The Effects of Prenatal Diet on Calf Performance and Perspectives for Fetal Programming Studies: A Meta-Analytical Investigation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
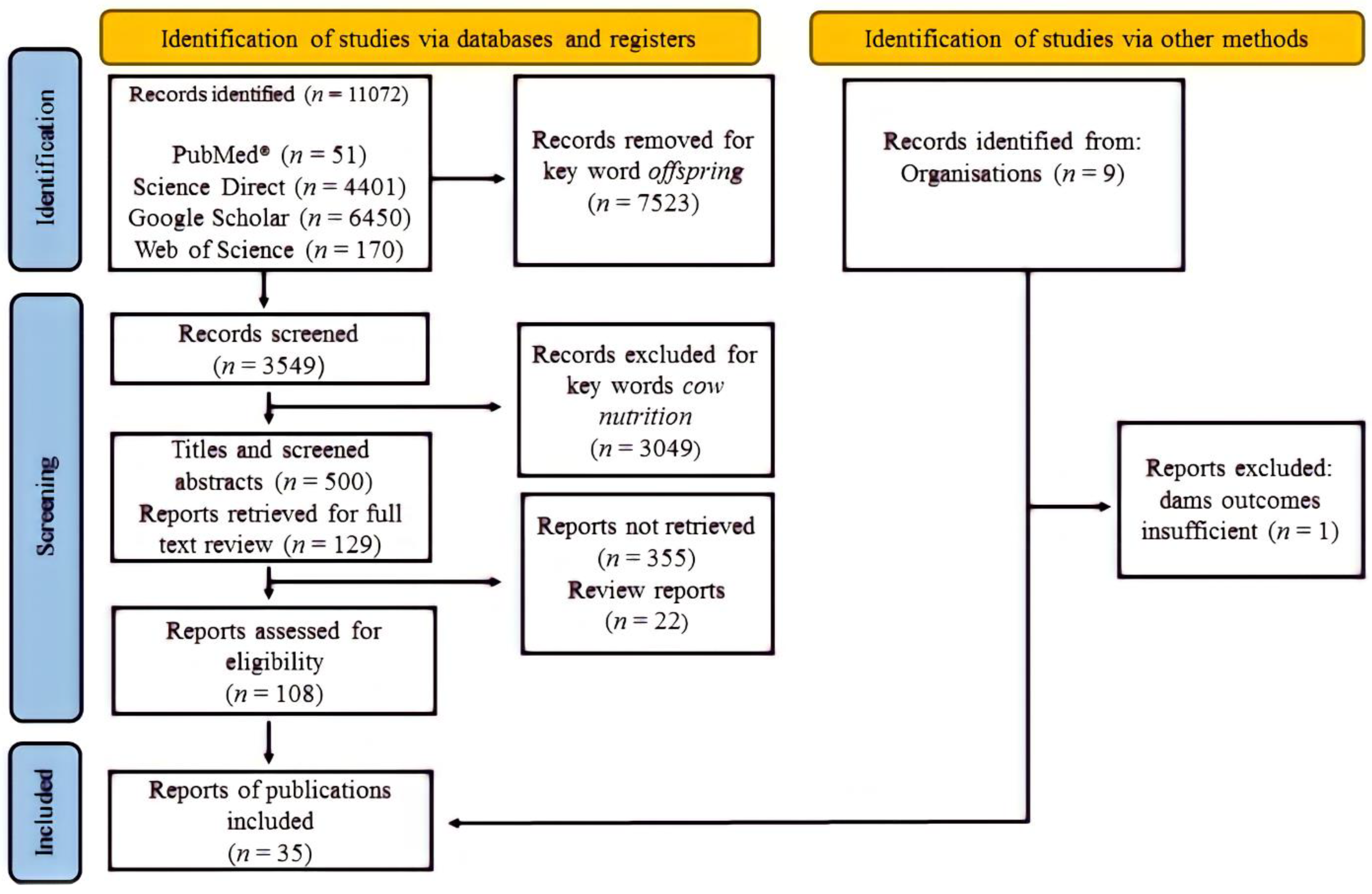
2.1. Study Selection Criteria and Relevance Screening
2.2. Process of Data Extraction
2.3. Data Analysis
3. Results
3.1. Dataset Characterization
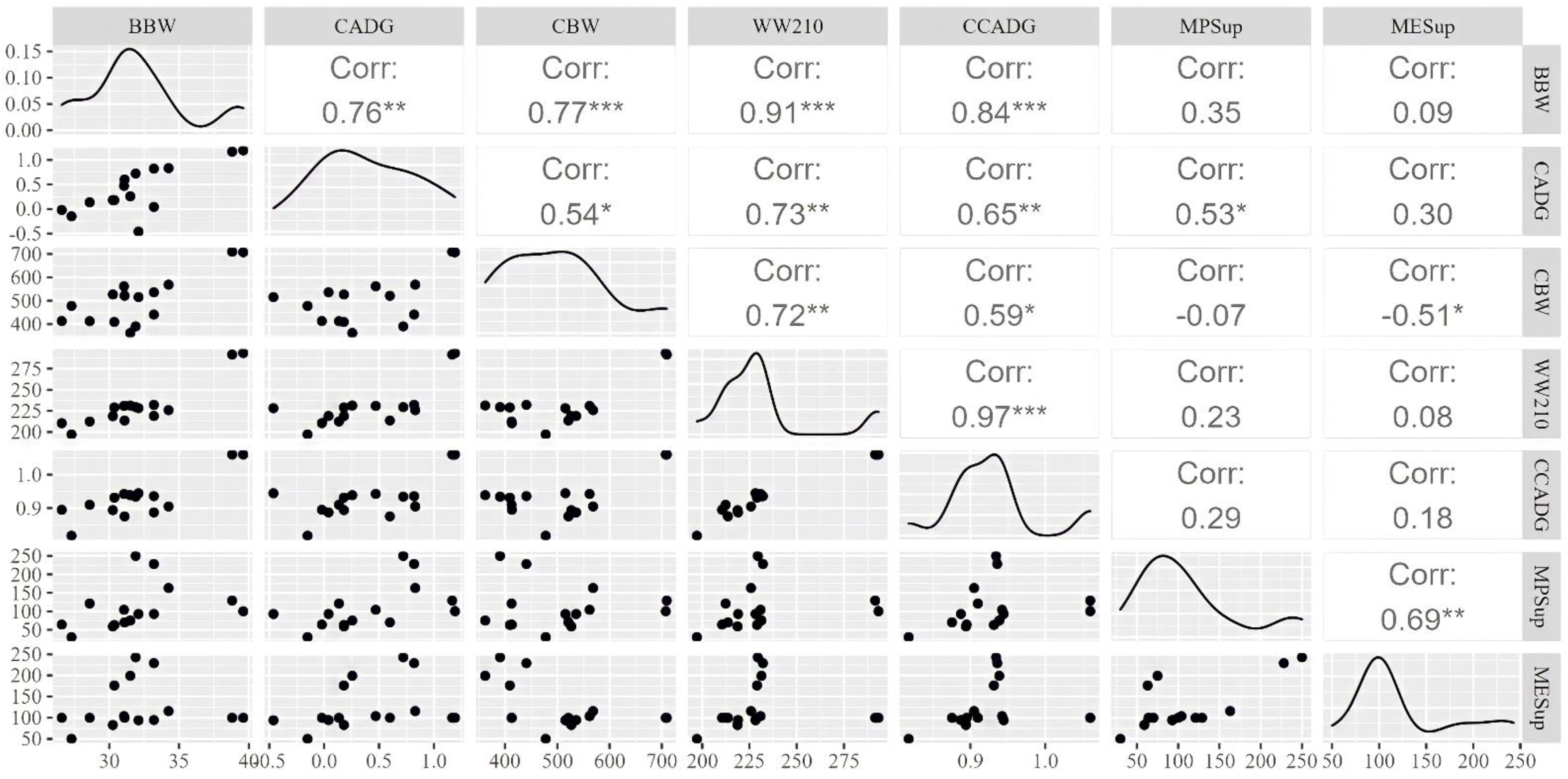
3.2. Correlations between Explanatory Variables and Interest Outcomes
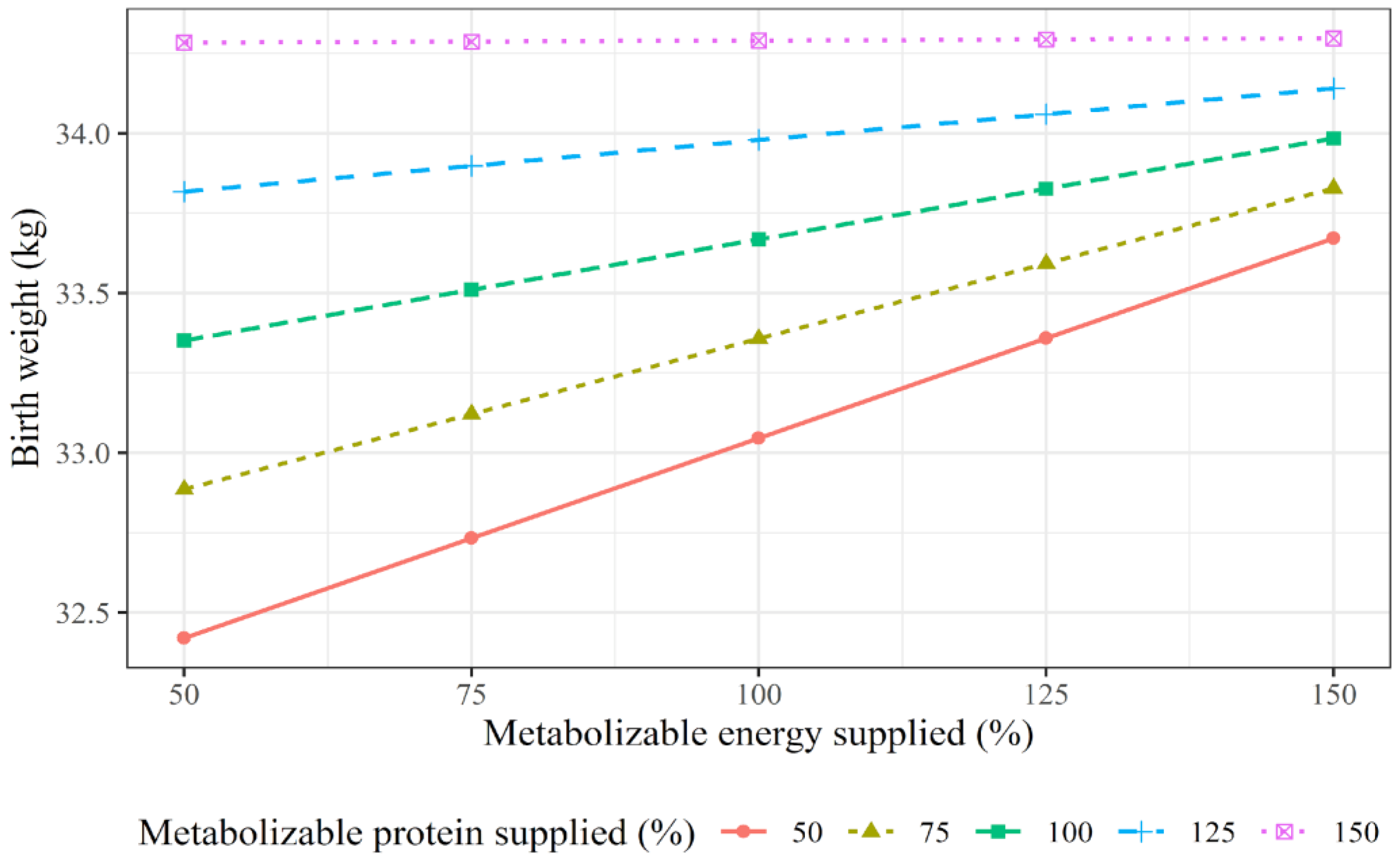
3.3. Predictor Variables of Cows’ ADG over Pregnancy and of Offspring Performance
4. Discussion
4.1. Factors That Can Affect the ADG of Beef Cows during Pregnancy
4.2. Maternal Aspects That May Affect the Offspring Performance Later in Life
4.3. Knowledge Gaps in the Scientific Literature Involving Studies concerning Gestational Nutrition of Beef Cattle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warner, R.; Greenwood, P.; Pethick, D.; Ferguson, D. Genetic and environmental effects on meat quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.; Cromie, A.; Berry, D. Genetic differences based on a beef terminal index are reflected in future phenotypic performance differences in commercial beef cattle. Animal 2016, 10, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.C.; Gionbelli, M.P.; Duarte, M.d.S. Fetal programming in ruminant animals: Understanding the skeletal muscle development to improve meat quality. Anim. Front. 2021, 11, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yan, X.; Tong, J.F.; Zhao, J.; Zhu, M.J. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol. Reprod. 2010, 82, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Zamudio, G.D.; da Cruz, W.F.; Schoonmaker, J.P.; de Resende, F.D.; Siqueira, G.R.; Neto, O.R.M.; Gionbelli, T.R.; Teixeira, P.D.; Rodrigues, L.M.; Gionbelli, M.P. Effect of rumen-protected fat on performance, carcass characteristics and beef quality of the progeny from Nellore cows fed by different planes of nutrition during gestation. Livest. Sci. 2022, 258, 104851. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathalielz, P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88, E51–E60. [Google Scholar] [CrossRef]
- Carvalho, E.B.; Costa, T.C.; Sanglard, L.P.; Nascimento, K.B.; Meneses, J.A.M.; Galvão, M.C.; Serão, N.V.L.; Duarte, M.S.; Gionbelli, M.P. Transcriptome profile in the skeletal muscle of cattle progeny as a function of maternal protein supplementation during mid-gestation. Livest. Sci. 2022, 263, 104995. [Google Scholar] [CrossRef]
- Hoffman, M.; Reed, S.; Pillai, S.; Jones, A.; McFadden, K.; Zinn, S.; Govoni, K. Physiology and endocrinology symposium: The effects of poor maternal nutrition during gestation on offspring postnatal growth and metabolism. J. Anim. Sci. 2017, 95, 2222–2232. [Google Scholar] [CrossRef]
- Zago, D.; Canozzi, M.E.A.; Barcellos, J.O.J. Pregnant beef cow’s nutrition and its effects on postnatal weight and carcass quality of their progeny. PLoS ONE 2020, 15, e0237941. [Google Scholar] [CrossRef]
- Bonnet, M.; Cassar-Malek, I.; Chilliard, Y.; Picard, B. Ontogenesis of muscle and adipose tissues and their interactions in ruminants and other species. Anim. Int. J. Anim. Biosci. 2010, 4, 1093. [Google Scholar] [CrossRef]
- McLean, K.J.; Boehmer, B.H.; Spicer, L.J.; Wettemann, R.P. The effects of protein supplementation of fall calving beef cows on pre-and postpartum plasma insulin, glucose and IGF-I, and postnatal growth and plasma insulin and IGF-I of calves. J. Anim. Sci. 2018, 96, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Maresca, S.; Valiente, S.L.; Rodriguez, A.M.; Long, N.M.; Pavan, E.; Quintans, G. Effect of protein restriction of bovine dams during late gestation on offspring postnatal growth, glucose-insulin metabolism and IGF-1 concentration. Livest. Sci. 2018, 212, 120–126. [Google Scholar] [CrossRef]
