Can Chromoendoscopy Improve the Early Diagnosis of Gastric Carcinoma in Dogs?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Ethical Licensing
2.2. Endoscopic Equipment and Image Acquisition
2.3. Targeted and Non-Targeted Biopsy Sampling and Histologic Assessment of Gastric Mucosal Microstructure
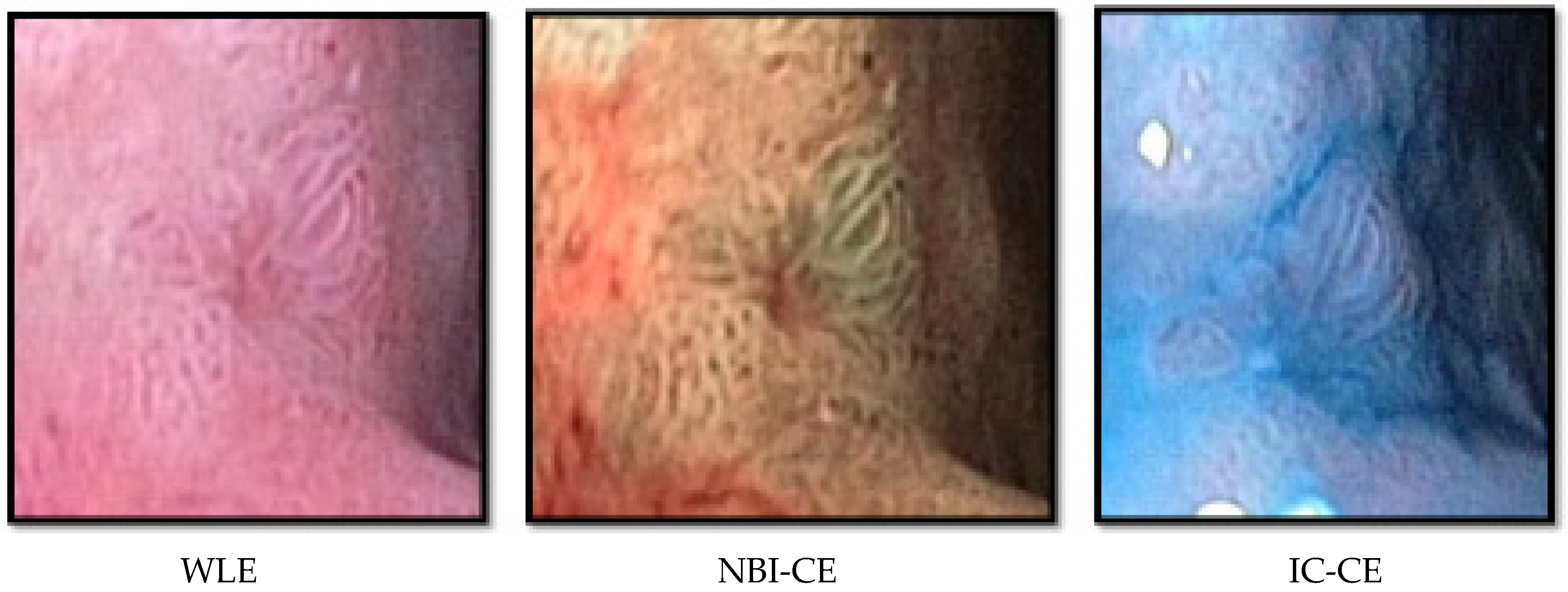
2.4. Real-Time Assessment of Endoscopic Findings during WLE, NBI-CE, and IC-CE
2.5. Template-Based NBI-CE and IC-CE Image Assessment of Gastric Mucosal Surface Patterns
2.6. Statistical Analysis
3. Results
3.1. Dogs and Endoscopic Findings
3.2. Histology of Targeted and Non-Targeted Gastric Mucosal Biopsies
3.3. Template-Based Assessment of Images Taken during NBI-CE
3.4. Template-Based Assessment of Images Taken during IC-CE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugen, S.; Thomas, R.E.; German, A.J.; Burgener, I.A.; Mandigers, P.J.J. Gastric carcinoma in canines and humans, a review. Vet. Comp. Oncol. 2017, 15, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nibe, K.; Chambers, J.K.; Uneyama, M.; Nakashima, K.; Ohno, K.; Tsujimoto, H.; Uchida, K.; Nakayama, H. A histopathological study on spontaneous gastrointestinal epithelial tumors in dogs. J. Toxicol. Pathol. 2020, 33, 105–113. [Google Scholar] [CrossRef]
- Abrams, B.; Wavreille, V.A.; Husbands, B.D.; Matz, B.M.; Massari, F.; Liptak, J.M.; Cray, M.T.; de Mello Souza, C.H.; Wustefeld-Janssens, B.G.; Oblak, M.L.; et al. Perioperative complications and outcome after surgery for treatment of gastric carcinoma in dogs: A Veterinary Society of Surgical Oncology retrospective study of 40 cases (2004–2018). Vet. Surg. 2019, 48, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Spillmann, T.; Candido, M. Stomach. In BSAVA Manual of Canine and Feline Gastroenterology, 3rd ed.; Hall, E.J., Williams, D.A., Kathrani, A., Eds.; BSAVA: Gloucester, UK, 2020; pp. 117–197. [Google Scholar]
- Coda, S.; Thillainayagam, A.V. State of the art in advanced endoscopic imaging for the detection and evaluation of dysplasia and early cancer of the gastrointestinal tract. Clin. Exp. Gastroenterol. 2014, 7, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Cândido, M.V.; Syrjä, P.; Hanifeh, M.; Lepajõe, J.; Salla, K.; Kilpinen, S.; Noble, P.J.; Spillmann, T. Gastric mucosal pathology in Belgian Shepherd dogs with and without clinical signs of gastric disease. Acta Vet. Scand. 2021, 63, 7. [Google Scholar] [CrossRef] [PubMed]
