Comprehensive Map of Canine Angiostrongylosis in Dogs in Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Climate
2.2. Sample Collection and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tieri, E.E.; Saletti, M.A.; D’Angelo, A.R.; Parisciani, G.; Pelini, S.; Cocco, A.; Di Teodoro, G.; Di Censo, E.; D’Alterio, N.; Latrofa, M.S.; et al. Angiostrongylus vasorum in foxes (Vulpes vulpes) and wolves (Canis lupus italicus) from Abruzzo region, Italy. Int. J. Parasitol. Parasites Wildl. 2021, 15, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Modry, D.; Paredes-Esquivel, C.; Foronda, P.; Traversa, D. Angiostrongylosis in Animals and Humans in Europe. Pathogens 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Shaw, S.E.; Brennan, S.F.; De Waal, T.D.; Jones, B.R.; Mulcahy, G. Angiostrongylus vasorum: A real heartbreaker. Trends Parasitol. 2005, 21, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Mozzer, L.R.; Lima, W.S. Gallus gallus domesticus: Paratenic host of Angiostrongylus vasorum. Vet. Parasitol. 2015, 207, 81–84. [Google Scholar] [CrossRef]
- Helm, J.R.; Morgan, E.R.; Jackson, M.W.; Wotton, P.; Bell, R. Canine angiostrongylosis: An emerging disease in Europe. J. Vet. Emerg. Crit. Care. (San Antonio) 2010, 20, 98–109. [Google Scholar] [CrossRef]
- Morgan, E.R.; Jefferies, R.; van Otterdijk, L.; McEniry, R.B.; Allen, F.; Bakewell, M.; Shaw, S.E. Angiostrongylus vasorum infection in dogs: Presentation and risk factors. Vet. Parasitol. 2010, 173, 255–261. [Google Scholar] [CrossRef]
- Morchón, R.; Montoya-Alonso, J.A.; Sánchez-Agudo, J.Á.; de Vicente-Bengochea, J.; Murcia-Martínez, X.; Carretón, E. Angiostrongylus vasorum in Domestic Dogs in Castilla y León, Iberian Peninsula, Spain. Animals 2021, 11, 1513. [Google Scholar] [CrossRef]
- Chapman, P.S.; Boag, A.K.; Guitian, J.; Boswood, A. Angiostrongylus vasorum infection in 23 dogs (1999–2002). J. Small Anim. Pract. 2004, 45, 435–440. [Google Scholar] [CrossRef]
- Colombo, M.; Traversa, D.; Grillotti, E.; Pezzuto, C.; De Tommaso, C.; Pampurini, F.; Schaper, R.; Drake, J.; Crisi, P.E.; Russi, I.; et al. Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum. Pathogens 2021, 10, 1372. [Google Scholar] [CrossRef]
- Willesen, J.L.; Langhorn, R.; Nielsen, L.N. Hemostatic Dysfunction in Dogs Naturally Infected with Angiostrongylus vasorum—A Narrative Review. Pathogens 2022, 11, 249. [Google Scholar] [CrossRef]
- Beugnet, F.; Halos, L.; Guillot, J. Textbook of Clinical Parasitology in Dogs and Cats; Boehringer Ingelheim: Ingelheim, Germany, 2018; p. 423. [Google Scholar]
- Bagardi, M.; Rabbogliatti, V.; Bassi, J.; Gioeni, D.; Oltolina, M.; Villa, L. Angiostrongylus vasorum in a Red Panda (Ailurus fulgens): Clinical Diagnostic Trial and Treatment Protocol. Acta Parasitológica 2021, 66, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Paradies, P.; Sasanelli, M.; Capogna, A.; Mercadante, A.; Rubino, G.T.R.; Bussadori, C.M. Is Pulmonary Hypertension a Rare Condition Associated to Angiostrongylosis in Naturally Infected Dogs? Top. Companion Anim. Med. 2021, 43, 100513. [Google Scholar] [CrossRef] [PubMed]
