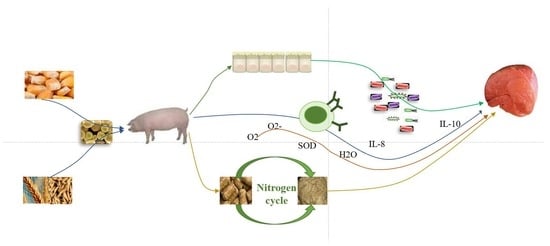
Effects of Yeast Culture Supplementation in Wheat–Rice-Based Diet on Growth Performance, Meat Quality, and Gut Microbiota of Growing–Finishing Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Diets and Yeast Culture
2.3. Sample Collection and Chemical Analyses
2.4. Intestinal Morphology
2.5. Bacterial Community Analysis
2.6. Real-Time Quantitative PCR
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Cost
3.2. Carcass Characteristics and Organ Development
3.3. Meat Quality
3.4. Blood Metabolites
3.5. Serum Antioxidant Capacity
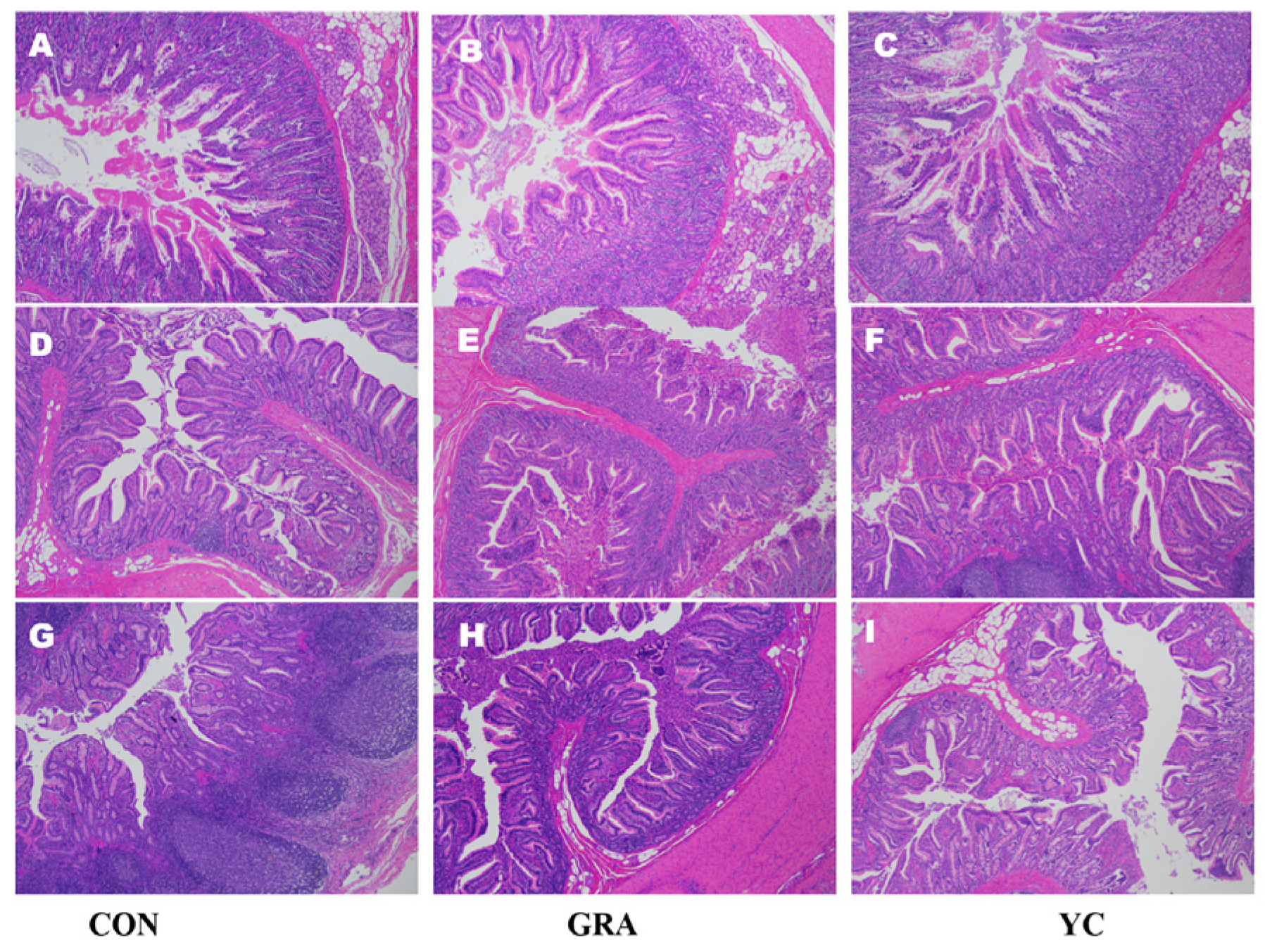
3.6. Intestinal Morphology
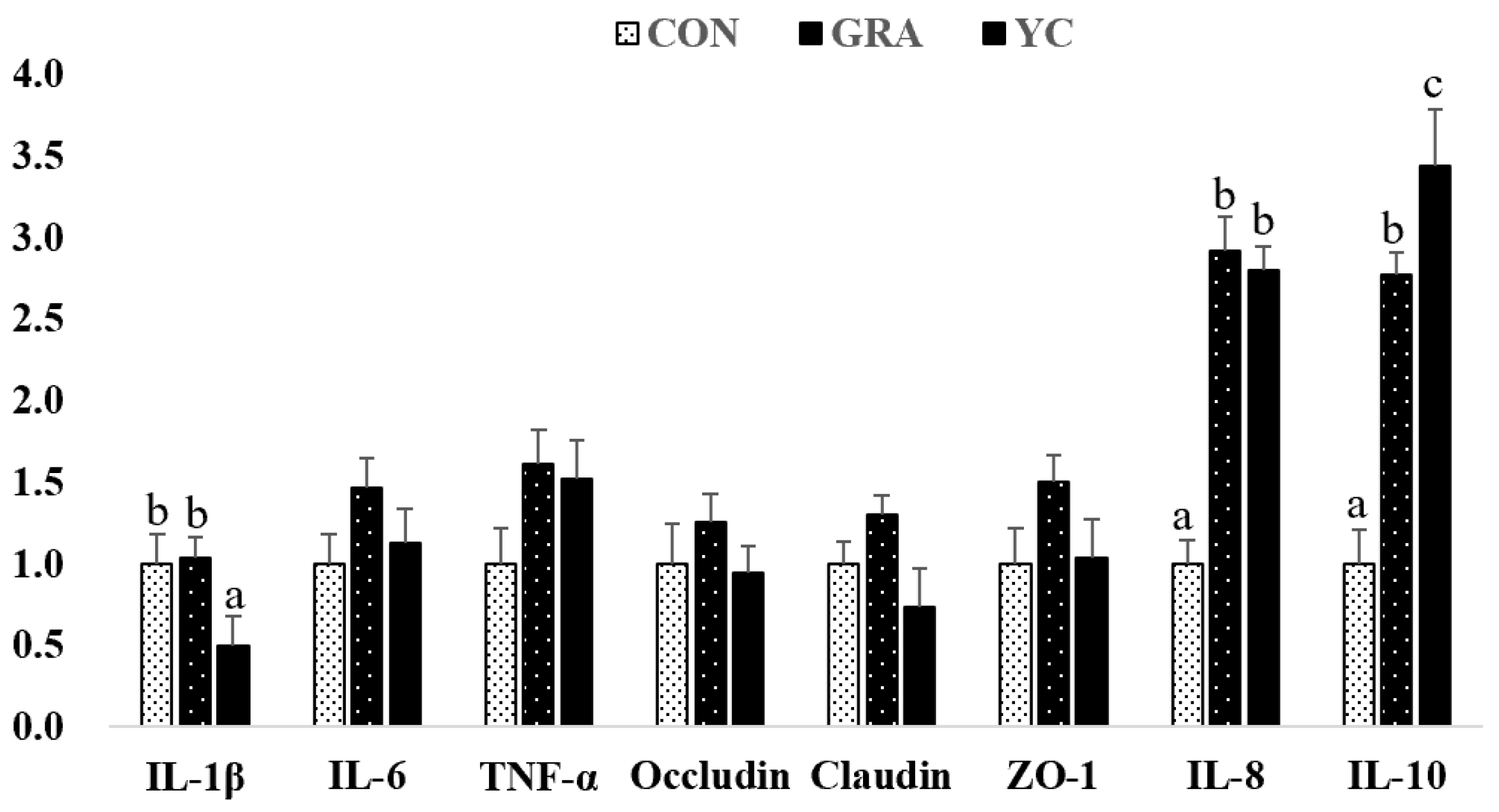
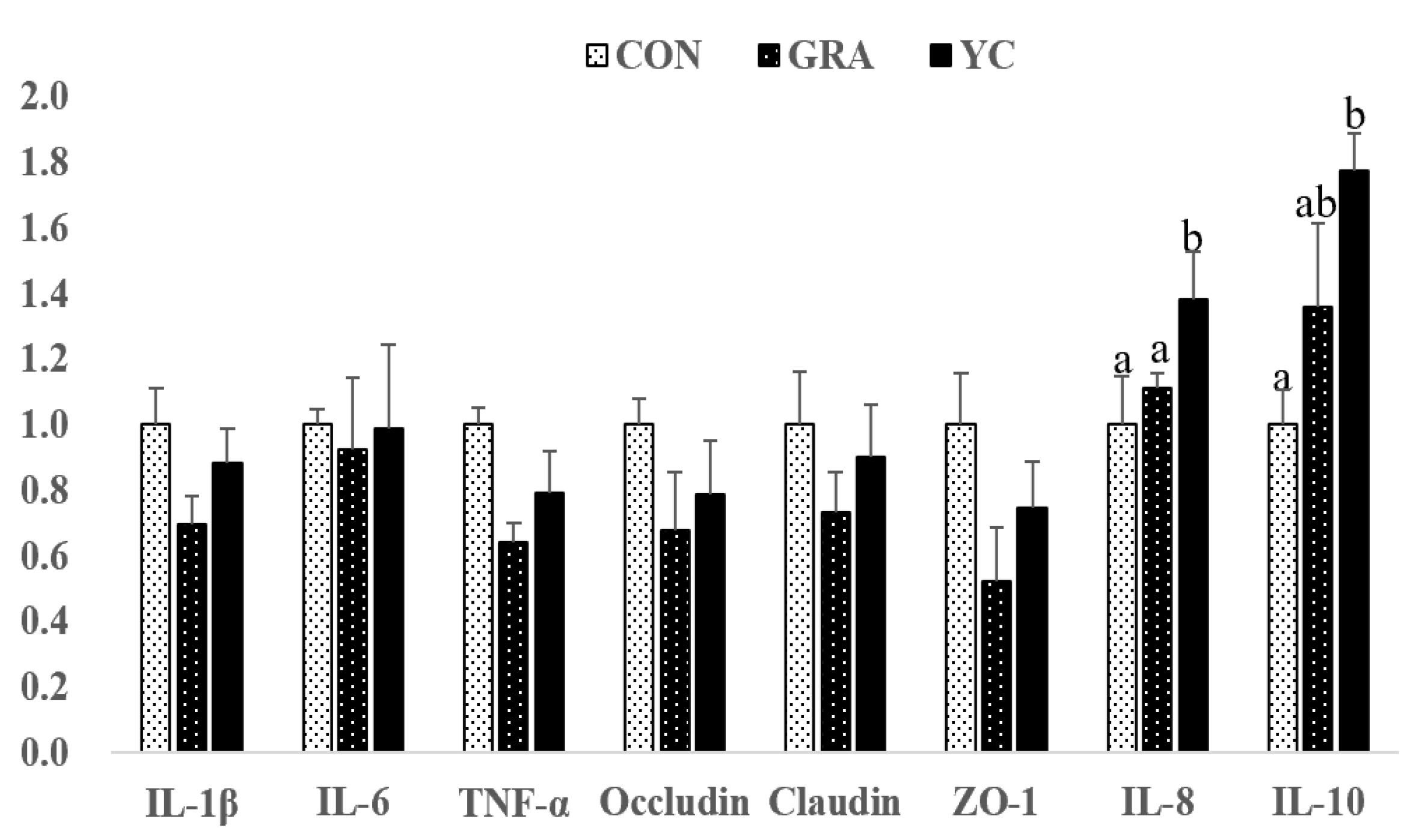
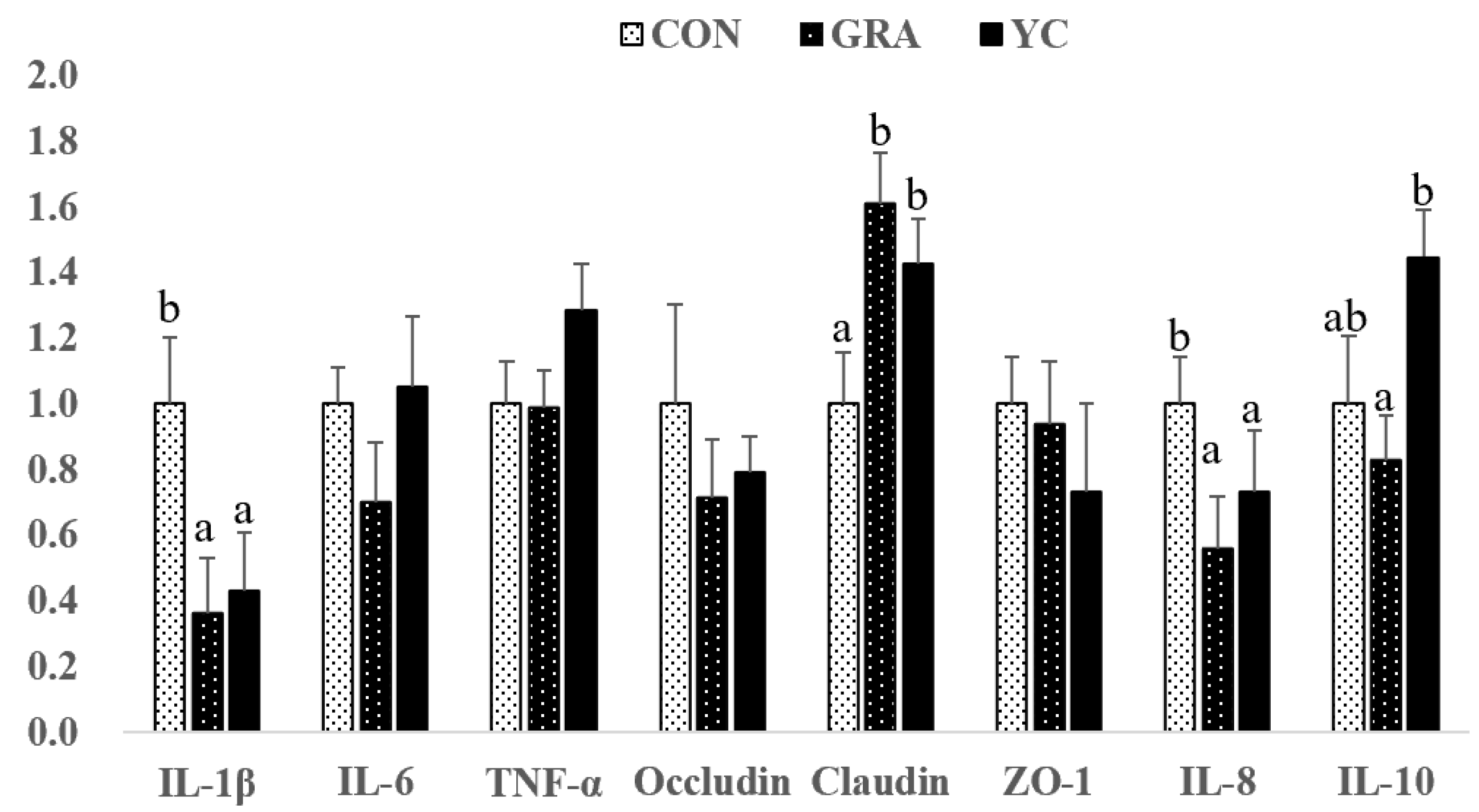
3.7. Gene Expression
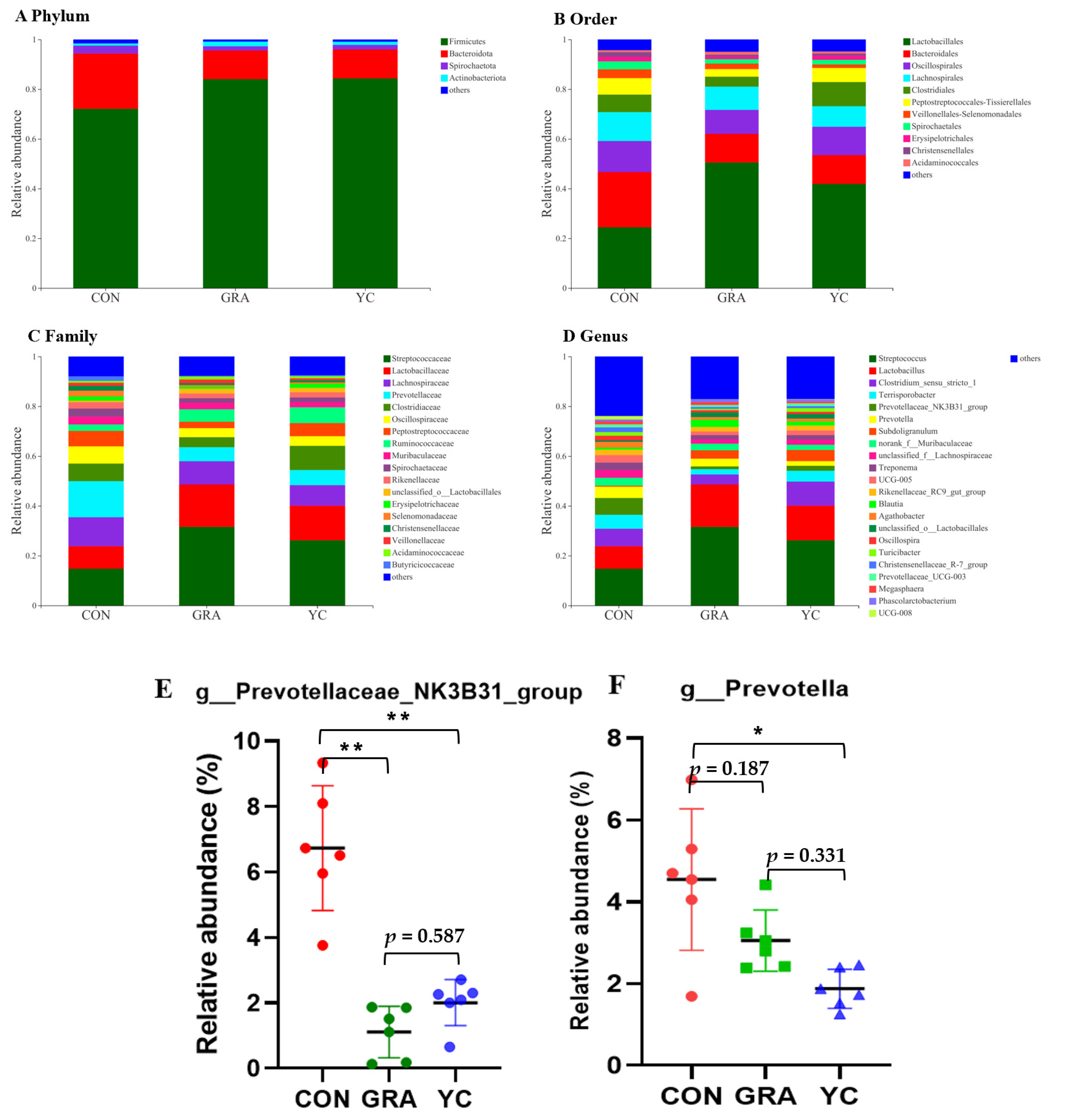
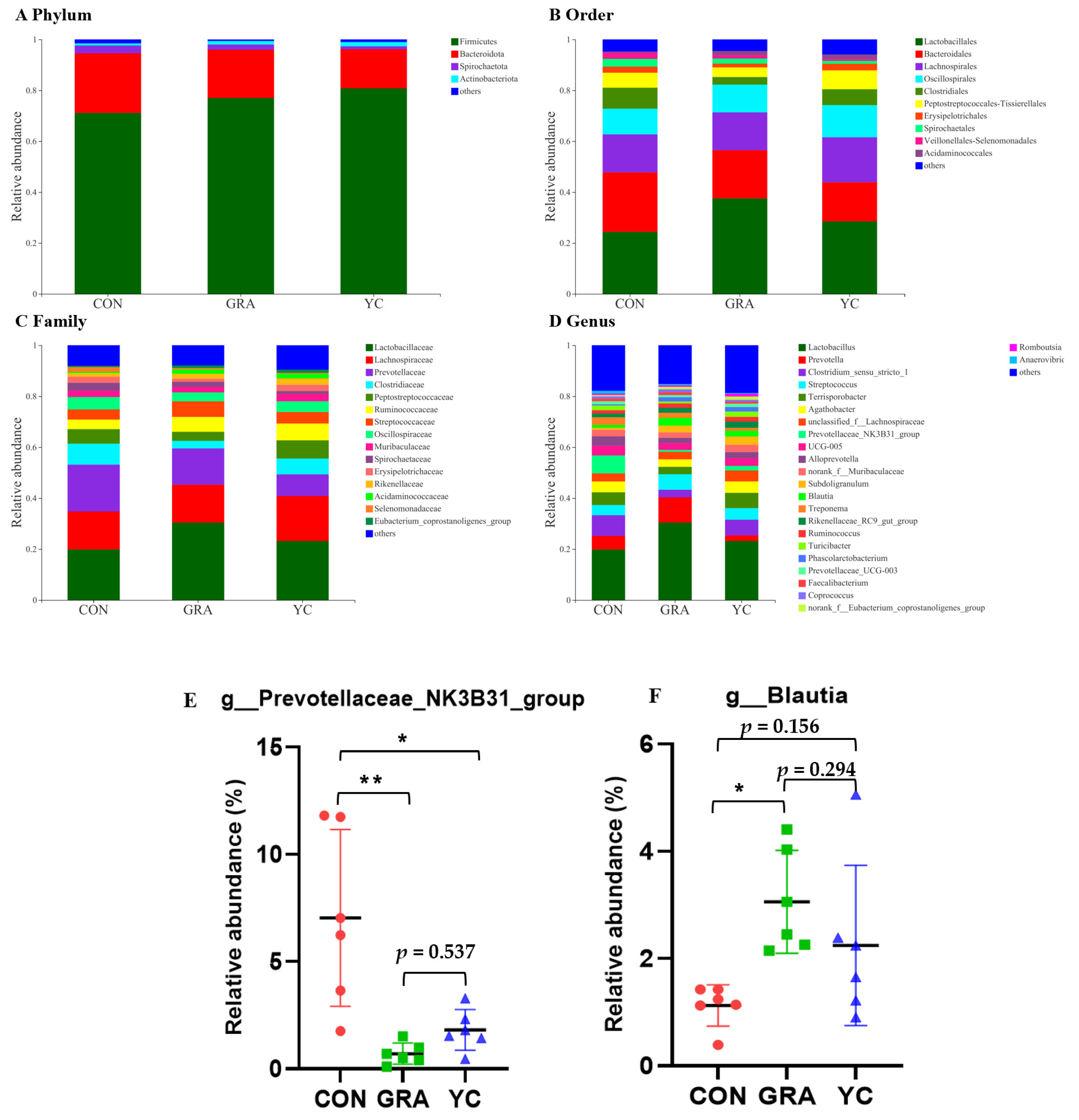
3.8. Overview of 16S rRNA Sequencing Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boontiam, W.; Bunchasak, C.; Kim, Y.Y.; Kitipongpysan, S.; Hong, J. Hydrolyzed Yeast Supplementation to Newly Weaned Piglets: Growth Performance, Gut Health, and Microbial Fermentation. Animals 2022, 12, 350. [Google Scholar] [CrossRef]
- Lee, J.; Kyoung, H.; Cho, J.; Choe, J.; Kim, Y.; Liu, Y.; Kang, J.; Lee, H.; Kim, H.; Song, M. Dietary Yeast Cell Wall Improves Growth Performance and Prevents of Diarrhea of Weaned Pigs by Enhancing Gut Health and Anti-Inflammatory Immune Responses. Animals 2021, 11, 2269. [Google Scholar] [CrossRef] [PubMed]
- Namted, S.; Poungpong, K.; Loongyai, W.; Rakangthong, C.; Bunchasak, C. Improving growth performance and blood profile by feeding autolyzed yeast to improve pork carcass and meat quality. Anim. Sci. J. 2021, 92, e13666. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.-Y.; Ren, L.-P.; Zhou, Z.-M.; Chang, Y.; Meng, Q.-X. Comparison of active dry yeast (Saccharomyces cerevisiae) and yeast culture for growth performance, carcass traits, meat quality and blood indexes in finishing bulls. Anim. Sci. J. 2016, 87, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Park, J.W.; Kim, I.H. Effect of supplementation with brewer’s yeast hydrolysate on growth performance, nutrients digestibility, blood profiles and meat quality in growing to finishing pigs. Asian-Australas. J. Anim. Sci. 2019, 32, 1565–1572. [Google Scholar] [CrossRef]
- Hoque, M.-R.; Jung, H.-I.; Kim, I.-H. Effect of Yeast Culture (Saccharomyces cerevisiae) Supplementation on Growth Performance, Excreta Microbes, Noxious Gas, Nutrient Utilization, and Meat Quality of Broiler Chicken. J. Poult. Sci. 2021, 58, 216–221. [Google Scholar] [CrossRef]
