Dose–Response Effects of Bamboo Leaves on Rumen Methane Production, Fermentation Characteristics, and Microbial Abundance In Vitro
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Bamboo Leaves Samples
2.2. Chemical Composition, Total Polyphenol, Flavonoid and Antioxidant Activity
2.3. Animals and In Vitro Experiments
2.4. In Vitro Fermentation and Gas Profiles
2.5. The DNA Extraction and Real-Time Polymerase Chain Reaction
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition and Antioxidant Activity
3.2. Effects of Bamboo Leaves on pH of Rumen Fluid and Dry Matter Digestibility
3.3. Effects of Bamboo Leaves on Gas Profiles
3.4. Effects of Bamboo Leaves on Volatile Fatty Acids Profiles
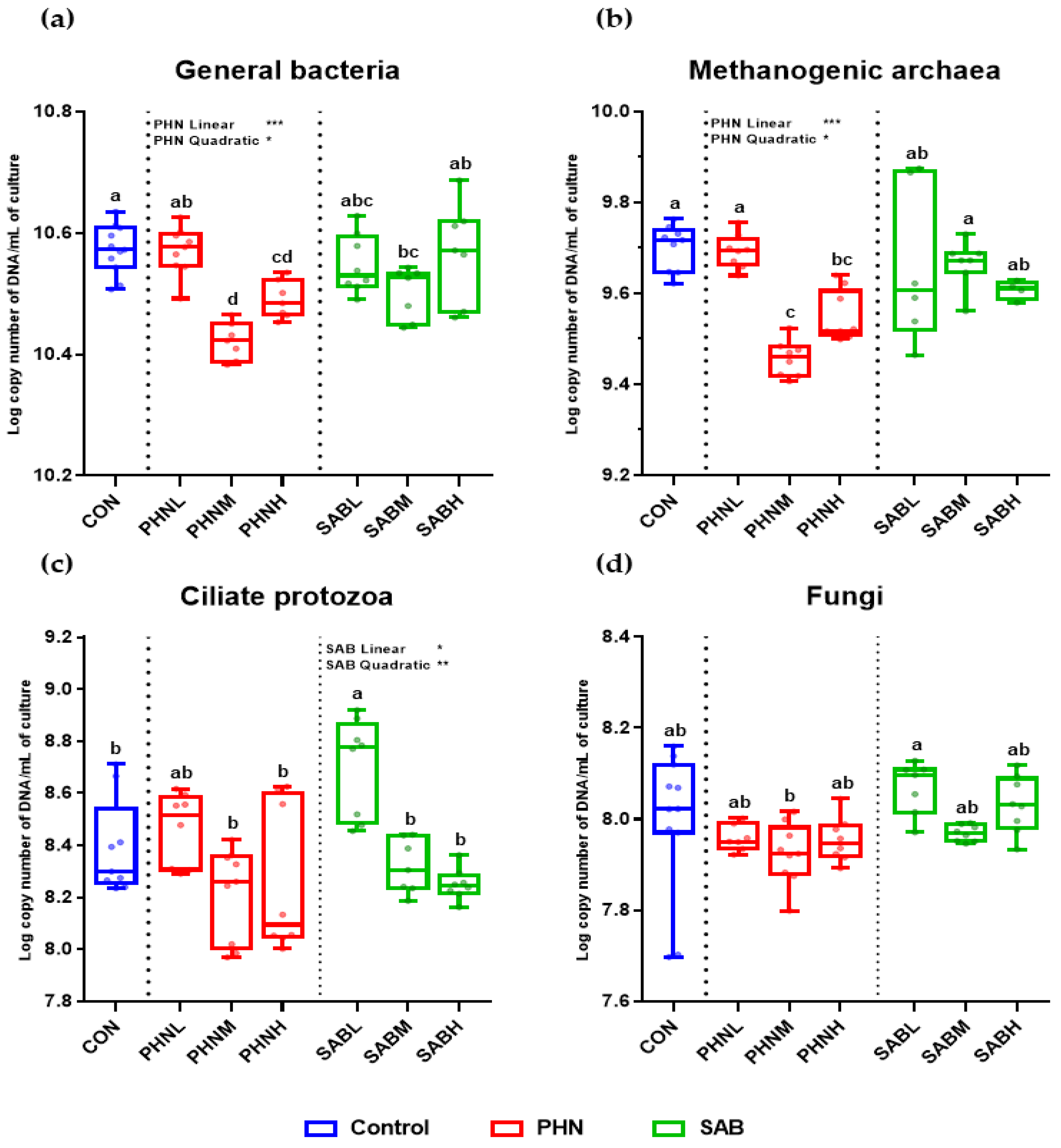
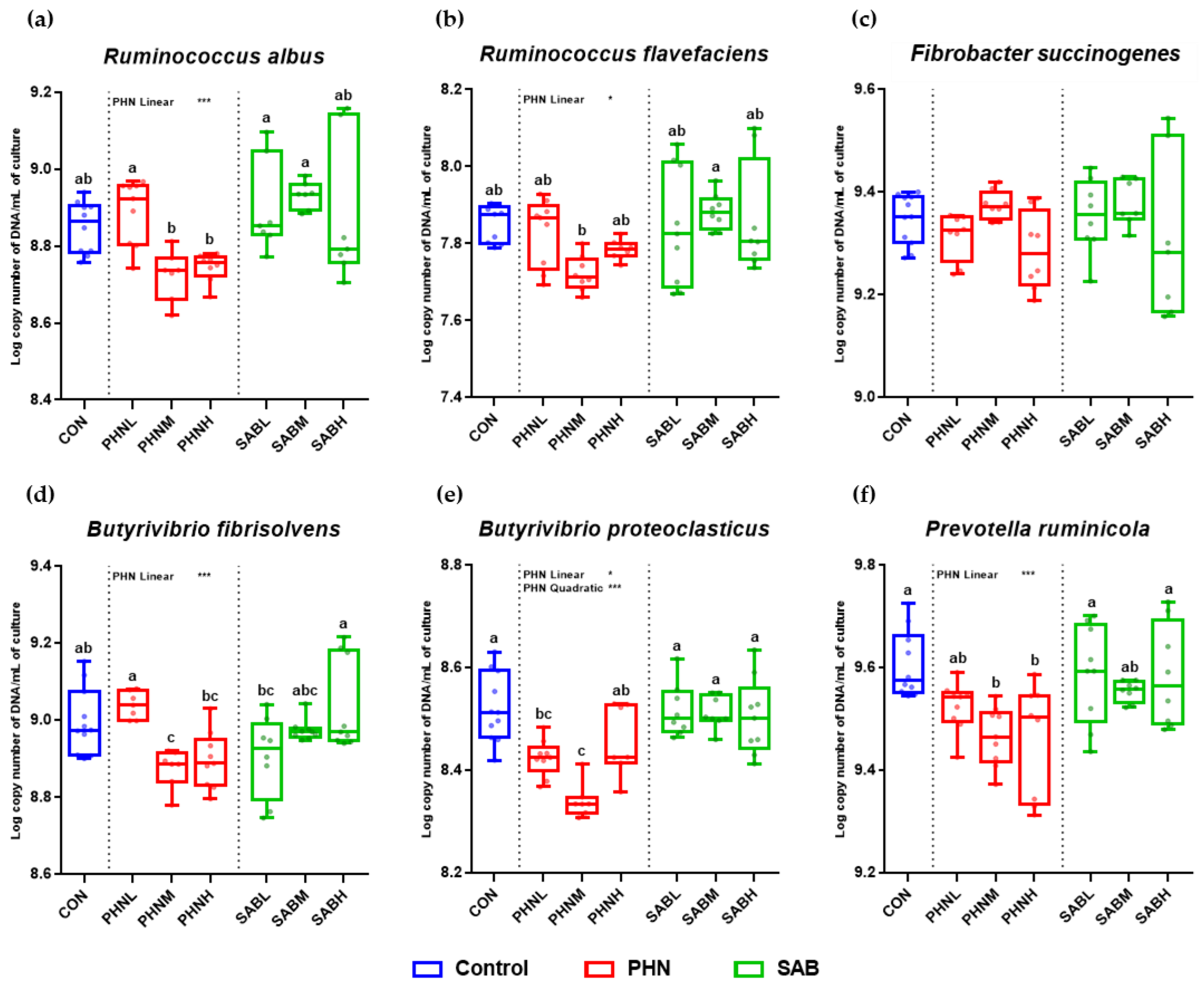
3.5. Effects of Bamboo Leaves on the Abundance of Microbial Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, K.A.; Johnson, D.E. Methane Emissions from Cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Opio, C.; Gerber, P.; Mottet, A.; Falcucci, A.; Tempio, G.; MacLeod, M.; Vellinga, T.; Henderson, B.; Steinfeld, H. Greenhouse Gas Emissions from Ruminant Supply Chains—A Global Life Cycle Assessment; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 9-25-107945-5. [Google Scholar]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 925107920X. [Google Scholar]
- Johnson, D.E.; Ward, G.M. Estimates of Animal Methane Emissions. Environ. Monit. Assess. 1996, 42, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Czerkawski, J.W. Methane Production in Ruminants and Its Significance. World Rev. Nutr. Diet. 1969, 11, 240–282. [Google Scholar] [PubMed]
- Chen, D.; Zhou, J.; Zhang, Q. Effects of Heating Rate on Slow Pyrolysis Behavior, Kinetic Parameters and Products Properties of Moso Bamboo. Bioresour. Technol. 2014, 169, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bahari, S.A.; Krause, A. Utilizing Malaysian Bamboo for Use in Thermoplastic Composites. J. Clean. Prod. 2016, 110, 16–24. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon Sequestration by Chinese Bamboo Forests and Their Ecological Benefits: Assessment of Potential, Problems, and Future Challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Benton, A. Priority Species of Bamboo. In Bamboo; Springer: Berlin/Heidelberg, Germany, 2015; pp. 31–41. [Google Scholar]
- Véras, R.M.L.; Gois, G.C.; Nascimento, D.B.; Magalhães, A.L.R.; Teodoro, A.L.; Pinto, C.S.; Oliveira, L.P.; Campos, F.S.; Andrade, A.P.; Lima, I.E. Potential Alternative Feed Sources for Ruminant Feeding from the Biodiesel Production Chain By-Products. S. Afr. J. Anim. Sci. 2020, 50, 69–77. [Google Scholar]
- Serrapica, F.; Masucci, F.; Raffrenato, E.; Sannino, M.; Vastolo, A.; Barone, C.M.A.; Di Francia, A. High Fiber Cakes from Mediterranean Multipurpose Oilseeds as Protein Sources for Ruminants. Animals 2019, 9, 918. [Google Scholar] [CrossRef]
