prediction Accuracies and Genetic Parameters for Test-Day Traits from Genomic and Pedigree-Based Random Regression Models with or without Heat Stress Interactions. J. Dairy Sci. 2019, 102, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Carrara, E.R.; Petrini, J.; Salvian, M.; de Oliveira, H.R.; Rovadoscki, G.A.; Iung, L.H.d.S.; Miquilini, M.; Machado, P.F.; Mourão, G.B. Genetic Parameters for Milk Yield and Quality Traits of Brazilian Holstein Cows as a Function of Temperature and Humidity Index. J. Anim. Breed. Genet. 2021, 138, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Shock, D.A.; LeBlanc, S.J.; Leslie, K.E.; Hand, K.; Godkin, M.A.; Coe, J.B.; Kelton, D.F. Studying the Relationship between On-Farm Environmental Conditions and Local Meteorological Station Data during the Summer. J. Dairy Sci. 2016, 99, 2169–2179. [Google Scholar] [CrossRef]
- Freitas, M.S.; Misztal, I.; Bohmanova, J.; West, J. Utility of On- and off-Farm Weather Records for Studies in Genetics of Heat Tolerance. Livest. Sci. 2006, 105, 223–228. [Google Scholar] [CrossRef]
- Santana, M.L.; Bignardi, A.B.; Pereira, R.J.; Menéndez-Buxadera, A.; el Faro, L. Random Regression Models to Account for the Effect of Genotype by Environment Interaction Due to Heat Stress on the Milk Yield of Holstein Cows under Tropical Conditions. J. Appl. Genet. 2016, 57, 119–127. [Google Scholar] [CrossRef]
| Trait | THI ≤ THIthreshold 1 | THI > THIthreshold 1 | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Milk yield (kg/d) | 30.26 | 6.27 | 30.12 | 6.37 |
| Fat yield (g/d) | 1190.88 | 254.02 | 1147.90 | 252.78 |
| Protein yield (g/d) | 987.42 | 188.88 | 963.90 | 190.19 |
| Milk Yield (kg2) | Fat Yield (g2) | Protein Yield (g2) | ||||
|---|---|---|---|---|---|---|
| Parameter 1 | THI < 70 | THI = 83 | THI < 57 | THI = 83 | THI < 58 | THI = 83 |
| 7.30 (±0.12) | 7.30 | 10,675.00 (±203.69) | 10,675.00 | 4874.80 (±106.31) | 4874.80 | |
| 0.02 (±0.01) | 3.95 | 5.89 (±0.50) | 4297.60 | 5.08 (±0.34) | 3439.62 | |
| 6.98 (±0.08) | 6.98 | 10,660.00 (±141.23) | 10,660.00 | 6872.50 (±76.07) | 6872.50 | |
| 0.03 (± 0.01) | 7.21 | 24.55 (±0.51) | 17,902.05 | 17.74 (±0.32) | 11,998.32 | |
| 0.21 (± 0.01) | 0.23 | 0.17 (± 0.01) | 0.17 | 0.14 (± 0.01) | 0.16 | |
| −0.13 (± 0.01) | −0.21 (± 0.01) | −0.20 (± 0.01) | ||||
| Milk Yield (kg) | Fat Yield (g) | Protein Yield (g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max |
| EBV_TC | −7.09 | 1.33 | 10.82 | −146.50 | 64.63 | 476.17 | −147.28 | 50.43 | 325.06 |
| EBV_HT | −0.24 | −0.02 | 0.22 | −8.23 | −2.14 | 1.93 | −5.64 | −1.34 | 4.10 |
| EBV_HS5 | −6.94 | 1.24 | 10.13 | −155,88 | 53.93 | 453.04 | −162.41 | 43.69 | 312.30 |
| EBV_HS10 | −6.80 | 1.15 | 10.92 | −170.18 | 43.23 | 429.92 | −177.54 | 36.94 | 303.40 |
| All Bulls 1 (n = 1916) | Top 100 Bulls | |||
|---|---|---|---|---|
| Trait | EBV_TC/EBV_HS5 | EBV_TC/EBV_HS10 | EBV_TC/EBV_HS5 | EBV_TC/EBV_THS10 |
| Milk | 0.98 | 0.95 | 0.85 | 0.59 |
| Fat | 0.99 | 0.99 | 0.96 | 0.82 |
| Protein | 0.99 | 0.98 | 0.91 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, I.L.; Chud, T.C.S.; Junior, G.A.O.; Baes, C.F.; Cánovas, Á.; Schenkel, F.S. Estimation of Genetic Parameters of Heat Tolerance for Production Traits in Canadian Holsteins Cattle. Animals 2022, 12, 3585. https://doi.org/10.3390/ani12243585
Campos IL, Chud TCS, Junior GAO, Baes CF, Cánovas Á, Schenkel FS. Estimation of Genetic Parameters of Heat Tolerance for Production Traits in Canadian Holsteins Cattle. Animals. 2022; 12(24):3585. https://doi.org/10.3390/ani12243585
Chicago/Turabian StyleCampos, Ivan L., Tatiane C. S. Chud, Gerson A. Oliveira Junior, Christine F. Baes, Ángela Cánovas, and Flavio S. Schenkel. 2022. "Estimation of Genetic Parameters of Heat Tolerance for Production Traits in Canadian Holsteins Cattle" Animals 12, no. 24: 3585. https://doi.org/10.3390/ani12243585
APA StyleCampos, I. L., Chud, T. C. S., Junior, G. A. O., Baes, C. F., Cánovas, Á., & Schenkel, F. S. (2022). Estimation of Genetic Parameters of Heat Tolerance for Production Traits in Canadian Holsteins Cattle. Animals, 12(24), 3585. https://doi.org/10.3390/ani12243585