- Han, T.H.; Hong, J.S.; Fang, L.H.; Do, S.H.; Kim, B.O.; Kim, Y.Y. Effects of wheat supplementation levels on growth performance, blood profiles, nutrient digestibility, and pork quality in growing-finishing pigs. Asian-Australas. J. Anim. Sci. 2017, 30, 1150–1159. [Google Scholar] [CrossRef]
- He, W.; Gao, Y.; Guo, Z.; Yang, Z.; Wang, X.; Liu, H.; Sun, H.; Shi, B. Effects of fermented wheat bran and yeast culture on growth performance, immunity, and intestinal microflora in growing-finishing pigs. J. Anim. Sci. 2022, 99, skab308. [Google Scholar] [CrossRef]
- Chance, J.A.; DeRouchey, J.M.; Amachawadi, R.G.; Ishengoma, V.; Nagaraja, T.G.; Goodband, R.D.; Woodworth, J.C.; Tokach, M.D.; Kang, Q.; Loughmiller, J.A.; et al. Influence of yeast-based pre- and probiotics in lactation and nursery diets on nursery pig performance and antimicrobial resistance of fecal Escherichia coli. J. Anim. Sci. 2022, 100, skac166. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Zhou, P.; Li, Z.; Zhong, W.; Zhuo, Y.; Che, L.; Xu, S.; Fang, Z.; Jiang, X.; et al. Effects of yeast culture supplementation from late gestation to weaning on performance of lactating sows and growth of nursing piglets. Animal 2022, 16, 100526. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Kim, I.-H. Dietary Supplementation of Mixed Yeast Culture Derived from Saccharomyces cerevisiae and Kluyveromyces maxianus: Effects on Growth Performance, Nutrient Digestibility, Meat Quality, Blood Parameters, and Gut Health in Broilers. J. Poult. Sci. 2019, 56, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, F.; Lin, X.; Wang, Y.; He, J.; Zhao, Y. Effect of Excessive or Restrictive Energy on Growth Performance, Meat Quality, and Intramuscular Fat Deposition in Finishing Ningxiang Pigs. Animals 2020, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2017, 73, 5261–5267. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nemechek, J.E.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; DeRouchey, J.M.; Woodworth, J.C. Effects of diet form and type on growth performance, carcass yield, and iodine value of finishing pigs1. J. Anim. Sci. 2015, 93, 4486–4499. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Zhong, R.; Gao, L.; Liu, Z.; Chen, L.; Zhang, H. Effects of Optimal Carbohydrase Mixtures on Nutrient Digestibility and Digestible Energy of Corn- and Wheat-Based Diets in Growing Pigs. Animals 2020, 10, 1846. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Mann, E.; Ertl, R.; Schmitz-Esser, S.; Wagner, M.; Klein, D.; Ritzmann, M.; Zebeli, Q. Dietary calcium concentration and cereals differentially affect mineral balance and tight junction proteins expression in jejunum of weaned pigs. Br. J. Nutr. 2015, 113, 1019–1031. [Google Scholar] [CrossRef]