- Park, H.-S.; Lim, J.H.; Kim, H.J.; Choi, H.J.; Lee, I.-S. Antioxidant Flavone Glycosides from the Leaves of Sasa borealis. Arch. Pharm. Res. 2007, 30, 161–166. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, H.J.; Jung, S.H.; Lee, E.H.; Cha, K.H.; Kang, S.W.; Pan, C.-H.; Um, B.-H. Rapid Identification of Radical Scavenging Phenolic Compounds from Black Bamboo Leaves Using High-Performance Liquid Chromatography Coupled to an Online ABTS+-Based Assay. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 613–619. [Google Scholar] [CrossRef]
- Formato, M.; Piccolella, S.; Zidorn, C.; Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Pacifico, S. UHPLC-ESI-Q q TOF Analysis and In Vitro Rumen Fermentation for Exploiting Fagus sylvatica Leaf in Ruminant Diet. Molecules 2022, 27, 2217. [Google Scholar] [CrossRef]
- Liu, M.-H.; Ko, C.-H.; Ma, N.; Tan, P.-W.; Fu, W.-M.; He, J.-Y. Chemical Profiles, Antioxidant and Anti-Obesity Effects of Extract of Bambusa textilis McClure Leaves. J. Funct. Foods 2016, 22, 533–546. [Google Scholar] [CrossRef]
- Xie, J.; Lin, Y.-S.; Shi, X.-J.; Zhu, X.-Y.; Su, W.-K.; Wang, P. Mechanochemical-Assisted Extraction of Flavonoids from Bamboo (Phyllostachys edulis) Leaves. Ind. Crops Prod. 2013, 43, 276–282. [Google Scholar] [CrossRef]
- Huang, H.; Szumacher-Strabel, M.; Patra, A.K.; Ślusarczyk, S.; Lechniak, D.; Vazirigohar, M.; Varadyova, Z.; Kozłowska, M.; Cieślak, A. Chemical and Phytochemical Composition, In Vitro Ruminal Fermentation, Methane Production, and Nutrient Degradability of Fresh and Ensiled Paulownia Hybrid Leaves. Anim. Feed Sci. Technol. 2021, 279, 115038. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.S.; Eom, J.S.; Choi, Y.Y.; Jo, S.U.; Chu, G.M.; Lee, Y.; Seo, J.; Kim, K.H.; Lee, S.S. Effects of Olive (Olea europaea L.) Leaves with Antioxidant and Antimicrobial Activities on In Vitro Ruminal Fermentation and Methane Emission. Animals 2021, 11, 2008. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, I.-C.; Kang, S.-S.; Moon, C.-J.; Kim, S.-H.; Shin, D.-H.; Kim, H.-C.; Yoo, J.-C.; Kim, J.-C. Effects of Bamboo Charcoal and Bamboo Leaf Supplementation on Performance and Meat Quality in Chickens. J. Life Sci. 2011, 21, 805–810. [Google Scholar] [CrossRef]
- Mekuriaw, Y.; Urge, M.; Animut, G. Intake, Digestibility, Live Weight Changes and Rumen Parameters of Washera Sheep Fed Mixtures of Lowland Bamboo (Oxytenanthera abyssinica) Leaves and Natural Pasture Grass Hay at Different Ratios. Pakistan J. Nutr. 2012, 11, 322–331. [Google Scholar] [CrossRef]
