Effect of Microbial Phytase on Ileal Digestibility of Minerals, Plasma and Urine Metabolites, and Bone Mineral Concentrations in Growing–Finishing Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Feed Analysis
2.4. Sampling and Measurements
2.5. Statistical Analysis
3. Results
3.1. Influence of Phytase Level on the Content of Minerals in Chyme from the Small Intestine of Pigs
3.2. Effect of Phytase Level on the Content of Minerals in the Blood Plasma of Pigs
3.3. Effect of Phytase Level on Femur Mineral Concentrations
3.4. Effect of Phytase Level on Urinary and Fecal Mineral Excretion
3.5. Correlations between the Content of Minerals in the Small Intestinal Chyme, Blood, Femur, Feces, and Urine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015, 95, 878–896. [Google Scholar] [CrossRef]
- Tsai, T.C.; Dove, R.; Bedford, M.R.; Azain, M.J. Effect of phytase on phosphorous balance in 20-kg barrows fed low or adequate phosphorous diets. Anim. Nutr. 2020, 6, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef]
- Létourneau-Montminy, M.P.; Narcy, A.; Lescoat, P.; Bernier, J.F.; Magnin, M.; Sauvant, D.; Jondreville, C.; Pomar, C. Modeling the fate of dietary phosphorus in the digestive tract of growing pigs. J. Anim. Sci. 2011, 89, 3596–3611. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.M.; Komori, M.; Hattori, R.; Matsui, T. Dietary phytase increases the true absorption and endogenous fecal excretion of zinc in growing pigs given a corn-soybean meal based diet. Anim. Sci. J. 2009, 80, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.M.; Parr, T.M.; Parr, E.N.; Webel, D.M.; Baker, D.H. The effects of phytase on growth performance, carcass characteristics, and bone mineralization of late-finishing pigs fed maize-soyabean meal diets containing no supplemental phosphorus, zinc, copper and manganese. Anim. Feed Sci. Technol. 2001, 94, 199–205. [Google Scholar] [CrossRef]
- Grela, E.R.; Kumek, R. Effect of feed supplementation with phytase and formic acid on piglet performance and composition of sow colostrum and milk. Vet. Med. 2002, 58, 375–377. [Google Scholar]
- Czech, A.; Grela, E.R. Biochemical and haematological blood parameters of sows during pregnancy and lactation fed the diet with different source and activity of phytase. Anim. Feed Sci. Technol. 2004, 116, 211–223. [Google Scholar] [CrossRef]
- Grela, E.R.; Muszyński, S.; Czech, A.; Donaldson, J.; Stanisławski, P.; Kapica, M.; Brezvyn, O.; Muzyka, V.; Kotsyumbas, I.; Tomaszewska, E. Influence of phytase supplementation at increasing doses from 0 to 1500 FTU/kg on growth performance, nutrient digestibility, and bone status in grower-finisher pigs fed phosphorus-deficient diets. Animals 2020, 10, 847. [Google Scholar] [CrossRef]
- Hong, B.; Kim, B.G. Supplemental phytase increases phosphorus digestibility in pigs regardless of phytase source or feed pelleting. Anim. Feed Sci. Technol. 2021, 276, 114901. [Google Scholar] [CrossRef]