- Ngamruengphong, S.; Abe, S.; Oda, I. Endoscopic management of early gastric adenocarcinoma and preinvasive gastric lesions. Surg. Clin. N. Am. 2017, 97, 371–385. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Lage, J.; Abrantes, D.; Coimbra, M.; Esposito, G.; Hormozdi, D.; Pepper, M.; Drasovean, S.; White, J.R.; et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016, 48, 723–730. [Google Scholar] [CrossRef]
- Salavati, M.; Pérez-Accino, J.; Tan, Y.L.; Liuti, T.; Smith, S.; Morrison, L.; Salavati Schmitz, S. Correlation of minimally invasive imaging techniques to assess intestinal mucosal perfusion with established markers of chronic inflammatory enteropathy in dogs. J. Vet. Intern. Med. 2021, 35, 162–171. [Google Scholar] [CrossRef]
- Yokoyama, T.; Miyahara, R.; Funasaka, K.; Furukawa, K.; Yamamura, T.; Ohno, E.; Nakamura, M.; Kawashima, H.; Watanabe, O.; Hirooka, Y.; et al. The utility of ultrathin endoscopy with flexible spectral imaging color enhancement for early gastric cancer. Nagoya J. Med. Sci. 2019, 81, 241–248. [Google Scholar]
- Capelle, L.G.; Haringsma, J.; de Vries, A.C.; Steyerberg, E.W.; Biermann, K.; van Dekken, H.; Kuipers, E.J. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig. Dis. Sci. 2010, 55, 3442–3448. [Google Scholar] [CrossRef]
- Gershman, G.; Ament, M. Chromoendoscopy. In Practical Pedriatric Gastrointestinal Endoscopy; Gershman, G., Ament, M., Eds.; John Wiley & Sons: Chichester, UK, 2008; pp. 181–193. [Google Scholar]
- Uedo, N.; Yao, K. Endoluminal diagnosis of early gastric cancer and its precursors: Bridging the gap between endoscopy and pathology. Adv. Exp. Med. Biol. 2016, 908, 293–316. [Google Scholar]
- Spillmann, T.; Willard, M.D.; Ruhnke, I.; Suchodolski, J.S.; Steiner, J.M. Feasibility of endoscopic retrograde cholangiopancreatography in healthy cats. Vet. Radiol. Ultrasound 2014, 55, 85–91. [Google Scholar] [CrossRef]
- Basford, P.J.; Brown, J.; Gadeke, L.; Fogg, C.; Haysom-Newport, B.; Ogollah, R.; Bhattacharyya, R.; Longcroft-Wheaton, G.; Thursby-Pelham, F.; Neale, J.R.; et al. A randomized controlled trial of pre-procedure simethicone and N-acetylcysteine to improve mucosal visibility during gastroscopy NICEVIS. Endosc. Int. Open 2016, 4, E1197–E1202. [Google Scholar] [CrossRef]
- Salla, K.M.; Lepajoe, J.; Candido, M.V.; Spillmann, T.; Casoni, D. Comparison of the effects of methadone and butorphanol combined with acepromazine for canine gastroduodenoscopy. Vet. Anaesth. Analg. 2020, 47, 748–756. [Google Scholar] [CrossRef]