- Jafari, S.; Goh, Y.M.; Rajion, M.A.; Ebrahimi, M. Effects of Polyphenol Rich Bamboo Leaf on Rumen Fermentation Characteristics and Methane Gas Production in an in Vitro Condition. Indian J. Anim. Res. 2020, 54, 322–326. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Xue, F.; Mao, S.; Xiong, B.; Ma, Z.; Jiang, L. Effects of Bamboo Leaf Extract on the Production Performance, Rumen Fermentation Parameters, and Rumen Bacterial Communities of Heat-Stressed Dairy Cows. Anim. Biosci. 2021, 34, 1784. [Google Scholar] [CrossRef]
- AOAC International. AOAC: Official Methods of Analysis; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990; Volume 1, pp. 69–90. [Google Scholar]
- Van Soest, P.J.; van Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of Procedures for Nitrogen Fractionation of Ruminant Feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Seleshe, S.; Lee, J.S.; Lee, S.; Lee, H.J.; Kim, G.R.; Yeo, J.; Kim, J.Y.; Kang, S.N. Evaluation of Antioxidant and Antimicrobial Activities of Ethanol Extracts of Three Kinds of Strawberries. Prev. Nutr. Food Sci. 2017, 22, 203. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A Simple Gas Production Method Using a Pressure Transducer to Determine the Fermentation Kinetics of Ruminant Feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- López, S.; Dhanoa, M.S.; Dijkstra, J.; al Bannink, A.; Kebreab, E.; France, J. Some Methodological and Analytical Considerations Regarding Application of the Gas Production Technique. Anim. Feed Sci. Technol. 2007, 135, 139–156. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Krueger, N.; Salawu, M.B.; Dean, D.B.; Staples, C.R. The Influence of Treatment with Dual Purpose Bacterial Inoculants or Soluble Carbohydrates on the Fermentation and Aerobic Stability of Bermudagrass. J. Dairy Sci. 2004, 87, 3407–3416. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Cho, S.; Kwon, I.; Seo, J. Dietary Lysophospholipids Supplementation Inhibited the Activity of Lipolytic Bacteria in Forage with High Oil Diet: An in Vitro Study. Asian-Australas. J. Anim. Sci. 2020, 33, 1590. [Google Scholar] [CrossRef]
- Hamid, M.M.A.; Moon, J.; Yoo, D.; Kim, H.; Lee, Y.K.; Song, J.; Seo, J. Rumen Fermentation, Methane Production, and Microbial Composition Following in Vitro Evaluation of Red Ginseng Byproduct as a Protein Source. J. Anim. Sci. Technol. 2020, 62, 801. [Google Scholar] [CrossRef]
- Denman, S.E.; McSweeney, C.S. Development of a Real-Time PCR Assay for Monitoring Anaerobic Fungal and Cellulolytic Bacterial Populations within the Rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen Microbiome Composition Determined Using Two Nutritional Models of Subacute Ruminal Acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, J.T.; Karnati, S.K.R.; Yu, Z.; Morrison, M.; Firkins, J.L. Development of an Assay to Quantify Rumen Ciliate Protozoal Biomass in Cows Using Real-Time PCR. J. Nutr. 2004, 134, 3378–3384. [Google Scholar] [CrossRef]