- Silversides, G.; Scott, T.A.; Bedford, M.R. The effect of phytase enzyme and level on nutrient extraction by broilers. Poult. Sci. 2004, 83, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Augspurger, N.R.; Webel, D.M.; Lei, X.G.; Baker, D.H. Efficacy of an E. coli phytase expressed in yeast for releasing phytate-bound phosphorus in young chicks and pigs. J. Anim. Sci. 2003, 81, 474–483. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Engelen, A.J.; van der Heeft, F.C.; Randsdorp, P.H.; Somers, W.A.; Schaefer, J.; van der Vat, B.J. Determination of phytase activity in feed by a colorimetric enzymatic method: Collaborative interlaboratory study. J. AOAC Int. 2001, 84, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, T.D.; Peo, E.R., Jr.; Lewis, A.J.; Moser, B.D.; Olson, D.G. Influence of age, sex and calcium and phosphorus levels on the mechanical properties of various bones in swine. J. Anim. Sci. 1981, 52, 1319–1329. [Google Scholar] [CrossRef]
- Pointillart, A.; Guéguen, L. Meal-feeding and phosphorus ingestion influence calcium bioavailability evaluated by calcium balance and bone breaking strength in pigs. Bone Miner. 1993, 21, 75–81. [Google Scholar] [CrossRef]
- Shaw, D.T.; Rozeboom, D.W.; Hill, G.M.; Orth, M.W.; Rosenstein, D.S.; Link, J.E. Impact of supplement withdrawal and wheat middling inclusion on bone metabolism, bone strength, and the incidence of bone fractures occurring at slaughter in pigs. J. Anim. Sci. 2006, 84, 1138–1146. [Google Scholar] [CrossRef][Green Version]
- Storskrubb, A.; Sevón-Aimonen, M.L.; Uimari, P. Genetic parameters for bone strength, osteochondrosis and meat percentage in Finnish Landrace and Yorkshire pigs. Animal 2010, 4, 1319–1324. [Google Scholar] [CrossRef]
- Saraiva, A.; Donzele, J.L.; Oliveira, R.F.M.; Abreu, M.L.T.; Silva, F.C.O.; Guimarães, S.E.F.; Kim, S.W. Phosphorus requirements for 60- to 100-kg pigs selected for high lean deposition under different thermal environments. J. Anim. Sci. 2012, 90, 1499–1505. [Google Scholar] [CrossRef]
- Santos, T.T.; Walk, C.L.; Wilcock, P.; Cordero, G.; Chewning, J. Performance and bone characteristics of growing pigs fed diets marginally deficient in available phosphorus and a novel microbial phytase. Can. J. Anim. Sci. 2014, 94, 493–497. [Google Scholar] [CrossRef]
- Teixeira, A.O.; Anderson Corassa, A.; Moreira, L.M.; Nogueira, E.T.; Lopes, J.B.; Rocha, C.M., Jr.; Ferreira, V.P.A. Bone characteristics of pigs fed different sources of phosphorus. Rev. Colom. Cienc. Pecua. 2016, 29, 245–254. [Google Scholar] [CrossRef]
- Arredondo, M.A.; Casas, G.A.; Stein, H.H. Increasing levels of microbial phytase increases the digestibility of energy and minerals in diets fed to pigs. Anim. Feed Sci. Technol. 2019, 248, 27–36. [Google Scholar] [CrossRef]
- Madrid, J.; Martínez, S.; López, C.; Hernández, F. Effect of phytase on nutrient digestibility, mineral utilization and performance in growing pigs. Livest. Sci. 2013, 154, 144–151. [Google Scholar] [CrossRef]