- Underwood, K.; Tong, J.; Price, P.; Roberts, A.; Grings, E.; Hess, B.; Means, W.; Du, M. Nutrition during mid to late gestation affects growth, adipose tissue deposition, and tenderness in cross-bred beef steers. Meat Sci. 2010, 86, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Schoonmaker, J.P.; Resende, F.D.; Siqueira, G.R.; Rodrigues Machado Neto, O.; Gionbelli, M.P.; Ramalho Santos Gionbelli, T.; Ladeira, M.M. Effects of protein supplementation on Nellore cows’ reproductive performance, growth, myogenesis, lipogenesis and intestine development of the progeny. Anim. Prod. Sci. 2020, 61, 371–380. [Google Scholar] [CrossRef]
- Gurevitch, J.; Koricheva, J.; Nakagawa, S.; Stewart, G. Meta-analysis and the science of research synthesis. Nature 2018, 555, 175–182. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 372. [Google Scholar] [CrossRef]
- Alvarenga, T.I.; Copping, K.J.; Han, X.; Clayton, E.H.; Meyer, R.J.; Rodgers, R.J.; McMillen, I.C.; Perry, V.E.; Geesink, G. The influence of peri-conception and first trimester dietary restriction of protein in cattle on meat quality traits of entire male progeny. Meat Sci. 2016, 121, 141–147. [Google Scholar] [CrossRef]
- Long, N.M.; Tousley, C.B.; Underwood, K.R.; Paisley, S.I.; Means, W.J.; Hess, B.W.; Du, M.; Ford, S.P. Effects of early- to mid-gestational undernutrition with or without protein supplementation on offspring growth, carcass characteristics, and adipocyte size in beef cattle1. J. Anim. Sci. 2012, 90, 197–206. [Google Scholar] [CrossRef]
- Micke, G.; Sullivan, T.; Gatford, K.; Owens, J.-A.; Perry, V. Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits. Anim. Reprod. Sci. 2010, 121, 208–217. [Google Scholar] [CrossRef]
- Stalker, L.A.; Adams, D.C.; Klopfenstein, T.J.; Feuz, D.M.; Funston, R.N. Effects of pre-and postpartum nutrition on reproduction in spring calving cows and calf feedlot performance. J. Anim. Sci. 2006, 84, 2582–2589. [Google Scholar] [CrossRef]
- Nascimento, K.B. Effects of Crude Protein Supplementation during Beef Cow’S Mid-Gestation on the Offspring Performance, Physiology and Metabolism. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2021. [Google Scholar]
- Meneses, J.A.M. Impacts of Protein Supplementation during Mid-Gestation of Beef Cows on Maternal Physiology, Skeletal Muscle, and Liver Tissues Metabolism. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2021. [Google Scholar]
- Oliveira, I. Production and Physiology of Pregnant Beef Cows Supplemented with Ruminally Degradable or Undegradable Protein under Low Protein Basal Diets during Mid-Gestation. Master’s Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2021. [Google Scholar]
- Summers, A.; Blair, A.; Funston, R. Impact of supplemental protein source offered to primiparous heifers during gestation on II. Progeny performance and carcass characteristics. J. Anim. Sci. 2015, 93, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Marquez, D.; Paulino, M.; Rennó, L.; Villadiego, F.; Ortega, R.; Moreno, D.; Martins, L.; De Almeida, D.; Gionbelli, M.; Manso, M. Supplementation of grazing beef cows during gestation as a strategy to improve skeletal muscle development of the offspring. Animal 2017, 11, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Maresca, S.; Valiente, S.L.; Rodriguez, A.M.; Testa, L.M.; Long, N.M.; Quintans, G.I.; Pavan, E. The influence of protein restriction during mid-to late gestation on beef offspring growth, carcass characteristic and meat quality. Meat Sci. 2019, 153, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.J.; Block, J.; Funston, R.; Underwood, K.; Legako, J.; Harty, A.; Salverson, R.; Olson, K.; Blair, A. Influence of maternal protein restriction in primiparous heifers during mid-and/or late-gestation on meat quality and fatty acid profile of progeny. Meat Sci. 2019, 152, 31–37. [Google Scholar] [CrossRef]