- Simone, A.; Casadei, A.; De Vergori, E.; Morgagni, P.; Saragoni, L.; Ricci, E. Rescue endoscopy to identify site of gastric dysplasia or carcinoma found at random biopsies. Dig. Liver Dis. 2011, 43, 721–725. [Google Scholar] [CrossRef]
- Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar]
- Amorim, I.; Taulescu, M.A.; Day, M.J.; Catoi, C.; Reis, C.A.; Carneiro, F.; Gärtner, F. Canine gastric pathology: A review. J. Comp. Pathol. 2016, 154, 9–37. [Google Scholar] [CrossRef]
- Plattner, B.; Hostetter, J.M.; Uzal, F.A. Alimentary system and peritoneum. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Volume 2, p. 47. [Google Scholar]
- Jergens, A.E.; Evans, R.B.; Ackermann, M.; Hostetter, J.; Willard, M.; Mansell, J.; Bilzer, T.; Wilcock, B.; Washabau, R.; Hall, E.J.; et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet. Pathol. 2014, 51, 946–950. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Soares, J.B.; Marcos-Pinto, R.; Santos, C.; Rolanda, C.; Bastos, R.P.; Areia, M.; Afonso, L.; Bergman, J.; et al. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy 2012, 44, 236–246. [Google Scholar] [CrossRef]
- Uedo, N.; Ishihara, R.; Iishi, H.; Yamamoto, S.; Yamada, T.; Imanaka, K.; Takeuchi, Y.; Higashino, K.; Ishiguro, S.; Tatsuta, M. A new method of diagnosing gastric intestinal metaplasia: Narrow-band imaging with magnifying endoscopy. Endoscopy 2006, 38, 819–824. [Google Scholar] [CrossRef]
- Waddingham, W.; Graham, D.; Banks, M.; Jansen, M. The evolving role of endoscopy in the diagnosis of premalignant gastric lesions. F1000Research 2018, 7, 715. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Fassan, M.; Pizzi, M.; Farinati, F.; Sturniolo, G.C.; Plebani, M.; Graham, D.Y. Operative link for gastritis assessment vs. operative link on intestinal metaplasia assessment. World J. Gastroenterol. 2011, 17, 4596–4601. [Google Scholar] [CrossRef] [PubMed]
- Seim-Wikse, T.; Jörundsson, E.; Nødtvedt, A.; Grotmol, T.; Bjornvad, C.R.; Kristensen, A.T.; Skancke, E. Breed predisposition to canine gastric carcinoma—A study based on the Norwegian canine cancer register. Acta Vet. Scand. 2013, 55, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candido, M.V.; Syrjä, P.; Kilpinen, S.; Spillmann, T. Canine breeds associated with gastric carcinoma, metaplasia and dysplasia diagnosed by histopathology of endoscopic biopsy samples. Acta Vet. Scand. 2018, 60, 37. [Google Scholar] [CrossRef]
- Munday, J.S.; Löhr, C.V.; Kiupel, M. Tumors of the alimentary tract. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley and Sons Inc.: Ames, IA, USA, 2020; pp. 499–600. [Google Scholar]