- Varley, P.F.; Callan, J.J.; O’Doherty, J.V. Effect of dietary phosphorus and calcium level and phytase addition on performance, bone parameters, apparent nutrient digestibility, mineral and nitrogen utilization of weaner pigs and the subsequent effect on finisher pig bone parameters. Anim. Feed Sci. Technol. 2011, 165, 201–209. [Google Scholar] [CrossRef]
- Lei, X.G.; Ku, P.K.; Miller, E.R.; Yokoyama, M.T. Supplementing corn-soybean meal diets with microbial phytase linearly improves phytate phosphorus utilization by weanling pigs. J. Anim. Sci. 1993, 71, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
- Gentile, J.M.; Roneker, K.R.; Crowe, S.E.; Pond, W.G.; Lei, X.G. Effectiveness of an experimental consensus phytase in improving dietary phytate phosphorus utilization by weanling pigs. J. Anim. Sci. 2003, 81, 2751–2757. [Google Scholar] [CrossRef]
- Jendza, J.A.; Dilger, R.N.; Adedokun, S.A.; Sands, J.S.; Adeola, O. Escherichia coli phytase improves growth performance of starter, grower, and finisher pigs fed phosphorus-deficient diets. J. Anim. Sci. 2005, 83, 1882–1889. [Google Scholar] [CrossRef]
- Rosenfelder-Kuon, P.; Siegert, W.; Rodehutscord, M. Effect of microbial phytase supplementation on P digestibility in pigs: A meta-analysis. Arch. Anim. Nutr. 2019, 74, 1–18. [Google Scholar] [CrossRef]
- Sung, J.Y.; Kim, B.G. Prediction models for apparent and standardized total tract digestible phosphorus in swine diets. Anim. Feed Sci. Technol. 2019, 255, 114224. [Google Scholar] [CrossRef]
- González-Vega, J.; Stein, H. Invited review—Calcium digestibility and metabolism in pigs. Anim. Biosci. 2014, 27, 1–9. [Google Scholar] [CrossRef]
- Stein, H.H.; Boersma, M.G.; Pedersen, C. Apparent and true total tract digestibility of phosphorus in field peas (Pisum sativum L.) by growing pigs. Can. J. Anim. Sci. 2006, 86, 523–525. [Google Scholar] [CrossRef]
- Stein, H.H.; Adeola, O.; Cromwell, G.L.; Kim, S.W.; Mahan, D.C.; Miller, P.S. Concentration of dietary calcium supplied by calcium carbonate does not affect the apparent total tract digestibility of calcium, but reduces digestibility of phosphorus by growing pigs. J. Anim. Sci. 2011, 89, 2139–2144. [Google Scholar] [CrossRef]
- Almeida, F.N.; Stein, H.H. Performance and phosphorus balance of pigs fed diets formulated on the basis of values for standardized total tract digestibility of phosphorus. Can. J. Anim. Sci. 2010, 88, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- González-Vega, J.C.; Walk, C.L.; Liu, Y.; Stein, H.H. The site of net absorption of Ca from the intestinal tract of growing pigs and effect of phytic acid, Ca level and Ca source on Ca digestibility. Arch. Anim. Nutr. 2014, 68, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Selle, P.H.; Ravindran, V. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 2008, 113, 99–122. [Google Scholar] [CrossRef]