- Fuehrer, H.P.; Morelli, S.; Unterköfler, M.S.; Bajer, A.; Bakran-Lebl, K.; Dwużnik-Szarek, D.; Farkas, R.; Grandi, G.; Heddergott, M.; Jokelainen, P.; et al. Dirofilaria spp. And Angiostrongylus vasorum: Current Risk of Spreading in Central and Northern Europe. Pathogens 2021, 10, 1268. [Google Scholar] [CrossRef]
- Carretón, E.; Morchón, R.; Falcón-Cordón, Y.; Matos, J.; Costa-Rodríguez, N.; Montoya-Alonso, J.A. First epidemiological survey of Angiostrongylus vasorum in domestic dogs from Spain. Parasit. Vectors. 2020, 13, 306. [Google Scholar] [CrossRef]
- Álvarez, F.; Iglesias, R.; Bos, J.; Rey, J.; San Martín Durán, M.L. Lung and hearth nematodes in some Spanish mammals. Wiad. Parazytol. 1991, 37, 481–490. [Google Scholar]
- Gotázar, C.; Villafuerte, R.; Lucientes, J.; Fernández-de-Luco, D. Habitat related differences in helminth parasites of red foxes in the Ebro valley. Vet. Parasitol. 1998, 80, 75–81. [Google Scholar] [CrossRef]
- Gerrikagoitia, X.; Barral, M.; Juste, R.A. Angiostrongylus species in wild carnivores in the Iberian Peninsula. Vet. Parasitol. 2010, 174, 175–180. [Google Scholar] [CrossRef]
- Mañas, S.; Ferrer, D.; Castellà, J.; Maria López-Martín, J. Cardiopulmonary helminth parasites of red foxes (Vulpes vulpes) in Catalonia, northeastern Spain. Vet. J. 2005, 169, 118–120. [Google Scholar] [CrossRef]
- Martínez-Carrasco, C.; Ruiz De Ybáñez, M.R.; Sagarminaga, J.L.; Garijo, M.M.; Moreno, F.; Acosta, I.; Hernández, S.; Alonso, F.D. Parasites of the red fox (Vulpes vulpes Linnaeus, 1758) in Murcia, southeast Spain. Revue Méd. Vét. 2007, 158, 331–335. [Google Scholar]
- Martínez-Rondán, F.J.; Ruiz de Ybáñez, M.R.; López-Beceiro, A.M.; Fidalgo, L.E.; Berriatua, E.; Lahat, L.; Sacristán, I.; Oleaga, Á.; Martínez-Carrasco, C. Cardiopulmonary nematode infections in wild canids: Does the key lie on host-prey-parasite evolution? Res. Vet. Sci. 2019, 126, 51–58. [Google Scholar] [CrossRef]
- Segovia, J.M.; Torres, J.; Miquel, J.; Llaneza, L.; Feliu, C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001, 75, 183–192. [Google Scholar] [PubMed]
- Torres, J.; Miquel, J.; Motjé, M. Helminth parasites of the eurasian badger (Meles meles L.) in Spain: A biogeographic approach. Parasitol. Res. 2001, 87, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística. Gobierno de España (INE). Available online: https://www.ine.es (accessed on 4 July 2022).
- Ministerio de Agricultura, Ganadería, Pesca y Alimentación. Available online: https://www.mapa.gob.es/es/default.aspx (accessed on 15 February 2022).
- Köppen’s Climate Classification. Available online: http://koeppen-geiger.vu-wien.ac.at/shifts.htm (accessed on 2 July 2022).
