Comparative Analysis of the Protein Composition of Goat Milk from French Alpine, Nubian, and Creole Breeds and Holstein Friesian Cow Milk: Implications for Early Infant Nutrition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Animal Feeding
2.3. Crude Protein Extraction from Skimmed Goat and Cow Milk by Conventional Solvent Precipitation
2.4. Total Protein Quantification by Fluorescence-Based Protein Assay
2.5. Resolution of Proteins by Gradient SDS-PAGE
2.6. Amino Acid Sequencing of BC Fraction by HPLC-MS/MS
2.7. Statistical Analysis
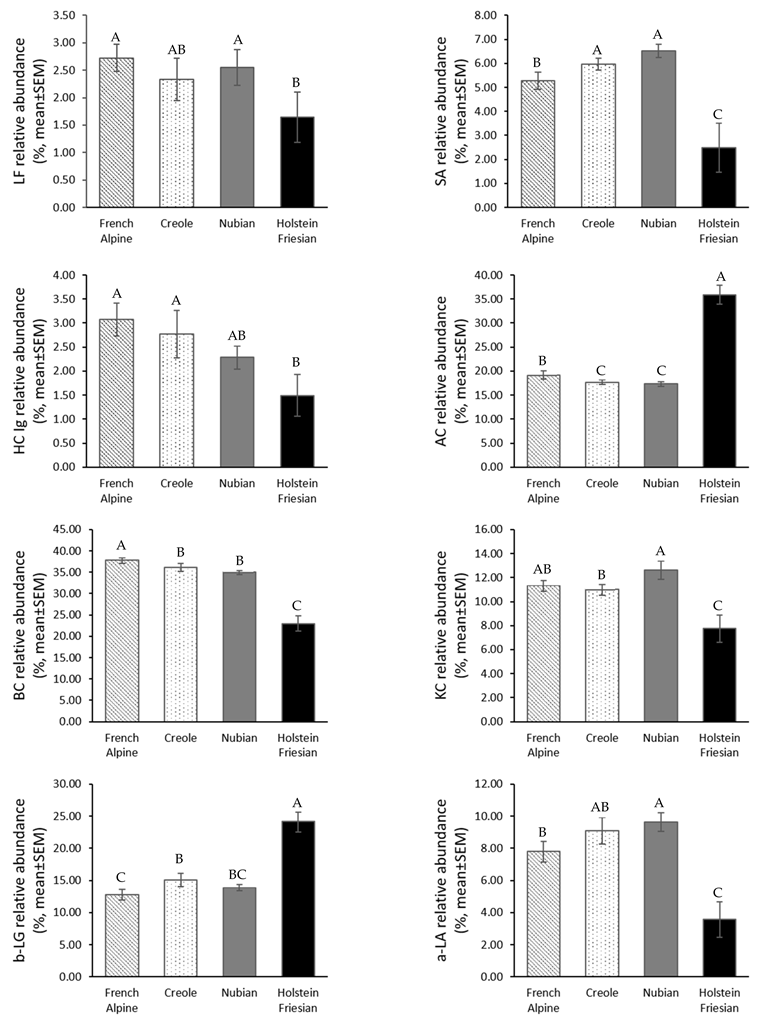
3. Results
3.1. Protein Quantification
3.2. Protein Profile of Goat Milk and Cow Milk
3.3. Amino Acid Sequencing of BC Fraction by HPLC-MS/MS
4. Discussion
4.1. Goat Milk and Cow Milk Protein Profile
4.2. Goat Milk BC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaelsen, K.F.; Greer, F.R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef] [PubMed]
- Haschke, F.; Binder, C.; Huber-Dangl, M.; Haiden, N. Early-life nutrition, growth trajectories, and long-term outcome. In Human Milk: Composition, Clinical Benefits and Future Opportunities; Karger Publishers: Basel, Switzerland, 2019; Volume 90, pp. 107–120. [Google Scholar]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012, 8, 1–87. [Google Scholar] [CrossRef]
- Feng, P.; Gao, M.; Holley, T.; Zhou, T.; Burgher, A.; Trabulsi, J.; Pramuk, K.; Nazzario, J. Amino Acid Composition and Protein Content of Mature Human Milk from Nine Countries. FASEB J. 2009, 23, LB448. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Erdmann, P.; Thakkar, S.K.; Sauser, J.; Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. J. Nutr. Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lönnerdal, B. Proteomic Characterization of Specific Minor Proteins in the Human Milk Casein Fraction. J. Proteome Res. 2011, 10, 5409–5415. [Google Scholar] [CrossRef]
- Niero, G.; Franzoi, M.; Manuelian, C.L.; Visentin, G.; Penasa, M.; De Marchi, M. Protein profile of cow milk from multibreed herds and its relationship with milk coagulation properties. Ital. J. Anim. Sci. 2021, 20, 2232–2242. [Google Scholar] [CrossRef]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lönnerdal, B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J. Proteome Res. 2011, 10, 1746–1754. [Google Scholar] [CrossRef]
- Park, Y.W.; Haenlein, G.F.W. A2 Bovine Milk and Caprine Milk as a Means of Remedy for Milk Protein Allergy. Dairy 2021, 2, 191–201. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, structure, and digestive dynamics of milk from different species—A review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef]
- Crowley, S.V.; Kelly, A.L.; Lucey, J.A.; O’Mahony, J.A. Potential Applications of Non-Bovine Mammalian Milk in Infant Nutrition. In Handbook of Milk of Non-Bovine Mammals; Wiley Online Books: Hoboken, NJ, USA, 2017; pp. 625–654. [Google Scholar] [CrossRef]