- Zeng, Z.K.; Li, Q.Y.; Zhao, P.F.; Xu, X.; Tian, Q.Y.; Wang, H.L.; Pan, L.; Yu, S.; Piao, X.S. A new Buttiauxella phytase continuously hydrolyzes phytate and improves amino acid digestibility and mineral balance in growing pigs fed phosphorous-deficient diet. J. Anim. Sci. 2016, 94, 629–638. [Google Scholar] [CrossRef]
- She, Y.; Liu, Y.; González-Vega, J.C.; Stein, H.H. Effects of graded levels of an Escherichia coli phytase on growth performance, apparent total tract digestibility of phosphorus, and on bone parameters of weanling pigs fed phosphorus-deficient corn-soybean meal based diets. Anim. Feed Sci. Technol. 2018, 232, 102–109. [Google Scholar] [CrossRef]
- Adeola, O. Digestive utilization of minerals by weanling pigs fed copper-and phytase-supplemented diets. Can. J. Anim. Sci. 1995, 75, 603–610. [Google Scholar] [CrossRef]
- Bikker, P.; Jongbloed, A.W.; Thissen, J.T.N.M. Meta-analysis of effects of microbial phytase on digestibility and bioavailability of copper and zinc in growing pigs. J. Anim. Sci. 2012, 90, 134–136. [Google Scholar] [CrossRef]
- Espinosa, C.D.; Stein, H.H. Digestibility and metabolism of copper in diets for pigs and influence of dietary copper on growth performance, intestinal health, and overall immune status: A review. J. Anim. Sci. Biotechnol. 2021, 12, 13. [Google Scholar] [CrossRef]
- Collins, J.F.; Prohaska, J.R.; Knutson, M.D. Metabolic crossroads of iron and copper. Nutr. Rev. 2010, 68, 133–147. [Google Scholar] [CrossRef]
- Jiao, L.F.; Zhang, Q.H.; Wu, H.; Wang, C.C.; Cao, S.T.; Feng, J.; Hu, C.H. Influences of copper/zinc-loaded montmorillonite on growth performance, mineral retention, intestinal morphology, mucosa antioxidant capacity, and cytokine contents in weaned piglets. Biol. Trace Elem. Res. 2018, 185, 356–363. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.V.; Gahan, D.A.; O’Shea, C.; Callan, J.J.; Pierce, K.M. Effects of phytase and 25-hydroxyvitamin D3 inclusions on the performance, mineral balance and bone parameters of grower-finisher pigs fed low-phosphorus diets. Animal 2010, 4, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Klem, T.B.; Bleken, E.; Morberg, H.; Thoresen, S.I.; Framstad, T. Hematologic and biochemical reference intervals for Norwegian crossbreed grower pigs. Vet. Clin. Path. 2010, 39, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.I.; Pedersen, C.; Lindemann, M.D.; Stein, H.H. Relative bioavailability of phosphorus in inorganic phosphorus sources fed to growing pigs. J. Anim. Sci. 2011, 89, 460–466. [Google Scholar] [CrossRef]
- Haeney, R.P.; Abrams, S.; Dawsonhughes, B.; Looker, A.; Marcus, R.; Matkovic, V.; Weaver, C. Peak Bone Mass. Osteoporos. Int. 2000, 11, 985–1009. [Google Scholar] [CrossRef]
- Calvo, M.S.; Park, Y.K. Changing phosphorus content of the U.S. Diet: Potential for adverse effects on bone. J. Nutr. 1996, 126, 1168–1180. [Google Scholar] [CrossRef]