- Guardone, L.; Schnyder, M.; Macchioni, F.; Deplazes, P.; Magi, M. Serological detection of circulating Angiostrongylus vasorum antigen and specific antibodies in dogs from central and northern Italy. Vet. Parasitol. 2013, 192, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Lurati, L.; Deplazes, P.; Hegglin, D.; Schnyder, M. Seroepidemiological survey and spatial analysis of the occurrence of Angiostrongylus vasorum in Swiss dogs in relation to biogeographic aspects. Vet. Parasitol. 2015, 212, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, M.; Schaper, R.; Pantchev, N.; Kowalska, D.; Szwedko, A.; Deplazes, P. Serological detection of circulating Angiostrongylus vasorum antigen- and parasite-specific antibodies in dogs from Poland. Parasitol. Res. 2013, 112 (Suppl. 1), 109–117. [Google Scholar] [CrossRef] [PubMed]
- Miterpáková, M.; Hurníková, Z.; Zalewski, A.P. The first clinically manifested case of angiostrongylosis in a dog in Slovakia. Acta Parasitol. 2014, 59, 661–665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deak, G.; Gillis-Germitsch, N.; Ionică, A.M.; Mara, A.; Păstrav, I.R.; Cazan, C.D.; Ioniță, M.; Mitrea, I.L.; Răileanu, C.; Bărburaș, D.; et al. The first seroepidemiological survey for Angiostrongylus vasorum in domestic dogs from Romania. Parasit. Vectors. 2019, 12, 224. [Google Scholar] [CrossRef]
- Globokar, M.; Pantchev, N.; Hinney, B.; Leschnik, M.; Peschke, R.; Schaper, R.; Schnyder, M. Serological and faecal detection of Angiostrongylus vasorum in dogs from Austria. Vet. Parasitol. Reg. Stud. Reports. 2021, 26, 100641. [Google Scholar] [CrossRef]