- Ferreira, R.L. Desempenho de Vacas Tabapuã Alimentadas Com Silage e Ração Total Contend Alta e Baixa Concentração de PNDRPNDR (Unpublished Data). Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2022. [Google Scholar]
- Larson, D.M.; Martin, J.L.; Adams, D.C.; Funston, R.N. Winter grazing system and supplementation during late gestation influence performance of beef cows and steer progeny. J. Anim. Sci. 2009, 87, 1147–1155. [Google Scholar] [CrossRef]
- Mulliniks, J.; Sawyer, J.; Mathis, C.; Cox, S.; Petersen, M. Winter protein management during late gestation alters range cow and steer progeny performance. J. Anim. Sci. 2012, 90, 5099–5106. [Google Scholar] [CrossRef]
- Mulliniks, J.; Mathis, C.; Cox, S.; Petersen, M. Supplementation strategy during late gestation alters steer progeny health in the feedlot without affecting cow performance. Anim. Feed Sci. Technol. 2013, 185, 126–132. [Google Scholar] [CrossRef]
- Bohnert, D.W.; Stalker, L.A.; Mills, R.R.; Nyman, A.; Falck, S.J.; Cooke, R.F. Late gestation supplementation of beef cows differing in BCS: Effects on cow and calf performance. J. Anim. Sci. 2013, 91, 5485–5491. [Google Scholar] [CrossRef]
- Wilson, T.; Schroeder, A.; Ireland, F.; Faulkner, D.B.; Shike, D. Effects of late gestation distillers grains supplementation on fall-calving beef cow performance and steer calf growth and carcass characteristics. J. Anim. Sci. 2015, 93, 4843–4851. [Google Scholar] [CrossRef]
- Wilson, T.; Faulkner, D.B.; Shike, D.W. Influence of late gestation drylot rations differing in protein degradability and fat content on beef cow and subsequent calf performance. J. Anim. Sci. 2015, 93, 5819–5828. [Google Scholar] [CrossRef]
- Wilson, T.; Long, N.; Faulkner, D.B.; Shike, D. Influence of excessive dietary protein intake during late gestation on drylot beef cow performance and progeny growth, carcass characteristics, and plasma glucose and insulin concentrations. J. Anim. Sci. 2016, 94, 2035–2046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cardenas, J.E.G. Nutritional and Metabolic Evaluation of Nellore Cows Supplemented or not during the Peripartum. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2017. [Google Scholar]
- Calderaro, L.V. Efeitos da Suplementação no Pré-Parto Sobre o Desempenho e Características Metabólicas de Vacas de Corte a Pasto. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2018. [Google Scholar]
- Moreno, D.P.S. Efeitos da Suplementação Estratégica Sobre a Resposta Produtiva e Status Nutricional em fêMeas Nelore em Pastagem Tropical. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2019. [Google Scholar]
- Adams, S.M. Nutrição Pré-Parto da Vaca e Seus Reflexos na Produção e Composição do Leite e no Desempenho do Bezerro. Master’s Thesis, Universidade Federal de Santa Maria, Santa Maria, Brazil, 2019. [Google Scholar]