- Hoffman, A.; Kiesslich, R.; Bender, A.; Neurath, M.F.; Nafe, B.; Herrmann, G.; Jung, M. Acetic acid-guided biopsies after magnifying endoscopy compared with random biopsies in the detection of Barrett’s esophagus: A prospective randomized trial with crossover design. Gastrointest Endosc. 2006, 64, 1–8. [Google Scholar] [CrossRef]
- Kara, M.A.; Peters, F.P.; Rosmolen, W.D.; Krishnadath, K.K.; Ten Kate, F.J.; Fockens, P.; Bergman, J.J. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: A prospective randomized crossover study. Endoscopy 2005, 37, 929–936. [Google Scholar] [CrossRef]
- Gruner, M.; Denis, A.; Masliah, C.; Amil, M.; Metivier-Cesbron, E.; Luet, D.; Kaasis, M.; Coron, E.; Le Rhun, M.; Lecleire, S.; et al. Narrow-band imaging versus Lugol chromoendoscopy for esophageal squamous cell cancer screening in normal endoscopic practice: Randomized controlled trial. Endoscopy 2021, 53, 674–682. [Google Scholar] [CrossRef]
- Bisschops, R.; Bessissow, T.; Joseph, J.A.; Baert, F.; Ferrante, M.; Ballet, V.; Willekens, H.; Demedts, I.; Geboes, K.; De Hertogh, G.; et al. Chromoendoscopy versus narrow band imaging in UC: A prospective randomised controlled trial. Gut 2018, 67, 1087–1094. [Google Scholar] [CrossRef]
- Ishioka, M.; Chino, A.; Ide, D.; Saito, S.; Igarashi, M.; Nagasaki, T.; Akiyoshi, T.; Nagayama, S.; Fukunaga, Y.; Ueno, M.; et al. Adding narrow-band imaging to chromoendoscopy for the evaluation of tumor response to neoadjuvant therapy in rectal cancer. Dis. Colon Rectum 2021, 64, 53–59. [Google Scholar] [CrossRef]
- Pennachi, C.M.P.S.; Moura, D.T.H.; Amorim, R.B.P.; Guedes, H.G.; Kumbhari, V.; Moura, E.G.H. Lugol’s iodine chromoendoscopy versus narrow band image enhanced endoscopy for the detection of esophageal cancer in patients with stenosis secondary to caustic/corrosive agent ingestion. Arq. Gastroenterol. 2017, 54, 250–254. [Google Scholar] [CrossRef]
- Lage, J.; Pimentel-Nunes, P.; Figueiredo, P.C.; Libanio, D.; Ribeiro, I.; Jacome, M.; Afonso, L.; Dinis-Ribeiro, M. Light-NBI to identify high-risk phenotypes for gastric adenocarcinoma: Do we still need biopsies? Scand. J. Gastroenterol. 2016, 51, 501–506. [Google Scholar] [CrossRef]
- Yoshida, N.; Doyama, H.; Yano, T.; Horimatsu, T.; Uedo, N.; Yamamoto, Y.; Kakushima, N.; Kanzaki, H.; Hori, S.; Yao, K.; et al. Early gastric cancer detection in high-risk patients: A multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut 2021, 70, 67–75. [Google Scholar] [CrossRef]
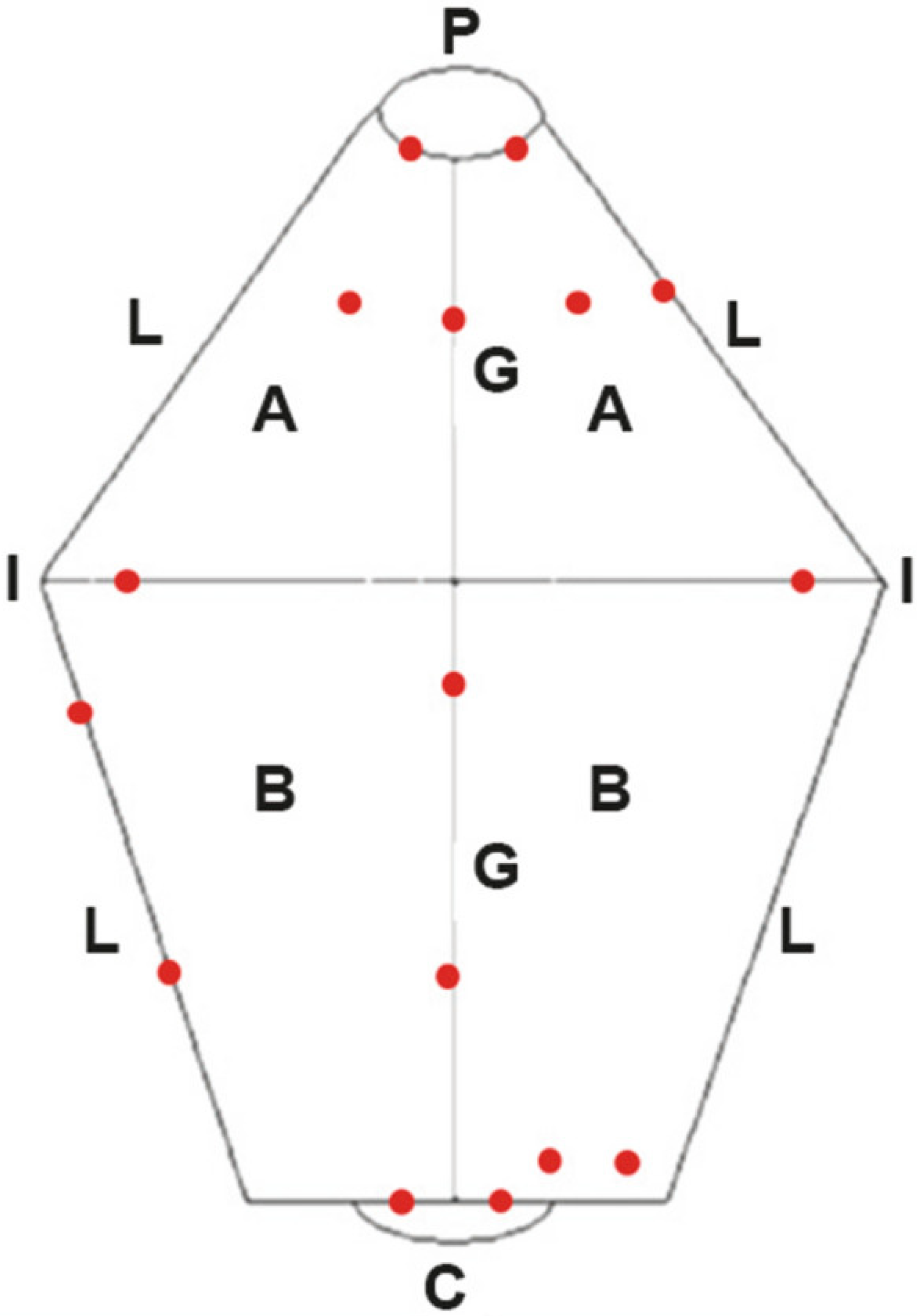