- Haenlein, G.F.W. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Carpenter, E.; Prosser, C.; Singh, H. Dynamic in vitro gastric digestion of infant formulae made with goat milk and cow milk: Influence of protein composition. Int. Dairy J. 2019, 97, 76–85. [Google Scholar] [CrossRef]
- Prosser, C.G. Compositional and functional characteristics of goat milk and relevance as a base for infant formula. J. Food Sci. 2021, 86, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Ceja, L.G.; Rosado, J.L.; Ronquillo, D.; García, O.P.; Caamaño, M.D.C.; García-Ugalde, C.; Viveros-Contreras, R.; Duarte-Vázquez, M. Lower Protein Intake Supports Normal Growth of Full-Term Infants Fed Formula: A Randomized Controlled Trial. Nutrients 2018, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on the suitability of goat milk protein as a source of protein in infant formulae and in follow-on formulae. EFSA J. 2012, 10, 2603. [Google Scholar]
- Muñoz, S.F. Comparison of the protein profile of goat milk from three breeds (French Alpine, Nubia, and Creole) with Holstein bovine milk. Master’s Thesis, Universidad Autónoma de Querétaro, Facultad de Ciencias Naturales, Juriquilla, Querétaro, Mexico, October 2016. [Google Scholar]
- Mayer, H.K.; Lenz, K.; Halbauer, E.M. “A2 milk” authentication using isoelectric focusing and different PCR techniques. Food Res. Int. 2021, 147, 110523. [Google Scholar] [CrossRef]
- Duarte-Vázquez, M.A.; García-Ugalde, C.; Villegas-Gutiérrez, L.M.; García-Almendárez, B.E.; Rosado, J.L. Production of cow´s milk free beta-casein A1 and its application in the manufacturing of specialized foods for early infant nutrition. Foods 2017, 6, 50. [Google Scholar] [CrossRef]
- Albenzio, M.; Campanozzi, A.; D’Apolito, M.; Santillo, A.; Mantovani, M.P.; Sevi, A. Differences in protein fraction from goat’s milk and cow’s milk and their role on cytokine production in children with cow’s milk protein allergy. Small Rumin. Res. 2012, 105, 202–205. [Google Scholar] [CrossRef]
- Comin, A.; Cassandro, M.; Chessa, S.; Ojala, M.; Dal Zotto, R.; De Marchi, M.; Carnier, P.; Gallo, L.; Pagnacco, G.; Bittante, G. Effects of composite β and κ-casein genotypes on milk coagulation, quality, and yield traits in Italian Holstein cows. J. Dairy Sci. 2008, 91, 4022–4027. [Google Scholar] [CrossRef]
- Azevedo, A.L.S.; Nascimento, C.S.; Steinberg, R.; Carvalho, M.; Peixoto, M.; Teodoro, R.; Verneque, R.; Guimaraes, S.; Machado, M. Genetic polymorphism of the kappa-casein gene in Brazilian cattle. Genet. Mol. Res. 2008, 7, 623–630. [Google Scholar] [CrossRef]
- Reichenwallner, J.; Hinderberger, D. Using bound fatty acids to disclose the functional structure of serum albumin. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 5382–5393. [Google Scholar] [CrossRef]
- Sreedhara, A.; Flengsrud, R.; Langsrud, T.; Kaul, P.; Prakash, V.; Vegarud, G.E. Structural characteristic, pH and thermal stabilities of apo and holo forms of caprine and bovine lactoferrins. BioMetals 2010, 23, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. BioMetals 2004, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Jiang, H.R.; Li, H.B.; Zhang, T.N.; Zhou, Q.; Liu, N. Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way lactoferrin. J. Dairy Sci. 2010, 93, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Le Maux, S.; Bouhallab, S.; Giblin, L.; Brodkorb, A.; Croguennec, T. Bovine β-lactoglobulin/fatty acid complexes: Binding, structural, and biological properties. Dairy Sci. Technol. 2014, 94, 409–426. [Google Scholar] [CrossRef]