- Wang, R.; Cao, W.; Cerniglia, C.E. A Universal Protocol for PCR Detection of 13 Species of Foodborne Pathogens in Foods. J. Appl. Microbiol. 1997, 83, 727–736. [Google Scholar] [CrossRef]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and Low Abundance of Classical Ruminal Bacterial Species in the Bovine Rumen Revealed by Relative Quantification Real-Time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef]
- Paillard, D.; McKain, N.; Chaudhary, L.C.; Walker, N.D.; Pizette, F.; Koppova, I.; McEwan, N.R.; Kopečný, J.; Vercoe, P.E.; Louis, P. Relation between Phylogenetic Position, Lipid Metabolism and Butyrate Production by Different Butyrivibrio-Like Bacteria from the Rumen. Antonie Van Leeuwenhoek 2007, 91, 417–422. [Google Scholar] [CrossRef]
- Su, B.; Chen, X. Current Status and Potential of Moringa Oleifera Leaf as an Alternative Protein Source for Animal Feeds. Front. Vet. Sci. 2020, 7, 53. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Cieślak, A. Potential of Phytofactors to Mitigate Rumen Ammonia and Methane Production. J. Anim. Feed Sci. 2010, 19, 319–337. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. A New Perspective on the Use of Plant Secondary Metabolites to Inhibit Methanogenesis in the Rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar] [CrossRef]
- Balcells, J.; Aris, A.; Serrano, A.; Seradj, A.R.; Crespo, J.; Devant, M. Effects of an Extract of Plant Flavonoids (Bioflavex) on Rumen Fermentation and Performance in Heifers Fed High-Concentrate Diets. J. Anim. Sci. 2012, 90, 4975–4984. [Google Scholar] [CrossRef] [PubMed]
- Broudiscou, L.-P.; Papon, Y.; Broudiscou, A.F. Effects of Dry Plant Extracts on Feed Degradation and the Production of Rumen Microbial Biomass in a Dual Outflow Fermenter. Anim. Feed Sci. Technol. 2002, 101, 183–189. [Google Scholar] [CrossRef]
- Teh, S.-S.; Bekhit, A.E.-D.; Birch, J. Antioxidative Polyphenols from Defatted Oilseed Cakes: Effect of Solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Cone, J.W. Degradation of Starch in Feed Concentrates by Enzymes, Rumen Fluid and Rumen Enzymes. J. Sci. Food Agric. 1991, 54, 23–34. [Google Scholar] [CrossRef]
- Hoover, W.H. Chemical Factors Involved in Ruminal Fiber Digestion. J. Dairy Sci. 1986, 69, 2755–2766. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane Production by Ruminants: Its Contribution to Global Warming. Annales de Zootechnie 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Henderson, C. The Influence of Extracellular Hydrogen on the Metabolism of Bacteroides ruminicola, Anaerovibrio lipolytica and Selenomonas ruminantium. Microbiology 1980, 119, 485–491. [Google Scholar] [CrossRef]
- Stein, D.R.; Allen, D.T.; Perry, E.B.; Bruner, J.C.; Gates, K.W.; Rehberger, T.G.; Mertz, K.; Jones, D.; Spicer, L.J. Effects of Feeding Propionibacteria to Dairy Cows on Milk Yield, Milk Components, and Reproduction. J. Dairy Sci. 2006, 89, 111–125. [Google Scholar] [CrossRef]