| Gastric Mucosal Pattern Category | NBI-CE Example Image |
|---|---|
| Normal round |  |
| Normal mosaic and polygonal |  |
| Enlarged mosaic and polygonal |  |
| Tubulo-villous | 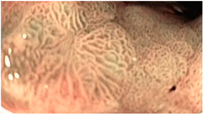 |
| Gastric Mucosal Pattern Category | IC-CE Example Image |
|---|---|
| Normal | 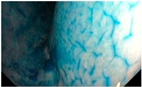 |
| Suspicious |  |
| Dog Nr. | Dog’s Study ID | Breed Type | Age in Years | Sex | Body Weight (kg) | Distribution of the Most Relevant Endoscopic Finding | Type of Inflammation | Mucous Metaplasia | Glandular Dysplasia | Carcinoma | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biopsy Set 1 | Biopsy Set 2 | Biopsy Set 1 | Biopsy Set 2 | Biopsy Set 1 | Biopsy Set 2 | ||||||||
| 1 | 641 | Tervuren | 10.8 | M | 26.1 | Diffuse Abnormal folding surrounded by irregular mucosal surface in corpus: distal greater curvature | LP | Yes | Yes | Yes | No | Yes | No |
| 2 | 642 | Tervuren | 9.9 | M | 22.7 | Diffuse Very prominent blood vessels in proximal antrum; abnormal folding/ thicker mucosa in corpus: distal greater curvature | LP, EO, NP | No | No | No | No | No | No |
| 3 | 644 | Tervuren | 10.1 | FS | 18.5 | Diffuse Major blood vessels visible in most of corpus, especially along greater curvature; marked mucosal folding in pylorus | LP, EO, NP | No | No | No | No | No | No |
| 4 | 645 | Tervuren | 11.6 | FS | 25.3 | Focal Sessile polyp 2 mm × 5 mm | LP, EO, NP | No | No | No | No | No | No |
| 5 | 646 | Groenendael | 11.2 | M | 23.0 | Diffuse Persistent folds after full insufflation in corpus: distal greater curvature | LP, NP | No | No | No | Yes | No | No |
| 6 | 647 | Tervuren | 10.1 | FS | 23.4 | Diffuse Abnormal folding in corpus: distal greater curvature | LP, NP | Yes | Yes | Yes | Yes | Yes | Yes |
| 7 | 649 | Tervuren | 7.9 | F | 20.5 | Diffuse Irregular mucosal surface in corpus: distal greater curvature | LP, EO, NP | No | No | No | No | No | No |
| 8 | 650 | Groenendael | 10.6 | M | 23.0 | Unremarkable | LP, NP | No | No | No | No | No | No |
| 9 | 702 | Malinois | 11.1 | MC | 31.6 | Focal Irregular mucosal surface in corpus: distal greater curvature | LP, EO | Yes | No | No | No | No | No |
| 10 | 703 | Tervuren | 9.0 | M | 21.3 | Diffuse More than 20 discolored patches 5 mm all over antrum | LP, EO | No | No | No | Yes | No | No |
| 11 | 652 | Tervuren | 10.1 | MC | 30.7 | Ulcerative More than 30 erosions 2 mm in proximal greater curvature | LP, EO | No | No * | Yes | No | No | No |