- Sutton, L.F.; Alston-Mills, B. Beta-Lactoglobulin as a potential modulator of intestinal activity and morphology in neonatal piglets. Anat. Rec. Discov. Mol. Cell. Evol. Biol. 2006, 288, 601–608. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.H.; Regester, G.O.; Le Leu, R.K.; Royle, P.J.; Smithers, G.W. Dairy proteins protect against dimethylhydrazine-induced intestinal cancers in rats. J. Nutr. 1995, 125, 809–816. [Google Scholar]
- Chatterton, D.E.W.; Smithers, G.; Roupas, P.; Brod Korb, A. Bioactivity of β-lactoglobulin and α-lactoalbumin technological implications for processing. Int. Dairy J. 2006, 16, 1240–1290. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Jiang, R.; Du, X. Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 606–614. [Google Scholar] [CrossRef]
- Ren, J.; Stuart, D.I.; Acharya, K.R. α-Lactalbumin possesses a distinct zinc binding site. J. Biol. Chem. 1993, 268, 19292–19298. [Google Scholar] [CrossRef]
- Hallgren, O.; Aits, S.; Brest, P.; Gustafsson, L.; Mossberg, A.-K.; Wullt, B.; Svanborg, C. Apoptosis and Tumor Cell Death in Response to HAMLET (Human α-Lactalbumin Made Lethal to Tumor Cells). Adv. Exp. Med. Biol. 2008, 606, 217–240. [Google Scholar] [CrossRef]
- Rammer, P.; Groth-Pedersen, L.; Kirkegaard, T.; Daugaard, M.; Rytter, A.; Szyniarowski, P.; Høyer-Hansen, M.; Povlsen, L.K.; Nylandsted, J.; Larsen, J.E.; et al. BAMLET Activates a Lysosomal Cell Death Program in Cancer Cells. Mol. Cancer Ther. 2010, 9, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Chao, P.; Gómez-Ruiz, J.A.; Rastall, R.A.; Mills, D.; Cramer, R.; Pihlanto, A.; Korhonen, H.; Jauregi, P. Production of novel ACE inhibitory peptides from β-lactoglobulin using Protease N Amano. Int. Dairy J. 2009, 19, 69–76. [Google Scholar] [CrossRef]
- Atanasova, J.; Ivanova, I. Antibacterial peptides from goat and sheep milk proteins. Biotechnol. Equip. 2010, 24, 1799–1803. [Google Scholar] [CrossRef]
- Chessa, S.; Chiatti, F.; Rignanese, G.; Ceriotti, G.; Caroli, A.; Pagnacco, G. Nutraceutical properties of goat’s milk: In silico analysis of the casein sequences. Options Mediterr. 2009, 91, 241–243. [Google Scholar]
- McLachlan, C.N.S. β-casein A1, ischaemic heart disease mortality, and other illnesses. Med. Hypotheses 2001, 56, 262–272. [Google Scholar] [CrossRef]
- European Food Safety Authority. Review of the potential health impact of β-casomorphins and related peptides. EFSA Sci. Rep. 2009, 231, 1–107. [Google Scholar]
- Ricci, I.; Artacho, R.; Olalla, M. Milk protein-peptides with angiotensin I-converting enzyme (ACE) inhibitory activity. Crit. Rev. Food Sci. Nutr. 2010, 50, 390–402. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A.; Rokka, T.; Korhonen, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Bovine Milk Proteins. Int. Dairy J. 1998, 8, 325–331. [Google Scholar] [CrossRef]