- Hussain, I.; Cheeke, P.R. Effect of Dietary Yucca Schidigera Extract on Rumen and Blood Profiles of Steers Fed Concentrate- or Roughage-Based Diets. Anim. Feed Sci. Technol. 1995, 51, 231–242. [Google Scholar] [CrossRef]
- Zdunic, G.; Godjevac, D.; Savikin, K.; Petrovic, S. Comparative Analysis of Phenolic Compounds in Seven Hypericum Species and Their Antioxidant Properties. Nat. Prod. Commun. 2017, 12, 1805–1811. [Google Scholar] [CrossRef]
- Makarova, K.; Sajkowska-Kozielewicz, J.J.; Zawada, K.; Olchowik-Grabarek, E.; Ciach, M.A.; Gogolewski, K.; Dobros, N.; Ciechowicz, P.; Freichels, H.; Gambin, A. Harvest Time Affects Antioxidant Capacity, Total Polyphenol and Flavonoid Content of Polish St John’s Wort’s (Hypericum perforatum L.) Flowers. Sci. Rep. 2021, 11, 3989. [Google Scholar] [CrossRef] [PubMed]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of Rumen Fermentation and Methane Production with Plant Secondary Metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Machmüller, A.; Soliva, C.R.; Kreuzer, M. Effect of Coconut Oil and Defaunation Treatment on Methanogenesis in Sheep. Reprod. Nutr. Dev. 2003, 43, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Wann, C.; Wanapat, M.; Mapato, C.; Ampapon, T.; Huang, B. Effect of Bamboo Grass (Tiliacora triandra, Diels) Pellet Supplementation on Rumen Fermentation Characteristics and Methane Production in Thai Native Beef Cattle. Asian-Australas. J. Anim. Sci. 2019, 32, 1153. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of Different Drying Methods on Extractable Phenolic Compounds and Antioxidant Properties from Lemon Myrtle Dried Leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef]
- Demeyer, D.I.; Van Nevel, C.J.; Van de Voorde, G. The Effect of Defaunation on the Growth of Lambs Fed Three Urea Containing Diets. Archiv für Tierernaehrung 1982, 32, 595–604. [Google Scholar] [CrossRef]
- Johnson, R.R. Influence of Carbohydrate Solubility on Non-Protein Nitrogen Utilization in the Ruminant. J. Anim. Sci. 1976, 43, 184–191. [Google Scholar] [CrossRef]
- Oh, S.; Shintani, R.; Koike, S.; Kobayashi, Y. Ginkgo Fruit Extract as an Additive to Modify Rumen Microbiota and Fermentation and to Mitigate Methane Production. J. Dairy Sci. 2017, 100, 1923–1934. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of Hydrogen on Rumen Methane Formation and Fermentation Balances through Microbial Growth Kinetics and Fermentation Thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of Short Chain Fatty Acids on Gut Morphology and Function. Gut 1994, 35, S35–S38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Items 1 | Timothy Hay | SD 2 |
|---|---|---|
| Chemical composition | ||
| Dry matter | 94.11 | 0.64 |