- Cashman, K.D. Diet, nutrition, and bone health. J. Nutr. 2007, 137 (Suppl. 11), 2507–2511. [Google Scholar] [CrossRef]
- Revy, P.S.; Jondreville, C.; Dourmad, J.Y.; Nys, Y. Effect of zinc supplemented as either an organic or an inorganic source and of microbial phytase on zinc and other minerals utilisation by weanling pigs. Anim. Feed Sci. Technol. 2004, 116, 93–112. [Google Scholar] [CrossRef]
- Adedokun, S.A.; Owusu-Asiedu, A.; Ragland, D.; Plumstead, P.; Adeola, O. The efficacy of a new 6-phytase obtained from Buttiauxella spp. expressed in Trichoderma reesei on digestibility of amino acids, energy, and nutrients in pigs fed a diet based on corn, soybean meal, wheat middlings, and corn distillers’ dried grains with solubles. J. Anim. Sci. 2015, 93, 168–175. [Google Scholar] [CrossRef]
- McCormick, K.; Walk, C.L.; Wyatt, C.L.; Adeola, O. Phosphorus utilization response of pigs and broiler chickens to diets supplemented with antimicrobials and phytase. Anim. Nutr. 2017, 3, 77–84. [Google Scholar] [CrossRef]
- Jendza, J.A.; Adeola, O. Water-soluble phosphorus excretion in pigs fed diets supplemented with microbial phytase. Anim. Sci. J. 2009, 80, 296–304. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Woyengo, T.A.; Cowieson, A.J.; Adeola, O.; Nyachoti, C.M. Ileal digestibility and endogenous flow of minerals and amino acids: Responses to dietary phytic acid in piglets. Br. J. Nutr. 2009, 102, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Principles of Animal Nutrition; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]


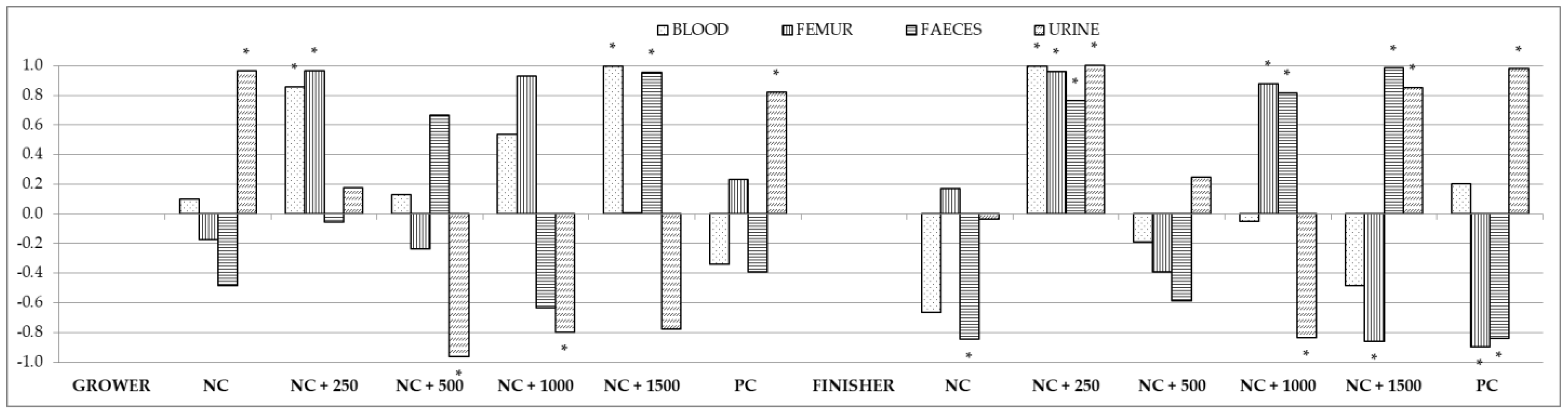
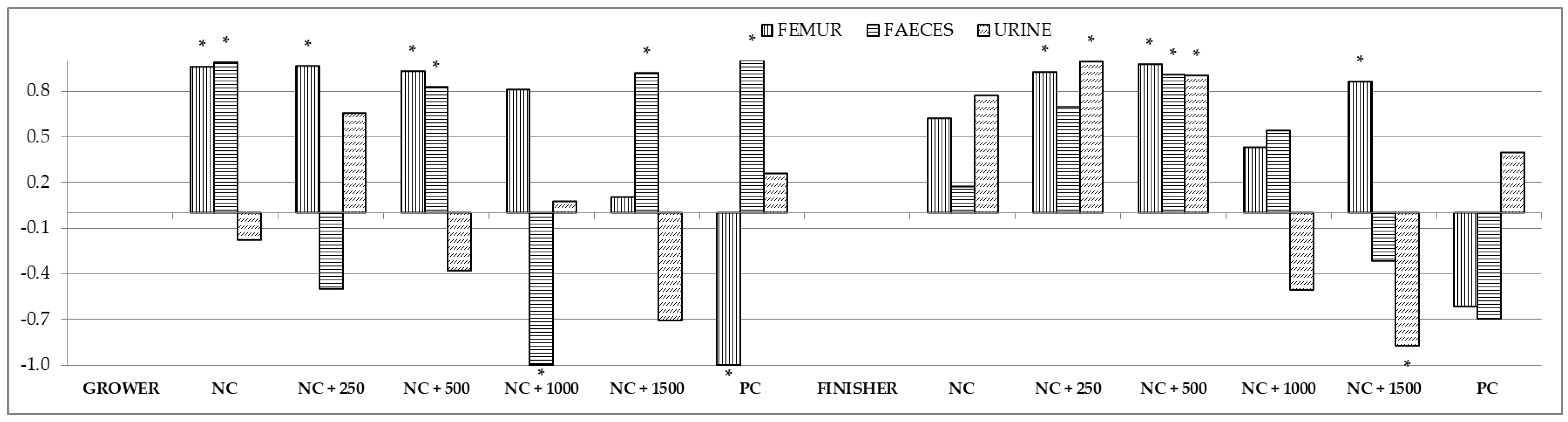
| Treatment 1 | NC | NC + 250 | NC + 500 | NC + 1000 | NC + 1500 | PC |
|---|---|---|---|---|---|---|
| Grower | ||||||
| Dry matter (g/kg) | 876.5 | 873.0 | 879.8 | 880.3 | 882.3 | 87.93 |
| Crude ash (g/kg) | 41.1 | 41.2 | 41.5 | 39.8 | 40.2 | 41.1 |
| Calcium (g/kg) | 5.51 | 5.45 | 5.47 | 5.49 | 5.57 | 6.62 |
| Total phosphorus (g/kg) | 4.82 | 4.74 | 4.75 | 4.78 | 4.83 | 5.69 |
| Magnesium (g/kg) | 1.82 | 1.83 | 1.82 | 1.83 | 1.84 | 1.82 |
| Iron (mg/kg) | 120.4 | 120.3 | 120.4 | 120.5 | 120.3 | 120.4 |
| Zinc (mg/kg) | 135.8 | 135.5 | 135.9 | 135.4 | 135.8 | 135.7 |
| Copper (mg/kg) | 20.3 | 20.1 | 20.2 | 20.3 | 20.2 | 20.3 |
| Phytase activity (FTU/kg) | 187 | 411 | 639 | 1081 | 1574 | 195 |
| Finisher | ||||||
| Dry matter (g/kg) | 871.6 | 867.7 | 869.2 | 871.3 | 868.9 | 869.7 |
| Crude ash (g/kg) | 42.6 | 43.2 | 42.8 | 43.9 | 40.1 | 43.6 |
| Calcium (g/kg) | 4.94 | 4.92 | 4.98 | 4.95 | 4.94 | 6.09 |
| Total phosphorus (g/kg) | 4.25 | 4.23 | 4.29 | 4.27 | 4.31 | 5.27 |
| Magnesium (g/kg) | 1.76 | 1.77 | 1.76 | 1.78 | 1.77 | 1.76 |
| Iron (mg/kg) | 105.4 | 105.7 | 105.3 | 105.8 | 105.7 | 105.4 |
| Zinc (mg/kg) | 124.3 | 125.1 | 124,6 | 125.2 | 124.6 | 124.7 |
| Copper (mg/kg) | 19.4 | 18.9 | 18.8 | 19.2 | 19.3 | 19.4 |