| 12 | 653 | Malinois | 6.7 | FS | 36.6 | Diffuse Hyperemic spots around cardia (lymphoid follicles) | LP, EO | No | No | No | No | No | No |
| 13 | 654 | Tervuren | 7.6 | M | 29.3 | Ulcerative Linear ulcers in corpus: distal lesser curvature | LP, EO, NP | No | No | No | No | No | No |
| 14 | 655 | Groenendael | 9.1 | FS | 22.2 | Diffuse Hyperemic spots around cardia | LP, EO | No | No | No | No | No | No |
| 15 | 658 | Tervuren | 7.2 | M | 27.8 | Ulcerative Massive elevation in mucosa, two large crater-like ulcers in corpus | LP, EO, NP | Yes | No | Yes | No | Yes | No |
| 16 | 664 | Tervuren | 10.4 | FS | 22.6 | Focal Tiny polyp in pylorus | LP, EO, NP | No | No | Yes | No | No | No |
| 17 | 677 | Groenendael | 8.3 | F | 17.4 | Ulcerative Long ulcer crater in corpus, closer to lesser curvature; gross (mural) thickening of the incisura | LP, EO, NP | No | No | Yes | Yes | Yes | Yes |
| 18 | 689 | Malinois | 8.8 | FS | 22.2 | Unremarkable | LP, EO, NP | No | No | Yes | Yes | No | No |
| 19 | 687 | Groenendael | 9.7 | FS | 27.7 | Unremarkable | LP, EO, NP | No | No | Yes | Yes | No | No |
| 20 | 686 | Tervuren | 5.6 | F | 21.5 | Unremarkable | LP, EO, NP | No | No | No | No | No | No |
| 21 | 679 | Tervuren | 9.0 | FS | 17.1 | Diffuse Visible blood vessels on gastric body | LP, EO, NP | No | No | No | No | No | No |
| 22 | 722 | Tervuren | 11.2 | M | 29.8 | Diffuse Greater curvature: persisting folds in distal corpus, irregular surface in antrum | LP, EO, NP | Yes | Yes | Yes | No | No | No |
| 23 | 721 | Tervuren | 11.2 | FS | 23.2 | Ulcerative Three small ulcers along lesser curvature and thickened mucosa in distal greater curvature of corpus; hyperemic antrum greater curvature | LP, EO, NP | No | Yes | Yes | Yes | Yes | Yes |
| 24 | 680 | Tervuren | 5.5 | F | 22.7 | Diffuse Distal greater curvature of corpus: very marked persistent folding with irregular surface and multifocal hyperemia, linear depression | LP, NP | Yes | No | Yes | Yes | No | No |
| 25 | 750 | Tervuren | 10.1 | FS | 25.5 | Diffuse Corncob texture along the left side of the corpus | LP, EO | Yes | No | Yes | Yes | No | No |
| 26 | 749 | Groenendael | 8.3 | MC | 27.2 | Unremarkable | LP | No | No | No | No | No | No |
| 27 | 747 | Tervuren | 8.2 | F | 29.5 | Unremarkable | LP, EO | Yes | Yes | No | No | No | No |
| 28 | 724 | Groenendael | 7.6 | F | 24.9 | Diffuse Corpus: elevated mucosa with multifocal, depressed darker spots along proximal greater curvature; visible small vessels in distal lesser curvature | LP, EO | No | No | No | No | No | No |