- Mejicanos, G.A.; González-Ortiz, G.; Nyachoti, C.M. Effect of dietary supplementation of xylanase in a wheat-based diet containing canola meal on growth performance, nutrient digestibility, organ weight, and short-chain fatty acid concentration in digesta when fed to weaned pigs. J. Anim. Sci. 2020, 98, skaa064. [Google Scholar] [CrossRef]
- Navarro, D.; Bruininx, E.; de Jong, L.; Stein, H.H. Effects of physicochemical characteristics of feed ingredients on the apparent total tract digestibility of energy, DM, and nutrients by growing pigs1. J. Anim. Sci. 2018, 96, 2265–2277. [Google Scholar] [CrossRef]
- Fu, R.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Yu, B. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Anim. Nutr. 2022, 5, 366–372. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Kvidera, S.K.; Horst, E.A.; Al-Qaisi, M.; McCarthy, C.S.; Abeyta, M.A.; Lei, S.; Elsasser, T.H.; Kahl, S.; Kiros, T.G.; et al. Effects of dietary live yeast supplementation on growth performance and biomarkers of metabolism and inflammation in heat-stressed and nutrient-restricted pigs. Transl. Anim. Sci. 2021, 5, txab072. [Google Scholar] [CrossRef]
- Kim, S.W.; Holanda, D.M.; Gao, X.; Park, I.; Yiannikouris, A. Efficacy of a Yeast Cell Wall Extract to Mitigate the Effect of Naturally Co-Occurring Mycotoxins Contaminating Feed Ingredients Fed to Young Pigs: Impact on Gut Health, Microbiome, and Growth. Toxins 2019, 11, 633. [Google Scholar] [CrossRef]
- Lu, H.; Wilcock, P.; Adeola, O.; Ajuwon, K.M. Effect of live yeast supplementation to gestating sows and nursery piglets on postweaning growth performance and nutrient digestibility. J. Anim. Sci. 2017, 97, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cheng, K.; Yu, C.; Tong, Y.; Yang, Z.; Wang, T. Effects of yeast hydrolysate on growth performance, serum parameters, carcass traits, meat quality and antioxidant status of broiler chickens. J. Sci. Food Agric. 2022, 102, 575–583. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhang, L.; Gao, F.; Zhou, G. Effects of dietary starch types on growth performance, meat quality and myofibre type of finishing pigs. Meat Sci. 2017, 131, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ran, T.; Fang, Y.; Wang, Y.; Yang, W.; Niu, Y.; Sun, X.; Zhong, R. Effects of grain type and conditioning temperature during pelleting on growth performance, ruminal fermentation, meat quality and blood metabolites of fattening lambs. Animal 2021, 15, 100146. [Google Scholar] [CrossRef] [PubMed]