| Ether extract | 5.48 | 0.12 |
| Crude protein | 10.46 | 0.68 |
| Crude ash | 5.93 | 0.06 |
| Crude fiber | 30.11 | 0.23 |
| NDF | 62.27 | 0.22 |
| ADF | 38.14 | 0.75 |
| Target Species | Primer 1 | Sequence | Size (bp) 2 | Efficiency 3 | References |
|---|---|---|---|---|---|
| General bacteria | F | CGGCAACGAGCGCAACCC | 130 | 1.98 | [36,37] |
| R | CCATTGTAGCACGTGTGTAGCC | ||||
| Methanogenic archaea | F | GAGGAAGGAGTGGACGACGGTA | 232 | 1.92 | [38] |
| R | ACGGGCGGTGTGTGCAAG | ||||
| Ciliate protozoa | F | GCTTTCGWTGGTAGTGTATT | 223 | 1.91 | [38] |
| R | CTTGCCCTCYAATCGTWCT | ||||
| Fungi | F | GAGGAAGTAAAAGTCGTAACAAGGTTTC | 120 | 1.83 | [36] |
| R | CAAATTCACAAAGGGTAGGATGATT | ||||
| Ruminococcus albus | F | CCCTAAAAGCAGTCTTAGTTCG | 176 | 1.93 | [39] |
| R | CCTCCTTGCGGTTAGAACA | ||||
| Ruminococcus flavefaciens | F | CGAACGGAGATAATTTGAGTTTACTTAGG | 132 | 1.81 | [36] |
| R | CGGTCTCTGTATGTTATGAGGTATTACC | ||||
| Fibrobacter succinogenes | F | GTTCGGAATTACTGGGCGTAAA | 121 | 1.88 | [36] |
| R | CGCCTGCCCCTGAACTATC | ||||
| Butyrivibrio fibrisolvens | F | ACCGCATAAGCGCACGGA | 65 | 1.89 | [40] |
| R | CGGGTCCATCTTGTACCGATAAAT | ||||
| Butyrivibrio proteoclasticus | F | TCCGGTGGTATGAGATGGGC | 185 | 2.22 | [41] |
| R | GTCGCTGCATCAGAGTTTCCT | ||||
| Prevotella ruminicola | F | GCGAAAGTCGGATTAATGCTCTATG | 78 | 2.04 | [37] |
| R | CCCATCCTATAGCGGTAAACCTTTG |
| Items 1 | Phyllostachys nigra Var. Henonis | SD 2 | Sasa borealis | SD |
|---|---|---|---|---|
| Chemical composition | ||||
| Dry matter (DM) | 45.39 | 0.24 | 54.84 | 0.22 |
| Ether extract | 2.49 | 0.56 | 0.65 | 0.15 |
| Crude protein (CP) | 13.15 | 0.32 | 14.82 | 0.20 |
| Crude ash | 12.12 | 0.11 | 7.99 | 0.05 |
| Crude fiber | 21.08 | 0.07 | 24.19 | 0.38 |
| NDF | 65.6 | 0.84 | 70.58 | 0.88 |
| ADF | 38.02 | 0.30 | 35.57 | 0.27 |
| NDICP (CP basis, %) | 12.83 | 0.06 | 14.26 | 0.07 |
| ADICP (CP basis, %) | 5.26 | 0.13 | 2.92 | 0.09 |
| Total polyphenol (mg CE/g extract) | 31.04 | 5.83 | 44.54 | 6.07 |
| Total flavonoid (mg QE/g extract) | 16.73 | 0.52 | 19.6 | 0.49 |
| IC50 for DPPH (µg/mL) | 115.58 | 6.06 | 125.43 | 3.83 |
| IC50 for ABTS (µg/mL) | 47.43 | 0.61 | 54.17 | 2.53 |
| Incubation Time (h) | CON 1 | Treatment 2 | SEM 3 | p Value | Contrasts 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHN | SAB | PHN Set | SAB Set | ||||||||||
| PHNL | PHNM | PHNH | SABL | SABM | SABH | L | Q | L | Q | ||||
| pH | |||||||||||||
| 12 | 6.91 d | 7.03 c | 7.09 bc | 7.08 bc | 7.14 ab | 7.12 abc | 7.20 a | 0.02 | <0.0001 | *** | * | *** | *** |
| 24 | 6.59 b | 6.72 a | 6.69 a | 6.71 a | 6.77 a | 6.72 a | 6.73 a | 0.02 | 0.0003 | *** | ** | ** | ** |
| 48 | 6.19 b | 6.30 a | 6.27 ab | 6.25 ab | 6.23 ab | 6.20 ab | 6.20 ab | 0.02 | 0.0218 | ns | * | ns | ns |
| Dry matter digestibility (%) | |||||||||||||