- Lopes, R.; Sampaio, C.; Trece, A.; Teixeira, P.; Gionbelli, T.; Santos, L.; Costa, T.; Duarte, M.; Gionbelli, M. Impacts of protein supplementation during late gestation of beef cows on maternal skeletal muscle and liver tissues metabolism. Animal 2020, 14, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Radunz, A.; Fluharty, F.; Relling, A.E.; Felix, T.; Shoup, L.; Zerby, H.N.; Loerch, S. Prepartum dietary energy source fed to beef cows: II. Effects on progeny postnatal growth, glucose tolerance, and carcass composition. J. Anim. Sci. 2012, 90, 4962–4974. [Google Scholar] [CrossRef] [PubMed]
- Mohrhauser, D.; Taylor, A.; Gonda, M.; Underwood, K.; Pritchard, R.; Wertz-Lutz, A.; Blair, A. The influence of maternal energy status during mid-gestation on beef offspring tenderness, muscle characteristics, and gene expression. Meat Sci. 2015, 110, 201–211. [Google Scholar] [CrossRef]
- Ramírez, M.; Testa, L.M.; Valiente, S.L.; Latorre, M.E.; Long, N.M.; Rodriguez, A.M.; Pavan, E.; Maresca, S. Maternal energy status during late gestation: Effects on growth performance, carcass characteristics and meat quality of steers progeny. Meat Sci. 2020, 164, 108095. [Google Scholar] [CrossRef]
- Wilson, T.; Faulkner, D.; Shike, D. Influence of prepartum dietary energy on beef cow performance and calf growth and carcass characteristics. Livest. Sci. 2016, 184, 21–27. [Google Scholar] [CrossRef]
- Shoup, L.; Wilson, T.; González-Peña, D.; Ireland, F.; Rodriguez-Zas, S.; Felix, T.; Shike, D. Beef cow prepartum supplement level and age at weaning: II. Effects of developmental programming on performance and carcass composition of steer progeny. J. Anim. Sci. 2015, 93, 4936–4947. [Google Scholar] [CrossRef]
- Moura, F.H.; Costa, T.C.; Trece, A.S.; de Melo, L.P.; Manso, M.R.; Paulino, M.F.; Rennó, L.N.; Fonseca, M.A.; Detmann, E.; Gionbelli, M.P. Effects of energy-protein supplementation frequency on performance of primiparous grazing beef cows during pre and postpartum. Asian-Australas. J. Anim. Sci. 2020, 33, 1430. [Google Scholar] [CrossRef]
- Ferreira, M.F.L.; Rennó, L.N.; Detmann, E.; Paulino, M.F.; de Campos Valadares Filho, S.; Moreira, S.S.; Martins, H.C.; de Oliveira, B.I.C.; Marquez, J.A.; de Paula Cidrine, I. Performance, metabolic and hormonal responses of grazing Nellore cows to an energy-protein supplementation during the pre-partum phase. BMC Vet. Res. 2020, 16, 108. [Google Scholar]
- Chenoweth, P. Aspects of reproduction in female Bos indicus cattle: A review. Aust. Vet. J. 1994, 71, 422–426. [Google Scholar] [CrossRef]
- Gionbelli, M.P.; de Campos Valadares Filho, S.; de Souza Duarte, M. Exigências nutricionais para vacas de corte vazias e gestantes. In Tabelas de Exigências Nutricionais de Zebuínos BR CORTE 3ª Edição, 3rd ed.; Filho, S.d.C.V., Silva, L.F.C.e., Gionbelli, M.P., Rotta, P.P., Marcondes, M.I., Chizzotti, M.L., Prados, L.F., Eds.; Produção Independente: Viçosa, Brazil, 2016; pp. 259–282. [Google Scholar]
- St-Pierre, N. Invited review: Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 2001, 84, 741–755. [Google Scholar] [CrossRef]
- Sauvant, D.; Letourneau-Montminy, M.; Schmidely, P.; Boval, M.; Loncke, C.; Daniel, J. Use and misuse of meta-analysis in Animal Science. Animal 2020, 14, s207–s222. [Google Scholar] [CrossRef] [PubMed]
- Kaps, M.; Lamberson, W.R. Biostatistics for Animal Science; CABI Publishing: Wallingford, UK, 2004; p. 445. [Google Scholar]
- Akaike, H. A new look at the statistical model indentification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Gionbelli, M.P.; Valadares Filho, S.; Duarte, M. Nutritional requirements for pregnant and non-pregnant beef cows. In Nutrient Requirements of Zebu and Crossbred Cattle, 3rd ed.; Valadares Filho, S.C., Costa e Silva, L.F.C., Gionbelli, M.P., Rotta, P.P., Marcondes, M.I., Chizzotti, M.L., Prados, L.F., Eds.; Suprema Gráfica Ltda: Viçosa, Brazil, 2016; pp. 251–272. [Google Scholar]