- Andriamialinirina, H.J.T.; Irm, M.; Taj, S.; Lou, J.H.; Jin, M.; Zhou, Q. The effects of dietary yeast hydrolysate on growth, hematology, antioxidant enzyme activities and non-specific immunity of juvenile Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2020, 101, 168–175. [Google Scholar] [CrossRef]
- Feng, Z.; Zhong, Y.; He, G.; Sun, H.; Chen, Y.; Zhou, W.; Lin, S. Yeast culture improved the growth performance, liver function, intestinal barrier and microbiota of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol. 2022, 120, 706–715. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, M.; Zhang, R.; Sun, Z.; Wang, C.; Yang, F.; Huang, T.; Qu, S.; Zhao, L.; Li, Y.; et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell Factories 2017, 16, 191. [Google Scholar] [CrossRef]
- Kim, E.; Kyoung, H.; Koh, N.H.; Lee, H.; Lee, S.; Kim, Y.; Park, K.I.; Heo, J.M.; Song, M. Supplementation of live yeast culture modulates intestinal health, immune responses, and microbiota diversity in broiler chickens. J. Anim. Sci. 2022, 100, skac122. [Google Scholar] [CrossRef]
- Taylor, H.B.; Vasu, C. Impact of Prebiotic β-glucan Treatment at Juvenile Age on the Gut Microbiota Composition and the Eventual Type 1 Diabetes Onset in Non-obese Diabetic Mice. Front. Nutr. 2021, 8, 769341. [Google Scholar] [CrossRef]
- Qiao, Y.; Ye, X.; Zhong, L.; Xia, C.; Zhang, L.; Yang, F.; Li, Y.; Fang, X.; Fu, L.; Huang, Y.; et al. Yeast β-1,3-glucan production by an outer membrane β-1,6-glucanase: Process optimization, structural characterization and immunomodulatory activity. Food Funct. 2022, 13, 3917–3930. [Google Scholar] [CrossRef]
- Lu, Q.; Stappenbeck, T.S. Local barriers configure systemic communications between the host and microbiota. Science 2022, 376, 950–955. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, S.; Cheng, Z.; Ding, F.; Fan, Q.; Lin, X.; Wang, Y.; Gou, Z. Feed Restriction Improves Lipid Metabolism by Changing the Structure of the Cecal Microbial Community and Enhances the Meat Quality and Flavor of Bearded Chickens. Animals 2022, 12, 970. [Google Scholar] [CrossRef]
- Sampath, V.; Shanmugam, S.; Park, J.H.; Kim, I.H. The Effect of Black Pepper (Piperine) Extract Supplementation on Growth Performance, Nutrient Digestibility, Fecal Microbial, Fecal Gas Emission, and Meat Quality of Finishing Pigs. Animals 2020, 10, 1965. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, J.; Wu, Q.; Chen, J.; Yang, X.; Wang, L.; Jiang, Z. Low Protein Diet Improves Meat Quality and Modulates the Composition of Gut Microbiota in Finishing Pigs. Front. Vet. Sci. 2022, 9, 843957. [Google Scholar] [CrossRef]
- Wu, C.; Lyu, W.; Hong, Q.; Zhang, X.; Yang, H.; Xiao, Y. Gut Microbiota Influence Lipid Metabolism of Skeletal Muscle in Pigs. Front. Nutr. 2021, 8, 675445. [Google Scholar] [CrossRef]