| Phytase activity (FTU/kg) | 209 | 394 | 699 | 1160 | 1577 | 205 |
| Treatment 1 | P | Ca | Mg | Cu | Zn | Fe | P | Ca | Mg | Cu | Zn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grower | Finisher | |||||||||||
| Ileum | g/kg DM | g/kg DM | g/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | g/kg DM | g/kg DM | g/kg DM | mg/kg DM | mg/kg DM | mg/kg DM |
| NC 2 | 9.60 b | 11.03 b | 2.69 b | 55.94 | 496.2 | 564.6 | 10.09 b | 11.97 b | 2.93 b | 56.91 | 536.8 | 573.6 |
| 250 3 | 9.30 b | 10.95 b | 2.63 b | 55.52 | 491.3 | 563.4 | 9.90 b | 11.77 b | 2.92 b | 55.28 | 531.2 | 572.8 |
| 500 3 | 9.13 b | 10.84 b | 2.38 c | 55.39 | 497.7 | 565.9 | 9.94 b | 11.54 b | 2.91 b | 51.78 | 532.1 | 580.6 |
| 1000 3 | 8.98 bc | 10.74 b | 2.47 c | 55.41 | 499.4 | 555.4 | 9.77 b | 11.45 b | 2.84 bc | 52.11 | 526.7 | 573.4 |
| 1500 3 | 8.92 c | 10.71 b | 2.16 d | 55.12 | 495.2 | 553.7 | 9.80 b | 11.61 b | 2.87 bc | 51.73 | 525.2 | 578.3 |
| PC 4 | 11.11 a | 12.56 a | 2.99 a | 55.97 | 497.4 | 571.9 | 12.04 a | 13.07 a | 3.03 a | 56.85 | 538.4 | 581.1 |
| SEM 5 | 0.089 | 0.320 | 0.041 | 0.981 | 7.70 | 10.94 | 0.396 | 0.486 | 0.018 | 3.63 | 8.46 | 6.50 |
| p-value | ||||||||||||
| TRT 6 | <0.001 | 0.003 | <0.001 | 0.987 | 0.988 | 0.610 | <0.001 | 0.027 | 0.026 | 0.547 | 0.992 | 0.998 |
| PHY 7 | 0.051 | 0.642 | 0.001 | 0.982 | 0.967 | 0.672 | 0.954 | 0.815 | 0.009 | 0.509 | 0.991 | 0.996 |
| Linear 8 | 0.003 | 0.125 | <0.001 | 0.557 | 0.840 | 0.207 | 0.471 | 0.328 | <0.001 | 0.109 | 0.626 | 0.766 |
| Quadratic 8 | 0.429 | 0.850 | <0.001 | 0.921 | 0.938 | 0.613 | 0.862 | 0.488 | <0.001 | 0.520 | 0.976 | 0.882 |
| Plasma | mmol/L | mmol/L | mmol/L | µmol/L | µmol/L | µmol/L | mmol/L | mmol/L | mmol/L | µmol/L | µmol/L | µmol/L |
| NC 2 | 1.63 c | 1.79 b | 0.694 | 30.27 b | 10.09 b | 40.10 | 1.93 c | 1.70 b | 0.741 | 31.40 b | 10.79 b | 40.45 |
| 250 3 | 2.39 ab | 2.17 a | 0.746 | 30.57 b | 11.38 b | 39.90 | 2.05 b | 2.18 a | 0.748 | 31.02 b | 11.11 b | 39.92 |
| 500 3 | 2.49 a | 2.16 a | 0.722 | 32.38 ab | 11.92 b | 41.17 | 2.14 ab | 2.05 a | 0.743 | 33.97 ab | 12.19 b | 41.93 |
| 1000 3 | 2.29 ab | 2.17 a | 0.744 | 35.93 a | 16.19 a | 41.28 | 2.10 ab | 2.12 a | 0.753 | 36.49 a | 14.37 a | 41.02 |
| 1500 3 | 2.13 b | 2.14 a | 0.723 | 34.27 a | 14.21 a | 41.88 | 2.15 ab | 2.14 a | 0.741 | 35.48 a | 14.56 a | 42.62 |
| PC 4 | 2.33 ab | 2.19 a | 0.743 | 33.59 ab | 11.51 b | 39.45 | 2.21 a | 2.17 a | 0.750 | 31.63 b | 11.38 b | 40.12 |
| SEM 5 | 0.050 | 0.025 | 0.009 | 0.687 | 0.533 | 0.305 | 0.018 | 0.017 | 0.005 | 0.506 | 0.342 | 0.320 |
| p-value | ||||||||||||
| TRT 6 | <0.001 | <0.001 | 0.591 | 0.011 | 0.002 | 0.071 | <0.001 | 0.009 | 0.973 | <0.001 | <0.001 | 0.072 |
| PHY 7 | <0.001 | <0.001 | 0.310 | 0.055 | 0.004 | 0.094 | <0.001 | 0.020 | 0.940 | 0.002 | <0.001 | 0.096 |
| Linear 8 | <0.001 | <0.001 | <0.001 | 0.009 | 0.001 | 0.039 | <0.001 | 0.097 | <0.001 | <0.001 | <0.001 | 0.028 |