- Rosa, G.; Valente, B. Breeding and genetics symposium: Inferring causal effects from observational data in livestock. J. Anim. Sci. 2013, 91, 553–564. [Google Scholar] [CrossRef]
- Jennings, T.; Gonda, M.; Underwood, K.; Wertz-Lutz, A.; Blair, A. The influence of maternal nutrition on expression of genes responsible for adipogenesis and myogenesis in the bovine fetus. Animal 2016, 10, 1697–1705. [Google Scholar] [CrossRef]
- Bell, A.; Ehrhardt, R. Regulation of macronutrient partitioning between maternal and conceptus tissues in the pregnant ruminant. In Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction; Cronjé, P.B., Ed.; CABI International: Wallingford, UK, 2000; pp. 275–293. [Google Scholar]
- Mellor, D.; Flint, D.; Vernon, R.; Forsyth, I. Relationships between plasma hormone concentrations, udder development and the production of early mammary secretions in twin-bearing ewes on different planes of nutrition. Q. J. Exp. Physiol. Transl. Integr. 1987, 72, 345–356. [Google Scholar] [CrossRef]
- Swanson, T.; Hammer, C.; Luther, J.; Carlson, D.; Taylor, J.; Redmer, D.; Neville, T.; Reed, J.; Reynolds, L.; Caton, J. Effects of gestational plane of nutrition and selenium supplementation on mammary development and colostrum quality in pregnant ewe lambs. J. Anim. Sci. 2008, 86, 2415–2423. [Google Scholar] [CrossRef]
- Pfeifer, L.F.M.; Rodrigues, W.B.; Nogueira, E. Relationship between body condition score index and fertility in beef cows subjected to timed artificial insemination. Livest. Sci. 2021, 248, 104482. [Google Scholar] [CrossRef]
- Pfeifer, L.F.; Castro, N.A.; Neves, P.M.; Cestaro, J.P.; Siqueira, L.G. Development and validation of an objective method for the assessment of body condition scores and selection of beef cows for timed artificial insemination. Livest. Sci. 2017, 197, 82–87. [Google Scholar] [CrossRef]
- Dickinson, S.E.; Elmore, M.F.; Kriese-Anderson, L.; Elmore, J.B.; Walker, B.N.; Dyce, P.W.; Rodning, S.P.; Biase, F.H. Evaluation of age, weaning weight, body condition score, and reproductive tract score in pre-selected beef heifers relative to reproductive potential. J. Anim. Sci. Biotechnol. 2019, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Keisler, D.H.; Lucy, M.C. Perception and interpretation of the effects of undernutrition on reproduction. J. Anim. Sci. 1996, 74, 1–17. [Google Scholar] [CrossRef]
- Barb, C.; Kraeling, R. Role of leptin in the regulation of gonadotropin secretion in farm animals. Anim. Reprod. Sci. 2004, 82, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Starbuck, M.J.; Dailey, R.A.; Inskeep, E.K. Factors affecting retention of early pregnancy in dairy cattle. Anim. Reprod. Sci. 2004, 84, 27–39. [Google Scholar] [CrossRef]
- Banchero, G.E.; Clariget, R.P.; Bencini, R.; Lindsay, D.R.; Milton, J.T.; Martin, G.B. Endocrine and metabolic factors involved in the effect of nutrition on the production of colostrum in female sheep. Reprod. Nutr. Dev. 2006, 46, 447–460. [Google Scholar] [CrossRef]
- Akers, R.M. A 100-Year Review: Mammary development and lactation. J. Dairy Sci. 2017, 100, 10332–10352. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Hadsell, D.L.; Haymond, M.W. Gene regulation of UDP-galactose synthesis and transport: Potential rate-limiting processes in initiation of milk production in humans. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E365–E376. [Google Scholar] [CrossRef]
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef]