| Sample | Total Protein (mg/mL) |
|---|---|
| Creole | 4.82 ± 0.80 |
| French Alpine | 3.17 ± 0.71 |
| Nubian | 3.57 ± 0.69 |
| Cow (Holstein) | 3.42 ± 0.44 |
| Protein: Beta-Casein Gene: CSN2 | ||
|---|---|---|
| Bos Taurus (Bovine) Molecular weight: 24 KDa Signal peptide: 15 amino acids Mature chain: 209 amino acids | Capra Hircus (Caprine) Molecular weight: 23.3 KDa Signal peptide: 9 amino acids Mature chain: 207 amino acids | |
Amino acid sequence | Amino acid sequence | Difference in 43 Amino acid |
| Breed of goats | ||
| Alpine French sequence | Nubian sequence | Creole sequence |
 |  |  |
| hydrolyzed peptides (20) | hydrolyzed peptides (20) | hydrolyzed peptides (21) |
| FQSEEQQQTEDELQDK | FQSEEQQQTEDELQDK | FQSEEQQQTEDELQDK |
| mVPKHKEmPFPKYP | mVPKHKEmPFPKYP | mVPKHKEmPFPKYP |
| ------------------ | ------------------ | HPFAQAQSLVYP |
| FTGPIPNSLPQN | FTGPIPNSLPQN | FTGPIPNSLPQN |
| REQEELN | REQEELN | REQEELN |
| AVPQRDmP | AVPQRDmP | AVPQRDmP |
| mHQPPQP | mHQPPQP | mHQPPQP |
| VLPVPQK | VLPVPQK | VLPVPQK |
| LSSSEES | LSSSEES | LSSSEES |
| mFPPQS | mFPPQS | mFPPQS |
| LTDVEK | LTDVEK | LTDVEK |
| FTESQS | FTESQS | FTESQS |
| LHLPLP | LHLPLP | LHLPLP |
| VRGPFP | VRGPFP | VRGPFP |
| LYQEP | LYQEP | LYQEP |
| LQPEI | LQPEI | LQPEI |
| LSQPK | LSQPK | LSQPK |
| LTQTP | LTQTP | LTQTP |
| mGVPK | mGVPK | mGVPK |
| VQSW | VQSW | VQSW |
| INKK | INKK | INKK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Salinas, F.; Andrade-Montemayor, H.M.; De la Torre-Carbot, K.; Duarte-Vázquez, M.Á.; Silva-Jarquin, J.C. Comparative Analysis of the Protein Composition of Goat Milk from French Alpine, Nubian, and Creole Breeds and Holstein Friesian Cow Milk: Implications for Early Infant Nutrition. Animals 2022, 12, 2236. https://doi.org/10.3390/ani12172236
Muñoz-Salinas F, Andrade-Montemayor HM, De la Torre-Carbot K, Duarte-Vázquez MÁ, Silva-Jarquin JC. Comparative Analysis of the Protein Composition of Goat Milk from French Alpine, Nubian, and Creole Breeds and Holstein Friesian Cow Milk: Implications for Early Infant Nutrition. Animals. 2022; 12(17):2236. https://doi.org/10.3390/ani12172236
Chicago/Turabian StyleMuñoz-Salinas, Florencia, Héctor Mario Andrade-Montemayor, Karina De la Torre-Carbot, Miguel Ángel Duarte-Vázquez, and Juan Carlos Silva-Jarquin. 2022. "Comparative Analysis of the Protein Composition of Goat Milk from French Alpine, Nubian, and Creole Breeds and Holstein Friesian Cow Milk: Implications for Early Infant Nutrition" Animals 12, no. 17: 2236. https://doi.org/10.3390/ani12172236
APA StyleMuñoz-Salinas, F., Andrade-Montemayor, H. M., De la Torre-Carbot, K., Duarte-Vázquez, M. Á., & Silva-Jarquin, J. C. (2022). Comparative Analysis of the Protein Composition of Goat Milk from French Alpine, Nubian, and Creole Breeds and Holstein Friesian Cow Milk: Implications for Early Infant Nutrition. Animals, 12(17), 2236. https://doi.org/10.3390/ani12172236