| 6 | 31.1 | 31.4 | 31.5 | 32.4 | 34.3 | 33.4 | 33.3 | 1.32 | 0.5560 | ns | ns | ns | ns |
| 12 | 38.7 b | 43.0 ab | 40.8 ab | 42.6 ab | 42.9 ab | 43.5 a | 42.0 ab | 1.01 | 0.0392 | ns | ns | * | * |
| 24 | 53.1 | 56.2 | 53.9 | 57.0 | 56.9 | 56.6 | 54.1 | 1.32 | 0.2069 | ns | ns | ns | * |
| 48 | 65.5 a | 61.8 b | 64.9 ab | 63.5 ab | 64.0 ab | 64.6 ab | 65.4 ab | 0.79 | 0.0466 | ns | ns | ns | ns |
| 72 | 68.7 | 66.4 | 67.5 | 66.4 | 67.3 | 67.8 | 69.3 | 0.85 | 0.1797 | ns | ns | ns | ns |
| Incubation Time (h) | CON 1 | Treatment 2 | SEM 3 | p Value | Contrasts 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHN | SAB | PHN Set | SAB Set | ||||||||||
| PHNL | PHNM | PHNH | SABL | SABM | SABL | L | Q | L | Q | ||||
| Total gas (mL∙g−1 DM 5) | |||||||||||||
| 12 | 87.0 a | 82.1 b | 82.0 b | 81.2 b | 82.2 b | 81.2 b | 77.5 c | 0.77 | <0.0001 | *** | * | *** | ns |
| 24 | 121 a | 115 ab | 115 abc | 112 bc | 116 ab | 115 bc | 109 c | 1.31 | 0.0002 | *** | ns | *** | ns |
| 48 | 156 a | 147 ab | 147 b | 146 b | 151 ab | 147 b | 147 ab | 1.97 | 0.0146 | ** | * | ** | ns |
| Methane (mL∙g−1 DM) | |||||||||||||
| 12 | 7.40 a | 5.84 ab | 4.87 b | 5.83 ab | 6.26 ab | 6.15 ab | 5.57 b | 0.37 | 0.0051 | ** | * | *** | ns |
| 24 | 14.8 | 12.7 | 13.7 | 14.6 | 14.2 | 14.1 | 13.8 | 0.79 | 0.6162 | ns | * | ns | ns |
| 48 | 23.7 a | 20.7 ab | 19.1 b | 18.5 b | 19.1 ab | 19.7 b | 19.4 ab | 0.73 | 0.0010 | *** | ns | ** | * |
| Methane/Total gas (%) | |||||||||||||
| 12 | 8.52 a | 7.12 ab | 5.94 b | 7.19 ab | 7.62 ab | 7.57 ab | 7.19 ab | 0.49 | 0.0488 | ns | * | * | ns |
| 24 | 12.2 | 11.0 | 11.9 | 13.0 | 12.3 | 12.3 | 12.7 | 0.64 | 0.4616 | ns | * | ns | ns |
| 48 | 15.2 a | 14.1 ab | 13.0 ab | 12.7 b | 12.6 b | 13.5 ab | 13.9 ab | 0.53 | 0.0249 | ** | ns | ns | * |
| Carbon dioxide (mL∙g−1 DM) | |||||||||||||
| 12 | 35.5 a | 27.3 ab | 22.6 b | 26.7 b | 30.7 ab | 28.5 ab | 27.2 ab | 1.89 | 0.0044 | ** | ** | ** | ns |
| 24 | 55.0 | 45.9 | 49.0 | 52.1 | 51.8 | 50.9 | 49.4 | 2.90 | 0.4603 | ns | * | ns | ns |
| 48 | 80.0 a | 70.8 ab | 63.5 b | 60.7 b | 63.5 b | 65.5 b | 67.6 b | 2.52 | 0.0005 | *** | ns | ** | * |
| Carbon dioxide/Total gas (%) | |||||||||||||
| 12 | 40.8 a | 33.3 ab | 27.6 b | 32.9 ab | 37.4 ab | 35.1 ab | 35.0 ab | 2.39 | 0.0272 | * | * | ns | ns |
| 24 | 45.7 | 39.8 | 42.7 | 46.6 | 44.6 | 44.3 | 45.4 | 2.44 | 0.5343 | ns | * | ns | ns |
| 48 | 51.3 a | 48.1 ab | 43.3 ab | 41.6 b | 42.0 b | 44.7 ab | 46.1 ab | 1.85 | 0.0123 | *** | ns | ns | * |
| Incubation Time (h) | CON 1 | Treatment 2 | SEM 3 | p Value | Contrasts 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHN | SAB | PHN Set | SAB Set | ||||||||||
| PHNL | PHNM | PHNH | SABL | SABM | SABH | L | Q | L | Q | ||||
| Total VFA (mM) | |||||||||||||
| 12 | 54.8 | 54.3 | 54.7 | 54.8 | 54.9 | 54.6 | 54.5 | 0.479 | 0.9634 | ns | ns | ns | ns |