- Meyer, A.; Reed, J.; Neville, T.; Thorson, J.; Maddock-Carlin, K.; Taylor, J.; Reynolds, L.; Redmer, D.; Luther, J.; Hammer, C. Nutritional plane and selenium supply during gestation affect yield and nutrient composition of colostrum and milk in primiparous ewes. J. Anim. Sci. 2011, 89, 1627–1639. [Google Scholar] [CrossRef]
- Prayaga, K. Evaluation of beef cattle genotypes and estimation of direct and maternal genetic effects in a tropical environment. 1. Growth traits. Aust. J. Agric. Res. 2003, 54, 1013–1025. [Google Scholar] [CrossRef]
- Domokos, Z.; Zandoki, R.V.; Tőzsér, J. Change of body condition of charolais cows in relation of birth and weaning weight of calves, process of calving and period until next pregnancy in two stock herds. Bull. UASVM Anim. Sci. Biotechnol. Lond. 2011, 133, 6–14. [Google Scholar]
- Greenwood, P.L.; Cafe, L.M. Prenatal and pre-weaning growth and nutrition of cattle: Long-term conseguences for beef production. Animal 2007, 1, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, L.; Wang, Z.; Deng, M.; Nie, H.; Zhang, G.; Ma, T.; Wang, F. N-carbamylglutamate and L-arginine improved maternal and placental development in underfed ewes. Reproduction 2016, 151, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.S.; Gionbelli, M.P.; Paulino, P.V.R.; Serão, N.V.L.; Nascimento, C.S.; Botelho, M.E.; Martins, T.S.; Filho, S.C.V.; Dodson, M.V.; Guimarães, S.E.F.; et al. Maternal overnutrition enhances mRNA expression of adipogenic markers and collagen deposition in skeletal muscle of beef cattle fetuses. J. Anim. Sci. 2014, 92, 3846–3854. [Google Scholar] [CrossRef] [PubMed]
- Cafe, L.M.; Hennessy, D.W.; Hearnshaw, H.; Morris, S.G.; Greenwood, P.L. Influences of nutrition during pregnancy an lactation on birth weights and growth to weaning of calves sired by Piedmontese or Wagyu bulls. Austr. J. Exp. Agric. 2006, 46, 245–255. [Google Scholar] [CrossRef]
- Thornton, K.J. Impacts of nutrition on the proliferation and differentiation of satellite cells in livestock species. J. Anim. Sci. 2019, 97, 2258–2269. [Google Scholar] [CrossRef]
- Bell, A.W.; Greenwood, P.L. Prenatal origins of postnatal variation in growth, development and productivity of ruminants. Anim. Prod. Sci. 2016, 56, 1217–1232. [Google Scholar] [CrossRef]
- Costa, T.C.; Du, M.; Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Schultz, E.B.; Gionbelli, M.P.; Duarte, M.d.S. Skeletal Muscle Development in Postnatal Beef Cattle Resulting from Maternal Protein Restriction during Mid-Gestation. Animals 2021, 11, 860. [Google Scholar] [CrossRef]
- Zhu, M.-J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol. Reprod. 2004, 71, 1968–1973. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key genes regulating skeletal muscle development and growth in farm animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef]
- Ladeira, M.; Schoonmaker, J.; Gionbelli, M.; Dias, J.; Gionbelli, T.; Carvalho, J.R.; Teixeira, P. Nutrigenomics and beef quality: A review about lipogenesis. Int. J. Mol. Sci. 2016, 17, 918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wong, C.; Liu, D.; Finegold, M.; Harper, J.W.; Elledge, S.J. p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev. 1999, 13, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.; Hoffman, M.; Govoni, K.; Zinn, S.; Reed, S. Restricted maternal nutrition alters myogenic regulatory factor expression in satellite cells of ovine offspring. Animal 2016, 10, 1200–1203. [Google Scholar] [CrossRef]
- Lazzarini, I.; Detmann, E.; Sampaio, C.B.; Paulino, M.F.; Valadares Filho, S.d.C.; Souza, M.A.d.; Oliveira, F.A. Intake and digestibility in cattle fed low-quality tropical forage and supplemented with nitrogenous compounds. Rev. Bras. Zootec. 2009, 38, 2021–2030. [Google Scholar] [CrossRef]