- Schnyder, M.; Schaper, R.; Lukácsc, Z.; Hornok, S.; Farkas, R. Combined serological detection of circulating Angiostrongylus vasorum antigen and parasite-specific antibodies in dogs from Hungary. Parasitol. Res. 2015, 114, S145–S154. [Google Scholar] [CrossRef]
- Segeritz, L.; Cardona, A.; Taubert, A.; Hermosilla, C.; Ruiz, A. Autochthonous Angiostrongylus cantonensis, Angiostrongylus vasorum and Aelurostrongylus abstrusus infections in native terrestrial gastropods from the Macaronesian Archipelago of Spain. Parasitol. Res. 2021, 120, 2671–2680. [Google Scholar] [CrossRef]
- Liu, J.; Schnyder, M.; Willesen, J.L.; Potter, A. Chandrashekar, R. Performance of the Angio Detect™ in-clinic test kit for detection of Angiostrongylus vasorum infection in dog samples from Europe. Vet. Parasitol. Reg. Stud. Rep. 2017, 7, 45–47. [Google Scholar]
- Schnyder, M.; Stebler, K.; Naucke, T.J.; Lorentz, S.; Deplazes, P. Evaluation of a rapid device for serological in-clinic diagnosis of canine angiostrongylosis. Parasit. Vectors 2014, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Venco, L.; Colaneri, G.; Formaggini, L.; De Franco, M.; Rishniw, M. Utility of thoracic ultrasonography in a rapid diagnosis of angiostrongylosis in young dogs presenting with respiratory distress. Vet. J. 2021, 271, 105649. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef]
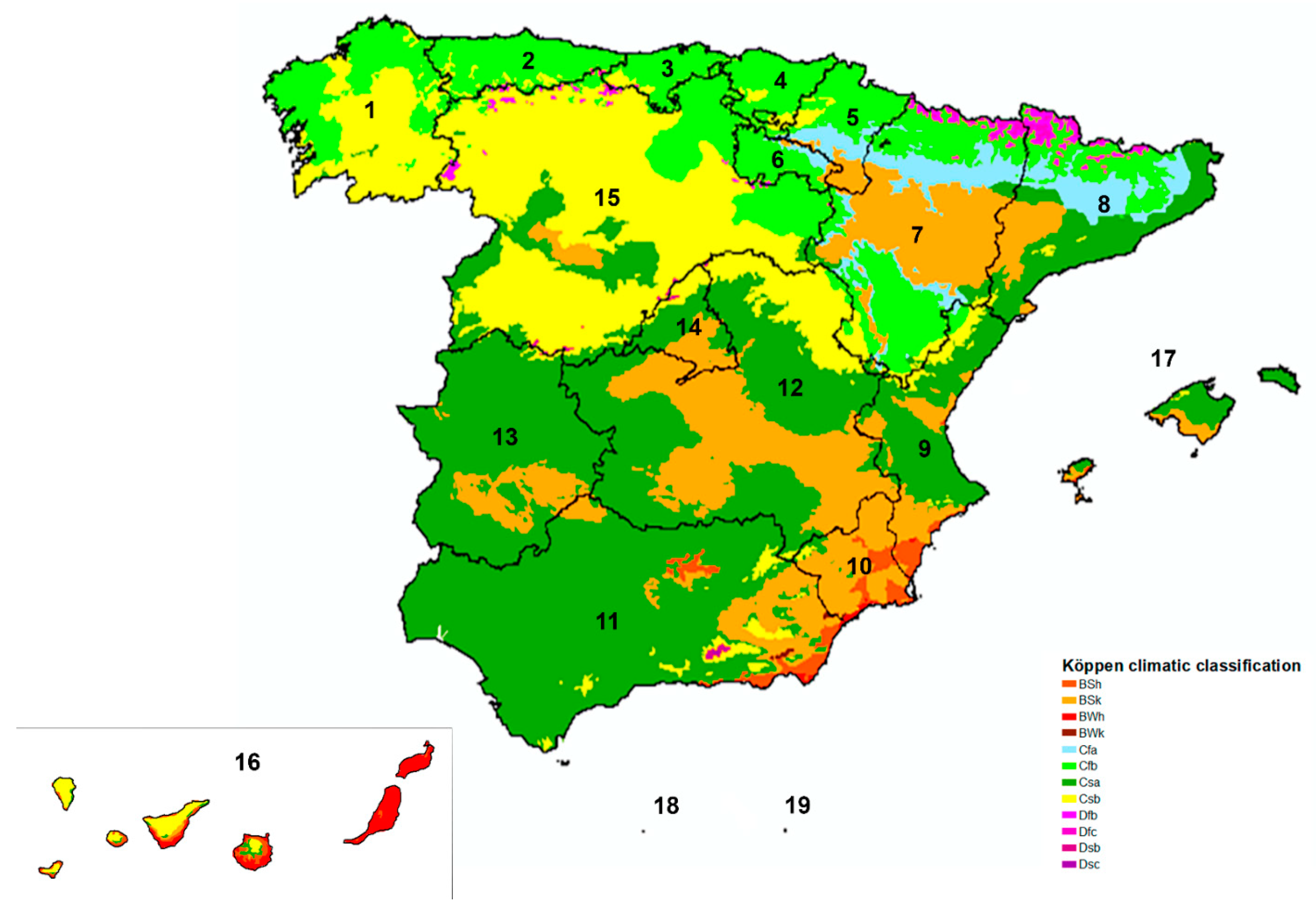
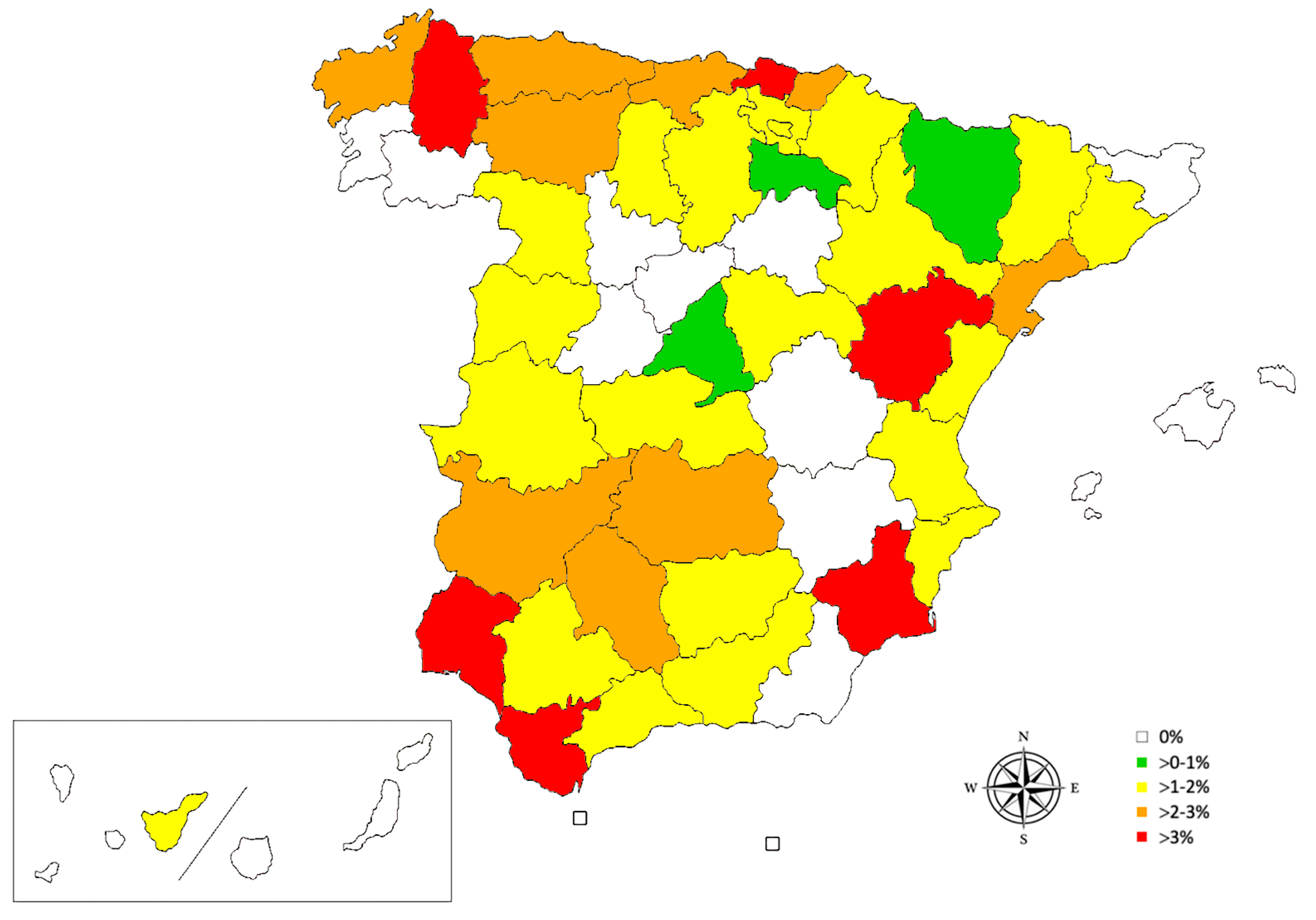
| n | + | % (CI 95%) | |
|---|---|---|---|
| 1. Galicia | 428 | 8 | 1.87 (0.95–3.64) |
| 2. Asturias | 120 | 3 | 2.50 (0.85–7.09) |
| 3. Cantabria | 125 | 3 | 2.40 (0.82–6.82) |
| 4. Basque Country | 308 | 10 | 3.25 (1.77–5.87) |
| 5. Navarre | 170 | 3 | 1.76 (0.60–5.06) |
| 6. La Rioja | 100 | 1 | 1.00 (0.18–5.45) |
| 7. Aragón | 209 | 3 | 1.44 (0.49–4.13) |