- Pinloche, E.; Williams, M.; D’Inca, R.; Auclair, E.; Newbold, C.J. Use of a colon simulation technique to assess the effect of live yeast on fermentation parameters and microbiota of the colon of pig. J. Anim. Sci. 2012, 90 (Suppl. 4), 353–355. [Google Scholar] [CrossRef]
- Jouany, J.-P.; Medina, B.; Bertin, G.; Julliand, V. Effect of live yeast culture supplementation on hindgut microbial communities and their polysaccharidase and glycoside hydrolase activities in horses fed a high-fiber or high-starch diet. J. Anim. Sci. 2009, 87, 2844–2852. [Google Scholar] [CrossRef]
- Hu, J.; Park, J.W.; Kim, I.H. Effect of dietary supplementation with brewer’s yeast hydrolysate on growth performance, faecal microbial counts, diarrhoea score, blood profile, rectal temperature in weanling pigs challenged with lipopolysaccharide. J. Anim. Physiol. Anim. Nutr. 2020, 104, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Kiros, T.G.; Luise, D.; Derakhshani, H.; Petri, R.; Trevisi, P.; D’Inca, R.; Auclair, E.; van Kessel, A.G. Effect of live yeast Saccharomyces cerevisiae supplementation on the performance and cecum microbial profile of suckling piglets. PLoS ONE 2019, 14, e0219557. [Google Scholar] [CrossRef] [PubMed]
- Borrello, K.; Lim, U.; Park, S.-Y.; Monroe, K.R.; Maskarinec, G.; Boushey, C.J.; Wilkens, L.R.; Randolph, T.W.; Le Marchand, L.; Hullar, M.A.; et al. Dietary Intake Mediates Ethnic Differences in Gut Microbial Composition. Nutrients 2022, 14, 660. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Rong, T.; Tian, Z.; Deng, D.; Lu, H.; Zhang, R.; Ma, X. Integrated metagenomics-metabolomics analysis reveals the cecal microbial composition, function, and metabolites of pigs fed diets with different starch sources. Food Res. Int. 2022, 154, 110951. [Google Scholar] [CrossRef]
| Ingredients, % | 50–75 kg | 75–100 kg | 100–110 kg | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | GRA | YC | CON | GRA | YC | CON | GRA | YC | |
| Corn | 74.59 | 10.00 | 10.00 | 74.90 | 0.00 | 0.00 | 79.90 | 0.00 | 0.00 |
| Wheat | 0.00 | 55.87 | 55.37 | 0.00 | 55.00 | 54.50 | 0.00 | 59.20 | 58.70 |
| Unhusked rice | 0.00 | 16.40 | 16.45 | 0.00 | 27.50 | 27.50 | 0.00 | 29.03 | 29.07 |
| Soybean oil | 1.01 | 2.00 | 2.00 | 1.00 | 2.00 | 2.00 | 1.00 | 2.00 | 2.00 |
| Soybean meal | 21.24 | 12.60 | 12.55 | 21.40 | 12.87 | 12.87 | 16.62 | 7.41 | 7.37 |
| Yeast culture | 0.00 | 0.00 | 0.50 | 0.00 | 0.00 | 0.50 | 0.00 | 0.00 | 0.50 |
| L-Lysine HCl | 0.30 | 0.48 | 0.48 | 0.12 | 0.30 | 0.30 | 0.10 | 0.30 | 0.30 |
| DL-methionine | 0.03 | 0.05 | 0.05 | 0.03 | 0.05 | 0.05 | 0.00 | 0.00 | 0.00 |
| L-Threonine | 0.07 | 0.17 | 0.17 | 0.02 | 0.10 | 0.10 | 0.00 | 0.10 | 0.10 |