- Detmann, E.; Paulino, M.F.; Mantovani, H.C.; Valadares Filho, S.d.C.; Sampaio, C.B.; de Souza, M.A.; Lazzarini, Í.; Detmann, K.S. Parameterization of ruminal fibre degradation in low-quality tropical forage using Michaelis–Menten kinetics. Livest. Sci. 2009, 126, 136–146. [Google Scholar] [CrossRef]
- Robinson, D.; Cafe, L.; Greenwood, P. Meat science and muscle biology symposium: Developmental programming in cattle: Consequences for growth, efficiency, carcass, muscle, and beef quality characteristics. J. Anim. Sci. 2013, 91, 1428–1442. [Google Scholar] [CrossRef]
- Gionbelli, T.R.S.; Veloso, C.M.; Rotta, P.P.; Filho, S.C.V.; Carvalho, B.C.; Marcondes, M.I.; Cunha, C.S.; Novaes, M.A.S.; Prezotto, L.D.; Duarte, M.S.; et al. Foetal development of skeletal muscle in bovines as a function of maternal nutrition, foetal sex and gestational age. J. Anim. Physiol. Anim. Nutr. 2018, 102, 545–556. [Google Scholar] [CrossRef]
- Baatar, D.; Hwang, S.G. Effect of testosterone on the differentiation control of stromal vascular cells isolated from longissimus muscle of Hanwoo beef cattle. Meat Sci. 2020, 159, 107916. [Google Scholar] [CrossRef]
- Sullivan, T.; Micke, G.; Greer, R.; Perry, V. Dietary manipulation of Bos indicus× heifers during gestation affects the prepubertal reproductive development of their bull calves. Anim. Reprod. Sci. 2010, 118, 131–139. [Google Scholar] [CrossRef]
- White, J.P.; Gao, S.; Puppa, M.J.; Sato, S.; Welle, S.L.; Carson, J.A. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol. Cell. Endocrinol. 2013, 365, 174–186. [Google Scholar] [CrossRef]
- Kruse, S.; Bridges, G.; Funnell, B.; Bird, S.; Lake, S.; Arias, R.; Amundson, O.; Larimore, E.; Keisler, D.; Perry, G. Influence of post-insemination nutrition on embryonic development in beef heifers. Theriogenology 2017, 90, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Redmer, D.; Wallace, J.; Reynolds, L. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest. Anim. Endocrinol. 2004, 27, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Redmer, D.A. Utero-placental vascular development and placental function. J. Anim. Sci. 1995, 73, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.O.; Vasconcelos, B.G.; Favaron, P.O.; Santos, A.C.; Leandro, R.M.; Pereira, F.T.; Maria, D.A.; Miglino, M.A. Desenvolvimento do sistema nervoso central de bovinos. Pesqui. Vet. Bras. 2018, 38, 147–153. [Google Scholar] [CrossRef]
- Hyttel, P.; Sinowatz, F.; Vejlsted, M. Embriologia Veterinária; Elsevier Ltda: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Duarte, M.S.; Gionbelli, M.P.; Paulino, P.V.R.; Serão, N.V.L.; Martins, T.S.; Tótaro, P.I.S.; Neves, C.A.; Valadares Filho, S.C.; Dodson, M.V.; Zhu, M.; et al. Effects of maternal nutrition on development of gastrointestinal tract of bovine fetus at different stages of gestation. Livest. Sci. 2013, 153, 60–65. [Google Scholar] [CrossRef]
- Gionbelli, T.; Rotta, P.; Veloso, C.; Valadares Filho, S.; Carvalho, B.; Marcondes, M.; Ferreira, M.; Souza, J.; Santos, J.; Lacerda, L. Intestinal development of bovine foetuses during gestation is affected by foetal sex and maternal nutrition. J. Anim. Physiol. Anim. Nutr. 2017, 101, 493–501. [Google Scholar] [CrossRef]
- Zaborski, D.; Grzesiak, W.; Szatkowska, I.; Dybus, A.; Muszynska, M.; Jedrzejczak, M. Factors affecting dystocia in cattle. Reprod. Domest. Anim. 2009, 44, 540–551. [Google Scholar] [CrossRef]