| 8. Catalonia | 521 | 6 | 1.15 (0.53–2.49) |
| 9. Valencian Community | 352 | 5 | 1.42 (0.61–3.28) |
| 10. Murcia | 97 | 4 | 4.12 (1.62–10.13) |
| 11. Andalusia | 502 | 10 | 1.99 (1.09–3.63) |
| 12. Castilla-La Mancha | 304 | 4 | 1.32 (0.51–3.33) |
| 13. Extremadura | 153 | 3 | 1.96 (0.67–5.61) |
| 14. Madrid | 389 | 3 | 0.77 (0.26–2.24) |
| 15. Castilla and León | 985 | 11 | 1.12 (0.62–1.99) |
| 16. Canary Islands | 552 | 1 | 0.18 (0.03–1.02) |
| 17. Balearic Islands | 229 | 0 | 0.00 (0.00–1.65) |
| 18. Ceuta | 27 | 0 | 0.00 (0.00–12.45) |
| 19. Melilla | 48 | 0 | 0.00 (0.00–7.41) |
| Total | 5619 | 78 | 1.39 (1.11–1,73) |
| Autonomous Community Province | n | + | % (CI 95%) | Autonomous Community Province | n | + | % (CI 95%) |
|---|---|---|---|---|---|---|---|
| Galicia | Extremadura | ||||||
| A Coruña | 224 | 5 | 2.23 (0.96–5.12) | Cáceres | 71 | 1 | 1.41 (0.25–7.56) |
| Lugo | 98 | 3 | 3.06 (1.05–8.62) | Badajoz | 82 | 2 | 2.44 (0.67–8.46) |
| Ourense | 47 | 0 | 0.00 (0.00–7.56) | Andalusia | |||
| Pontevedra | 59 | 0 | 0.00 (0.00–6.11) | Cádiz | 58 | 2 | 3.45 (0.95–11.73) |
| Asturias | Seville | 70 | 1 | 1.43 (0.25–7.66) | |||
| Oviedo | 120 | 3 | 2.50 (0.85–7.09) | Málaga | 54 | 1 | 1.85 (0.33–9.77) |
| Cantabria | Granada | 87 | 1 | 1.15 (0.20–6.23) | |||
| Santander | 125 | 3 | 2.40 (0.82–6.82) | Jaén | 67 | 1 | 1.49 (0.26–7.98) |
| Basque Country | Huelva | 78 | 3 | 3.85 (1.32–10.71) | |||
| Araba | 81 | 1 | 1.23 (0.22–6.67) | Almería | 41 | 0 | 0.00 (0.00–8.57) |
| Bizkaia | 125 | 6 | 4.80 (2.22–10.08) | Córdoba | 47 | 1 | 2.13 (0.38–11.11) |
| Gipuzkoa | 102 | 3 | 2.94 (1.01–8.29) | Castilla-La Mancha | |||
| Navarre | Albacete | 40 | 0 | 0.00 (0.00–8.76) | |||