| 29 | 711 | Groenendael | 8.3 | FS | 24.6 | Ulcerative Elongated, small transversal, non-bleeding crater in transition between corpus and antrum, left of greater curvature | LP, EO | No | No | Yes | No | Yes | No |
| 30 | 719 | Tervuren | 11.3 | FS | 18.2 | Unremarkable | LP, EO | No | No | No | No | No | No |
| Histopathological Diagnosis | Type of Mucosal Pattern at NBI-CE | |||
|---|---|---|---|---|
| Normal | Abnormal | |||
| Round (n = 44) | Mosaic and Polygonal (n = 21) | Enlarged Mosaic and Polygonal (n = 12) | Tubulo-Villous (n = 10) | |
| Normal (n = 24) | 14 | 7 | 3 | 0 |
| Inflammation (n = 32) | 19 | 6 | 4 | 3 |
| Mucous metaplasia (n = 2) | 1 | 1 | 0 | 0 |
| Glandular dysplasia (n = 16) | 8 | 4 | 4 | 0 |
| Carcinoma (n = 13) | 2 | 3 | 1 | 7 |
| (4.1) | ||||
| NBI-CE Mucosal Pattern | Histopathological Diagnosis | |||
| Normal/Inflammation (n = 56) | Meta-/Dysplasia/Carcinoma (n = 31) | |||
| Normal (n = 65) | 46 | 19 | ||
| Abnormal (n = 22) | 10 | 12 | ||
| Histopathological Diagnosis | IC-CE Mucosal Appearance | |||
|---|---|---|---|---|
| Normal (n = 109) | Suspicious (n = 20) | Abnormal (n = 9) | ||
| Normal (n = 46) | 40 | 6 | 0 | |
| Inflammation (n = 50) | 40 | 8 | 2 | |
| Mucous metaplasia (n = 1) | 1 | 0 | 0 | |
| Glandular dysplasia (n = 23) | 20 | 2 | 1 | |
| Carcinoma (n = 18) | 8 | 4 | 6 | |
| (5.1) | ||||
| IC-CE Mucosal Appearance | Histopathological Diagnosis | |||
| Normal/Inflammation (n = 96) | Meta-/Dysplasia/Carcinoma (n = 42) | |||
| Normal (n = 109) | 80 | 29 | ||
| Suspicious/Abnormal (n = 29) | 16 | 13 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candido, M.V.; Syrjä, P.; Kilpinen, S.; Meisner, S.; Hanifeh, M.; Spillmann, T. Can Chromoendoscopy Improve the Early Diagnosis of Gastric Carcinoma in Dogs? Animals 2022, 12, 2253. https://doi.org/10.3390/ani12172253
Candido MV, Syrjä P, Kilpinen S, Meisner S, Hanifeh M, Spillmann T. Can Chromoendoscopy Improve the Early Diagnosis of Gastric Carcinoma in Dogs? Animals. 2022; 12(17):2253. https://doi.org/10.3390/ani12172253
Chicago/Turabian StyleCandido, Marcus Vinicius, Pernilla Syrjä, Susanne Kilpinen, Søren Meisner, Mohsen Hanifeh, and Thomas Spillmann. 2022. "Can Chromoendoscopy Improve the Early Diagnosis of Gastric Carcinoma in Dogs?" Animals 12, no. 17: 2253. https://doi.org/10.3390/ani12172253
APA StyleCandido, M. V., Syrjä, P., Kilpinen, S., Meisner, S., Hanifeh, M., & Spillmann, T. (2022). Can Chromoendoscopy Improve the Early Diagnosis of Gastric Carcinoma in Dogs? Animals, 12(17), 2253. https://doi.org/10.3390/ani12172253