| Quadratic 8 | <0.001 | <0.001 | 0.173 | 0.684 | 0.453 | 0.554 | 0.036 | 0.458 | 0.619 | 0.542 | 0.538 | 0.621 |
| Treatment 1 | P g/kg | Ca g/kg | Mg g/kg | Cu mg/kg | Zn mg/kg | Fe mg/kg | P g/kg | Ca g/kg | Mg g/kg | Cu mg/kg | Zn mg/kg | Fe mg/kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grower | Finisher | |||||||||||
| NC 2 | 196.6 b | 329.0 | 4.18 | 0.397 b | 167.6 c | 11.66 a | 213.7 b | 356.5 | 4.54 | 0.394 b | 185.3 b | 10.77 a |
| 250 3 | 197.8 b | 330.6 | 4.19 | 0.446 b | 170.9 bc | 12.04 a | 216.9 b | 359.0 | 4.56 | 0.591 a | 198.0 ab | 11.91 a |
| 500 3 | 204.7 a | 333.1 | 4.17 | 0.508 a | 177.2 a | 12.65 a | 245.2 a | 363.0 | 4.53 | 0.688 a | 207.8 ab | 12.85 a |
| 1000 3 | 198.3 b | 330.0 | 4.20 | 0.470 a | 180.8 a | 10.47 b | 222.7 b | 356.0 | 4.71 | 0.425 b | 222.5 a | 7.14 b |
| 1500 3 | 198.9 ab | 331.8 | 4.19 | 0.462 ab | 175.5 ab | 10.21 b | 230.1 ab | 367.5 | 4.63 | 0.352 b | 198.3 ab | 8.21 b |
| PC 4 | 204.3 a | 333.6 | 4.18 | 0.399 b | 169.1 c | 11.76 a | 239.6 a | 349.5 | 4.72 | 0.581 a | 189.0 ab | 11.83 a |
| SEM 5 | 0.635 | 0.807 | 0.014 | 0.008 | 0.806 | 0.131 | 4.34 | 3.93 | 0.153 | 0.051 | 7.71 | 0.747 |
| p-value | ||||||||||||
| TRT 6 | <0.001 | 0.546 | 0.994 | <0.001 | <0.001 | <0.001 | 0.001 | 0.146 | 0.833 | <0.001 | 0.033 | 0.001 |
| PHY 7 | <0.001 | 0.655 | 0.980 | <0.001 | <0.001 | <0.001 | 0.004 | 0.394 | 0.842 | <0.001 | 0.040 | 0.002 |
| Linear 8 | 0.178 | 0.447 | 0.764 | 0.002 | <0.001 | <0.001 | 0.030 | 0.213 | 0.427 | 0.139 | 0.050 | 0.004 |
| Quadratic 8 | 0.003 | 0.508 | 0.976 | <0.001 | <0.001 | <0.001 | 0.041 | 0.691 | 0.980 | <0.001 | 0.029 | 0.044 |
| Treatment 1 | P | Ca | Mg | Cu | Zn | Fe | P | Ca | Mg | Cu | Zn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grower | Finisher | |||||||||||
| Feces | g/kg DM | g/kg DM | g/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | g/kg DM | g/kg DM | g/kg DM | mg/kg DM | mg/kg DM | mg/kg DM |
| NC 2 | 4.64 b | 5.31 b | 2.41 b | 14.05 | 105.9 a | 91.60 | 3.40 b | 3.80 ab | 2.09 b | 8.23 | 56.86 a | 61.54 |
| 250 3 | 4.47 bc | 5.22 b | 2.18 bc | 13.96 | 102.7 a | 90.76 | 3.21 c | 3.61 c | 1.93 b | 8.19 | 56.21 ab | 61.49 |
| 500 3 | 4.30 cd | 5.20 b | 2.27 bc | 13.81 | 95.75 b | 90.01 | 3.18 c | 3.67 bc | 1.98 b | 8.16 | 54.59 ab | 62.11 |
| 1000 3 | 4.08 d | 5.16 b | 2.25 bc | 13.61 | 91.91 bc | 89.31 | 3.07 c | 3.62 bc | 1.78 c | 8.15 | 53.63 b | 62.10 |
| 1500 3 | 4.02 d | 5.18 b | 2.00 c | 13.38 | 89.04 c | 89.11 | 3.00 c | 3.82 ab | 1.83 c | 8.11 | 53.05 b | 62.43 |
| PC 4 | 5.05 a | 5.71 a | 2.31 a | 14.06 | 106.2 a | 91.45 | 3.68 a | 3.95 a | 2.31 a | 8.21 | 56.95 a | 61.61 |
| SEM 5 | 0.057 | 0.032 | 0.052 | 0.071 | 1.07 | 0.513 | 0.033 | 0.025 | 0.029 | 0.041 | 0.371 | 0.501 |
| p-value | ||||||||||||
| TRT 6 | <0.001 | <0.001 | <0.001 | 0.295 | <0.001 | 0.632 | <0.001 | <0.001 | <0.001 | 0.101 | 0.001 | 0.994 |
| PHY 7 | <0.001 | 0.097 | <0.001 | 0.337 | <0.001 | 0.632 | <0.001 | 0.001 | <0.001 | 0.966 | 0.005 | 0.984 |