| L-Tryptophan | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Choline 50% | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Limestone | 0.64 | 0.80 | 0.80 | 0.60 | 0.80 | 0.80 | 0.50 | 0.78 | 0.78 |
| Dicalcium phosphate | 1.18 | 0.70 | 0.70 | 1.00 | 0.45 | 0.45 | 0.95 | 0.25 | 0.25 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Vitamin premix1 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Mineral premix1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Calculated Values | |||||||||
| DE, Mcal/kg | 3.37 | 3.29 | 3.29 | 3.38 | 3.23 | 3.22 | 3.39 | 3.23 | 3.22 |
| CP, % | 15.75 | 15.77 | 15.77 | 15.63 | 15.62 | 15.63 | 13.87 | 13.86 | 13.86 |
| Total Lysine | 0.98 | 0.98 | 0.98 | 0.85 | 0.85 | 0.85 | 0.72 | 0.73 | 0.73 |
| Total methionine | 0.28 | 0.29 | 0.29 | 0.28 | 0.29 | 0.29 | 0.23 | 0.22 | 0.22 |
| Total Threonine | 0.65 | 0.67 | 0.67 | 0.60 | 0.60 | 0.60 | 0.52 | 0.52 | 0.53 |
| Total Tryptophan | 0.18 | 0.18 | 0.18 | 0.17 | 0.18 | 0.18 | 0.14 | 0.16 | 0.16 |
| Ca, % | 0.59 | 0.59 | 0.59 | 0.53 | 0.54 | 0.54 | 0.47 | 0.47 | 0.47 |
| TP, % | 0.55 | 0.52 | 0.52 | 0.52 | 0.49 | 0.49 | 0.49 | 0.44 | 0.44 |
| Gene | Primers | Accession No | Product Length (bp) |
|---|---|---|---|
| IL-10 | F: CACTGCTCTATTGCCTGATCTTCC R: AAACTCTTCACTGGGCCGAAG | NM_214041.1 | 136 |
| TNF-α | F: TGGCCCCTTGAGCATCA R: CGGGCTTATCTGAGGTTTGAGA | NM_214022.1 | 68 |
| IL-1β | F: TACCCTCTCCAGCCAGTCTTCA R: AGGTCCAGGTTTTGGGTGCAG | NM_214055.1 | 167 |
| IL-6 | F: AGGGAAATGTCGAGGCTGTGC R: CCGGCATTTGTGGTGGGGTT | NM_214399.1 | 112 |
| IL-8 | F: AGGACCAGAGCCAGGAAGAGAC R: CACAGAGAGCTGCAGAAAGCAG | NM_213867.1 | 108 |
| ZO-1 | F: CAGCCCCCGTACATGGAGA R: GCGCAGACGGTGTTCATAGTT | XM_021098896.1 | 114 |
| Occludin | F: ATGCCTCCTCCCCTTTCGGA R: CGCCCGTCGTGTAGTCTGTC | NM_001163647.2 | 295 |
| Claudin-1 | F: GATCGGCTCCA TCGTCAGCA R: CATTGACTGGGGTCATGGGGTC | NM_001244539.1 | 416 |
| β-actin | F: TCTGGCACCACACCTTCT R: TGATCTGGGTCATCTTCTCAC | XM_021086047.1 | 114 |
| Item | CON | GRA | YC | p Value |
|---|---|---|---|---|
| BW (kg) | ||||
| 0 d | 45.61 ± 0.98 | 45.76 ± 1.02 | 46.82 ± 0.91 | 0.642 |
| 30 d | 79.04 ± 1.06 | 79.01 ± 1.31 | 80.21 ± 1.56 | 0.771 |
| 60 d | 111.55 ± 0.70 | 111.40 ± 2.42 | 112.46 ± 2.36 | 0.921 |
| ADG (g) | ||||
| 0–30 d, g | 1078.28 ± 20.56 | 1072.69 ± 14.73 | 1076.99 ± 22.69 | 0.978 |
| 31–60 d, g | 1083.78 ± 31.27 | 1079.56 ± 38.19 | 1075.11 ± 38.19 | 0.987 |
| 0–60 d, g | 1080.98 ± 18.56 | 1076.07 ± 24.19 | 1076.07 ± 26.13 | 0.985 |
| ADFI (g) | ||||
| 0–30 d | 2960.69 ± 47.00 | 2860.75 ± 84.15 | 2926.50 ± 77.37 | 0.615 |
| 31–60 d | 3340.67 ± 137.53 | 3389.89 ± 173.17 | 3269.53 ± 155.70 | 0.862 |