| 24 | 73.4 bc | 73.7 bc | 76.6 ab | 73.1 c | 78.0 a | 77.4 a | 75.0 abc | 0.741 | 0.0002 | ns | ** | ns | *** |
| 48 | 78.4 | 78.2 | 78.4 | 78.2 | 80.3 | 80.5 | 83.6 | 1.259 | 0.0534 | ns | ns | * | ns |
| Acetate (mM) | |||||||||||||
| 12 | 35.3 | 35.0 | 35.5 | 35.5 | 35.2 | 35.0 | 34.9 | 0.354 | 0.7824 | ns | ns | ns | ns |
| 24 | 45.6 c | 47.4 bc | 49.7 ab | 46.8 bc | 52.1 a | 50.9 a | 48.9 abc | 0.765 | <0.0001 | * | ** | * | *** |
| 48 | 51.5 b | 51.2 b | 51.3 b | 51.7 ab | 54.3 ab | 53.0 ab | 56.4 a | 1.017 | 0.0115 | ns | ns | * | ns |
| Propionate (mM) | |||||||||||||
| 12 | 13.0 ab | 12.7 ab | 12.5 b | 12.8 ab | 13.1 a | 12.8 ab | 12.5 ab | 0.127 | 0.0188 | ns | * | * | ns |
| 24 | 19.3 a | 18.4 bc | 18.8 b | 18.3 c | 18.0 c | 18.4 bc | 18.1 c | 0.108 | <0.0001 | *** | ns | *** | *** |
| 48 | 18.8 ab | 17.1 c | 17.0 c | 17.2 bc | 17.8 abc | 18.0 abc | 19.0 a | 0.370 | 0.0023 | * | * | ns | * |
| Butyrate (mM) | |||||||||||||
| 12 | 6.43 | 6.64 | 6.74 | 6.57 | 6.65 | 6.80 | 7.07 | 0.140 | 0.1014 | ns | ns | ** | ns |
| 24 | 8.43 a | 7.88 b | 8.09 ab | 7.94 ab | 7.87 b | 8.07 ab | 8.05 ab | 0.117 | 0.0463 | * | ns | ns | * |
| 48 | 8.09 b | 9.94 a | 10.12 a | 9.29 ab | 8.17 b | 9.40 ab | 8.20 b | 0.312 | 0.0002 | ** | *** | ns | ns |
| Acetate-to-propionate ratio | |||||||||||||
| 12 | 2.71 b | 2.77 ab | 2.84 a | 2.78 ab | 2.69 b | 2.72 b | 2.78 ab | 0.026 | 0.0064 | * | ns | * | ns |
| 24 | 2.36 c | 2.57 bc | 2.64 b | 2.56 bc | 2.90 a | 2.77 ab | 2.71 ab | 0.046 | <0.0001 | ** | ** | ** | *** |
| 48 | 2.74 b | 2.99 ab | 3.02 ab | 3.02 ab | 3.06 a | 2.94 ab | 2.96 ab | 0.064 | 0.0445 | ** | * | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.U.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Choi, Y.; Lee, Y.; Lee, S.S. Dose–Response Effects of Bamboo Leaves on Rumen Methane Production, Fermentation Characteristics, and Microbial Abundance In Vitro. Animals 2022, 12, 2222. https://doi.org/10.3390/ani12172222
Jo SU, Lee SJ, Kim HS, Eom JS, Choi Y, Lee Y, Lee SS. Dose–Response Effects of Bamboo Leaves on Rumen Methane Production, Fermentation Characteristics, and Microbial Abundance In Vitro. Animals. 2022; 12(17):2222. https://doi.org/10.3390/ani12172222
Chicago/Turabian StyleJo, Seong Uk, Shin Ja Lee, Hyun Sang Kim, Jun Sik Eom, Youyoung Choi, Yookyung Lee, and Sung Sill Lee. 2022. "Dose–Response Effects of Bamboo Leaves on Rumen Methane Production, Fermentation Characteristics, and Microbial Abundance In Vitro" Animals 12, no. 17: 2222. https://doi.org/10.3390/ani12172222
APA StyleJo, S. U., Lee, S. J., Kim, H. S., Eom, J. S., Choi, Y., Lee, Y., & Lee, S. S. (2022). Dose–Response Effects of Bamboo Leaves on Rumen Methane Production, Fermentation Characteristics, and Microbial Abundance In Vitro. Animals, 12(17), 2222. https://doi.org/10.3390/ani12172222