| Linear 8 | <0.001 | 0.016 | 0.002 | 0.049 | <0.001 | 0.123 | <0.001 | 0.682 | 0.149 | 0.463 | <0.001 | 0.573 |
| Quadratic 8 | 0.220 | 0.192 | <0.001 | 0.698 | 0.390 | 0.785 | 0.003 | <0.001 | 0.002 | 0.976 | 0.778 | 0.982 |
| Urine | mmol/L | mmol/L | mmol/L | µmol/L | µmol/L | µmol/L | mmol/L | mmol/L | mmol/L | µmol/L | µmol/L | µmol/L |
| NC 2 | 1.46 ab | 1.11 a | 0.924 | 0.511 a | 3.87 c | 4.55 bc | 1.16 b | 1.19 a | 0.953 b | 0.633 a | 5.69 | 6.52 a |
| 250 3 | 1.35 b | 1.10 a | 0.859 | 0.501 b | 4.08 b | 4.35 bc | 1.10 c | 1.18 a | 0.950 b | 0.618 abc | 5.70 | 6.33 a |
| 500 3 | 1.35 b | 0.844 b | 0.842 | 0.487 b | 5.03 a | 5.63 a | 1.19 b | 1.13 ab | 0.943 b | 0.607 bc | 5.49 | 6.33 a |
| 1000 3 | 1.23 bc | 0.710 bc | 0.886 | 0.474 bc | 4.83 a | 5.69 a | 0.959 c | 1.04 b | 1.03 a | 0.592 c | 5.42 | 6.07 a |
| 1500 3 | 1.17 c | 0.655 c | 0.869 | 0.411 d | 4.68 a | 4.25 c | 0.852 d | 0.946 b | 1.04 a | 0.509 d | 4.86 | 5.70 b |
| PC 4 | 1.58 a | 1.10 a | 0.936 | 0.520 a | 3.88 c | 4.67 b | 1.46 a | 1.21 a | 0.961 b | 0.638 a | 5.79 | 6.53 a |
| SEM 5 | 0.035 | 0.048 | 0.015 | 0.009 | 0.023 | 0.144 | 0.047 | 0.025 | 0.010 | 0.011 | 0.083 | 0.077 |
| p-value | ||||||||||||
| TRT 6 | <0.001 | <0.001 | 0.422 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| PHY 7 | 0.002 | <0.001 | 0.647 | <0.001 | 0.002 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.002 | 0.001 |
| Linear 8 | <0.001 | <0.001 | 0.517 | <0.001 | 0.001 | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Quadratic 8 | 0.931 | 0.750 | 0.307 | 0.001 | 0.013 | <0.001 | <0.001 | 0.080 | 0.058 | <0.001 | 0.032 | 0.115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czech, A.; Samolińska, W.; Tomaszewska, E.; Muszyński, S.; Grela, E.R. Effect of Microbial Phytase on Ileal Digestibility of Minerals, Plasma and Urine Metabolites, and Bone Mineral Concentrations in Growing–Finishing Pigs. Animals 2022, 12, 1294. https://doi.org/10.3390/ani12101294
Czech A, Samolińska W, Tomaszewska E, Muszyński S, Grela ER. Effect of Microbial Phytase on Ileal Digestibility of Minerals, Plasma and Urine Metabolites, and Bone Mineral Concentrations in Growing–Finishing Pigs. Animals. 2022; 12(10):1294. https://doi.org/10.3390/ani12101294
Chicago/Turabian StyleCzech, Anna, Wioletta Samolińska, Ewa Tomaszewska, Siemowit Muszyński, and Eugeniusz R. Grela. 2022. "Effect of Microbial Phytase on Ileal Digestibility of Minerals, Plasma and Urine Metabolites, and Bone Mineral Concentrations in Growing–Finishing Pigs" Animals 12, no. 10: 1294. https://doi.org/10.3390/ani12101294
APA StyleCzech, A., Samolińska, W., Tomaszewska, E., Muszyński, S., & Grela, E. R. (2022). Effect of Microbial Phytase on Ileal Digestibility of Minerals, Plasma and Urine Metabolites, and Bone Mineral Concentrations in Growing–Finishing Pigs. Animals, 12(10), 1294. https://doi.org/10.3390/ani12101294