| 0–60 d | 3147.57 ± 89.82 | 3120.98 ± 117.20 | 3095.20 ± 113.70 | 0.943 |
| F:G ratio | ||||
| 0–30 d | 2.75 ± 0.05 | 2.73 ± 0.02 | 2.72 ± 0.03 | 0.851 |
| 31–60 d | 3.09 ± 0.12 | 3.14 ± 0.10 | 3.04 ± 0.10 | 0.828 |
| 0–60 d | 2.91 ± 0.06 | 2.90 ± 0.08 | 2.88 ± 0.05 | 0.928 |
| Cost/kg of gain (US$) | ||||
| 0–30 d | 1.52 ± 0.03 a | 1.42 ± 0.01 b | 1.45 ± 0.02 ab | 0.040 |
| 31–60 d | 1.78 ± 0.07 | 1.66 ± 0.05 | 1.55 ± 0.05 | 0.130 |
| 0–60 d | 1.65 ± 0.07 A | 1.54 ± 0.06 B | 1.58 ± 0.06 AB | 0.080 |
| Item | CON | GRA | YC | p Value |
|---|---|---|---|---|
| Eye muscle area, cm2 | 53.63 ± 2.66 | 54.39 ± 2.61 | 49.32 ± 1.73 | 0.301 |
| Backfat thickness, mm | 17.03 ± 0.47 | 18.96 ± 1.89 | 19.07 ± 1.38 | 0.627 |
| Dressing percentage, % | 74.15 ± 0.23 A | 75.58 ± 0.52 AB | 76.30 ± 0.31 B | 0.060 |
| Carcass length/cm | 83.70 ± 1.93 | 84.36 ± 0.86 | 85.68 ± 0.63 | 0.612 |
| Abdominal fat index, % | 1.05 ± 0.07 | 1.06 ± 0.09 | 1.15 ± 0.15 | 0.789 |
| Heart index, % | 0.36 ± 0.01 | 0.34 ± 0.01 | 0.34 ± 0.01 | 0.474 |
| Liver index, % | 1.46 ± 0.04 | 1.45 ± 0.05 | 1.43 ± 0.03 | 0.878 |
| Spleen index, % | 0.14 ± 0.01 | 0.13 ± 0.00 | 0.15 ± 0.01 | 0.370 |
| Lung index, % | 0.65 ± 0.07 | 0.62 ± 0.03 | 0.62 ± 0.07 | 0.912 |
| Kidney index, % | 0.31 ± 0.01 a | 0.28 ± 0.01 ab | 0.27 ± 0.01 b | 0.011 |
| Items | CON | GRA | YC | p Value |
|---|---|---|---|---|
| pH45min | 6.34 ± 0.09 | 6.08 ± 0.16 | 6.16 ± 0.18 | 0.475 |
| pH24h | 5.43 ± 0.03 | 5.42 ± 0.03 | 5.41 ± 0.02 | 0.888 |
| pH48h | 5.45 ± 0.02 | 5.42 ± 0.03 | 5.44 ± 0.02 | 0.727 |
| Drip loss, % | 2.33 ± 0.41 | 2.95 ± 0.30 | 3.05 ± 0.24 | 0.303 |
| Cooking loss, % | 34.87 ± 1.07 | 34.49 ± 0.82 | 34.67 ± 1.00 | 0.965 |
| Marbling score | 3.75 ± 0.22 | 3.25 ± 0.31 | 3.20 ± 0.15 | 0.189 |
| 45 min | ||||
| L*(lightness) | 42.19 ± 0.47 A | 44.80 ± 0.50 B | 44.35 ± 0.37 B | 0.063 |
| a* (redness) | 8.27 ± 0.40 | 7.66 ± 0.39 | 7.39 ± 0.40 | 0.330 |
| b* (yellowness) | 6.77 ± 0.33 | 7.29 ± 0.19 | 7.18 ± 0.12 | 0.421 |
| 24 h | ||||
| L*(lightness) | 51.41 ± 0.37 | 52.65 ± 0.30 | 52.06 ± 0.25 | 0.129 |
| a* (redness) | 11.88 ± 0.57 A | 12.54 ± 0.31 AB | 12.82 ± 0.26 B | 0.067 |
| b* (yellowness) | 9.53 ± 0.15 B | 9.96 ± 0.12 B | 8.74 ± 0.27 A | 0.052 |
| Item | CON | GRA | YC | p Value |
|---|---|---|---|---|
| D 30 | ||||
| Total protein, g/L | 70.43 ± 3.45 | 68.33 ± 5.46 | 68.45 ± 3.63 | 0.927 |
| Albumin, g/L | 38.58 ± 1.81 | 35.90 ± 2.17 | 37.55 ± 0.73 | 0.539 |
| GLU, mmol/L | 4.23 ± 0.39 | 3.78 ± 0.35 | 5.13 ± 0.62 | 0.151 |
| UN, mmol/L | 6.95 ± 0.78 | 5.95 ± 0.69 | 6.70 ± 0.54 | 0.693 |
| D 60 | ||||
| Total protein, g/L | 67.87 ± 3.02 a | 61.35 ± 2.81 ab | 55.00 ± 1.66 b | 0.048 |