| Navarre | 170 | 3 | 1.76 (0.60–5.06) | Guadalajara | 87 | 1 | 1.15 (0.20–6.23) |
| La Rioja | Cuenca | 38 | 0 | 0.00 (0.00–9.18) | |||
| La Rioja | 100 | 1 | 1.00 (0.18–5.45) | Ciudad Real | 85 | 2 | 2.35 (0.65–8.18) |
| Aragón | Toledo | 54 | 1 | 1.85 (0.33–9.77) | |||
| Huesca | 112 | 1 | 0.89 (0.16–4.88) | Castilla y León | |||
| Zaragoza | 67 | 1 | 1.49 (0.26–7.98) | León | 148 | 3 | 2.03 (0.69–5.79) |
| Teruel | 30 | 1 | 3.33 (0.59–16.67) | Zamora | 94 | 1 | 1.06 (0.19–5.78) |
| Catalonia | Salamanca | 137 | 2 | 1.46 (0.40–5.17) | |||
| Girona | 75 | 0 | 0.00 (0.00–4.87) | Valladolid | 85 | 0 | 0.00 (0.00–4.32) |
| Barcelona | 285 | 3 | 1.05 (0.36–3.05) | Palencia | 112 | 2 | 1.79 (0.49–6.28) |
| Tarragona | 91 | 2 | 2.20 (0.60–7.66) | Burgos | 154 | 3 | 1.95 (0.66–5.57) |
| Lleida | 70 | 1 | 1.43 (0.25–7.66) | Segovia | 98 | 0 | 0.00 (0.00–3.77) |
| Valencian Community | Soria | 82 | 0 | 0.00 (0.00–4.47) | |||
| Castellón | 60 | 1 | 1.67 (0.29–8.85) | Ávila | 75 | 0 | 0.00 (0.00–4.87) |
| Valencia | 207 | 3 | 1.45 (0.49–4.17) | Canary Islands | |||
| Alicante | 85 | 1 | 1.18 (0.21–6.37) | Las Palmas | 240 | 0 | 0.00 (0.00–1.58) |
| Murcia | S/C Tenerife | 312 | 1 | 0.32 (0.06–1.79) | |||
| Murcia | 97 | 4 | 4.12 (1.62–10.13) | Balearic Islands | |||
| Madrid | Balearic Islands | 229 | 0 | 0.00 (0.00–1.65) | |||
| Madrid | 389 | 3 | 0.77 (0.26–2.24) | Ceuta | 27 | 0 | 0.00 (0.00–12.45) |
| Melilla | 48 | 0 | 0.00 (0.00–7.41) | ||||
| Total | 5619 | 78 | 1.39 |
| n | + | % (CI 95%) | |
|---|---|---|---|
| Sex | |||
| Female | 2826 | 41 | 1.45 (1.07–1.96) |
| Male | 2793 | 37 | 1.32 (0.96–1.82) |
| Age | |||
| <1 year | 915 | 7 | 0.77 (0.37–1.57) |