| Albumin, g/L | 39.25 ± 2.47 a | 32.67 ± 2.23 ab | 28.28 ± 2.61 b | 0.041 |
| GLU, mmol/L | 4.25 ± 0.51 B | 3.14 ± 0.26 A | 3.04 ± 0.36 A | 0.085 |
| UN, mmol/L | 7.92 ± 0.73 a | 6.35 ± 0.73 ab | 4.84 ± 0.57 b | 0.038 |
| Item | CON | GRA | YC | p Value |
|---|---|---|---|---|
| D 30 | ||||
| CAT, U/mL | 1.93 ± 0.29 | 1.68 ± 0.27 | 1.48 ± 0.28 | 0.557 |
| T-AOC, U/mL | 4.70 ± 0.29 | 5.05 ± 0.43 | 4.57 ± 0.37 | 0.643 |
| T-SOD, U/mL | 73.69 ± 1.52 a | 80.13 ± 1.86 b | 82.13 ± 0.49 b | 0.002 |
| MDA, nmol/mL | 3.08 ± 0.27 | 2.92 ± 0.19 | 3.02 ± 0.43 | 0.936 |
| D 60 | ||||
| CAT, U/mL | 1.73 ± 0.16 a | 1.71 ± 0.43 a | 3.50 ± 0.36 b | 0.048 |
| T-AOC, U/mL | 4.68 ± 0.28 | 4.53 ± 0.23 | 4.04 ± 0.34 | 0.279 |
| T-SOD, U/mL | 82.79 ± 1.47 | 82.69 ± 1.99 | 79.13 ± 1.50 | 0.243 |
| MDA, nmol/mL | 2.50 ± 0.21 A | 2.74 ± 0.17 AB | 2.26 ± 0.19 B | 0.050 |
| Items | CON | GRA | YC | p Value |
|---|---|---|---|---|
| Jejunum | ||||
| VH, μm | 359.65 ± 17.10 | 346.78 ± 19.98 | 351.95 ± 24.60 | 0.908 |
| CD, μm | 108.94 ± 6.13 | 104.44 ± 10.54 | 91.32 ± 4.32 | 0.286 |
| VH/CD | 2.98 ± 0.24 | 3.42 ± 0.34 | 3.68 ± 0.05 | 0.104 |
| Ileum | ||||
| VH, μm | 370.16 ± 10.16 | 377.86 ± 13.79 | 330.29 ± 26.14 | 0.178 |
| CD, μm | 115.70 ± 7.71 | 117.15 ± 5.65 | 105.15 ± 8.84 | 0.489 |
| VH/CD | 3.26 ± 0.22 | 3.27 ± 0.25 | 3.24 ± 0.40 | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Yu, C.; Ma, Z.; Che, L.; Feng, B.; Fang, Z.; Xu, S.; Zhuo, Y.; Li, J.; Zhang, J.; et al. Effects of Yeast Culture Supplementation in Wheat–Rice-Based Diet on Growth Performance, Meat Quality, and Gut Microbiota of Growing–Finishing Pigs. Animals 2022, 12, 2177. https://doi.org/10.3390/ani12172177
Lin Y, Yu C, Ma Z, Che L, Feng B, Fang Z, Xu S, Zhuo Y, Li J, Zhang J, et al. Effects of Yeast Culture Supplementation in Wheat–Rice-Based Diet on Growth Performance, Meat Quality, and Gut Microbiota of Growing–Finishing Pigs. Animals. 2022; 12(17):2177. https://doi.org/10.3390/ani12172177
Chicago/Turabian StyleLin, Yan, Chenglong Yu, Zhao Ma, Lianqiang Che, Bin Feng, Zhengfeng Fang, Shengyu Xu, Yong Zhuo, Jian Li, Junjie Zhang, and et al. 2022. "Effects of Yeast Culture Supplementation in Wheat–Rice-Based Diet on Growth Performance, Meat Quality, and Gut Microbiota of Growing–Finishing Pigs" Animals 12, no. 17: 2177. https://doi.org/10.3390/ani12172177
APA StyleLin, Y., Yu, C., Ma, Z., Che, L., Feng, B., Fang, Z., Xu, S., Zhuo, Y., Li, J., Zhang, J., Yang, M., Chen, P., & Wu, D. (2022). Effects of Yeast Culture Supplementation in Wheat–Rice-Based Diet on Growth Performance, Meat Quality, and Gut Microbiota of Growing–Finishing Pigs. Animals, 12(17), 2177. https://doi.org/10.3390/ani12172177