| 1–4.9 years | 1183 | 21 | 1.78 (1.16–2.70) |
| 5–9.9 years | 1389 | 24 | 1.73 (1.16–2.56) |
| 10–15 years | 1206 | 17 | 1.41 (0.88–2.25) |
| >15 years | 926 | 9 | 0.97 (0.51–1.84) |
| Habitat | |||
| Outdoor | 3327 | 59 | 1.77 (1.38–2.28) |
| Indoor | 431 | 4 | 0.93 (0.36–2.36) |
| Indoor/Outdoor | 1861 | 15 | 0.81 (0.49–1.33) |
| Climates | |||
| Csa | 2242 | 29 | 1.29 (0.90–1.85) |
| Csb | 1197 | 16 | 1.34 (0.82–2.26) |
| Cfb | 1271 | 26 | 2.05 (1.40–2.98) |
| BSk | 417 | 7 | 1.68 (0.82–3.42) |
| BWh | 143 | 0 | 0.00 (0.00–2.62) |
| BSh | 349 | 0 | 0.00 (0.00–1.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carretón, E.; Morchón, R.; García-Rodríguez, S.N.; Rodríguez-Escolar, I.; Matos, J.I.; Costa-Rodríguez, N.; Montoya-Alonso, J.A. Comprehensive Map of Canine Angiostrongylosis in Dogs in Spain. Animals 2022, 12, 2217. https://doi.org/10.3390/ani12172217
Carretón E, Morchón R, García-Rodríguez SN, Rodríguez-Escolar I, Matos JI, Costa-Rodríguez N, Montoya-Alonso JA. Comprehensive Map of Canine Angiostrongylosis in Dogs in Spain. Animals. 2022; 12(17):2217. https://doi.org/10.3390/ani12172217
Chicago/Turabian StyleCarretón, Elena, Rodrigo Morchón, Sara Nieves García-Rodríguez, Iván Rodríguez-Escolar, Jorge Isidoro Matos, Noelia Costa-Rodríguez, and José Alberto Montoya-Alonso. 2022. "Comprehensive Map of Canine Angiostrongylosis in Dogs in Spain" Animals 12, no. 17: 2217. https://doi.org/10.3390/ani12172217
APA StyleCarretón, E., Morchón, R., García-Rodríguez, S. N., Rodríguez-Escolar, I., Matos, J. I., Costa-Rodríguez, N., & Montoya-Alonso, J. A. (2022). Comprehensive Map of Canine Angiostrongylosis in Dogs in Spain. Animals, 12(17), 2217. https://doi.org/10.3390/ani12172217