- Fontes, P.; Oosthuizen, N.; Ciriaco, F.; Sanford, C.; Canal, L.; Cooke, R.; Pohler, K.; Henry, D.; Mercadante, V.; Ealy, A. Effects of nutrient restriction on the metabolic profile of Bos indicus-influenced and B. taurus suckled beef cows. Animal 2021, 15, 100166. [Google Scholar] [CrossRef]
- Trivers, R.L.; Willard, D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science 1973, 179, 90–92. [Google Scholar] [CrossRef]
- Hinde, K.; Carpenter, A.J.; Clay, J.S.; Bradford, B.J. Holsteins favor heifers, not bulls: Biased milk production programmed during pregnancy as a function of fetal sex. PLoS ONE 2014, 9, e86169. [Google Scholar] [CrossRef]
- Koskela, E.; Mappes, T.; Niskanen, T.; Rutkowska, J. Maternal investment in relation to sex ratio and offspring number in a small mammal—A case for Trivers and Willard theory? J. Anim. Ecol. 2009, 78, 1007–1014. [Google Scholar] [CrossRef]
- Hewison, A.M.; Gaillard, J.-M. Successful sons or advantaged daughters? The Trivers–Willard model and sex-biased maternal investment in ungulates. Trends Ecol. Evol. 1999, 14, 229–234. [Google Scholar] [CrossRef]
- Ithurralde, J.; Pérez-Clariget, R.; Corrales, F.; Fila, D.; López-Pérez, Á.; de Jesús Marichal, M.; Saadoun, A.; Bielli, A. Sex-dependent effects of maternal undernutrition on growth performance, carcass characteristics and meat quality of lambs. Livest. Sci. 2019, 221, 105–114. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Roberts, R.M. Maternal diet and other factors affecting offspring sex ratio: A review. Biol. Reprod. 2004, 71, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Peabody, J.; Turnier, V.; Clark, R.H. A new look at intrauterine growth and the impact of race, altitude, and gender. Pediatrics 2000, 106, e21. [Google Scholar] [CrossRef] [PubMed]
- Meneses, J.A.M.; Galvão, M.C.; Moreira, G.M.; Chalfun, L.H.L.; de Souza, S.P.; Ramírez-Zamudio, G.D.; Ladeira, M.M.; Duarte, M.d.; Casagrande, D.R.; Gionbelli, M.P. Protein supplementation during mid-gestation affects maternal voluntary feed intake, performance, digestibility and uterine blood flow in Zebu beef cows. Animals, 2022; in press. [Google Scholar]
- Love, O.P.; Chin, E.H.; Wynne-Edwards, K.E.; Williams, T.D. Stress hormones: A link between maternal condition and sex-biased reproductive investment. Am. Nat. 2005, 166, 751–766. [Google Scholar] [CrossRef]
- Pike, T.W.; Petrie, M. Maternal body condition and plasma hormones affect offspring sex ratio in peafowl. Anim. Behav. 2005, 70, 745–751. [Google Scholar] [CrossRef]
- Copping, K.; Hoare, A.; Callaghan, M.; McMillen, I.; Rodgers, R.; Perry, V. Fetal programming in 2-year-old calving heifers: Peri-conception and first trimester protein restriction alters fetal growth in a gender-specific manner. Anim. Prod. Sci. 2014, 54, 1333–1337. [Google Scholar] [CrossRef]
- Batistel, F.; Alharthi, A.S.; Yambao, R.R.; Elolimy, A.A.; Pan, Y.-X.; Parys, C.; Loor, J.J. Methionine supply during late-gestation triggers offspring sex-specific divergent changes in metabolic and epigenetic signatures in bovine placenta. J. Nutr. 2019, 149, 6–17. [Google Scholar] [CrossRef]
- Nugent, B.M.; Bale, T.L. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front. Neuroendocrinol. 2015, 39, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Notaro, U.S.; Recce, S.; Rodríguez, F.M.; Ortega, H.H.; Salvetti, N.R.; Rey, F. Fetal programming in dairy cows: Effect of heat stress on progeny fertility and associations with the hypothalamic-pituitary-adrenal axis functions. Anim. Reprod. Sci. 2020, 216, 106348. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Ehrhardt, R.A. Regulation of placental nutrient transport and implications for fetal growth. Nutr. Res. Rev. 2002, 15, 211–230. [Google Scholar] [CrossRef] [PubMed]
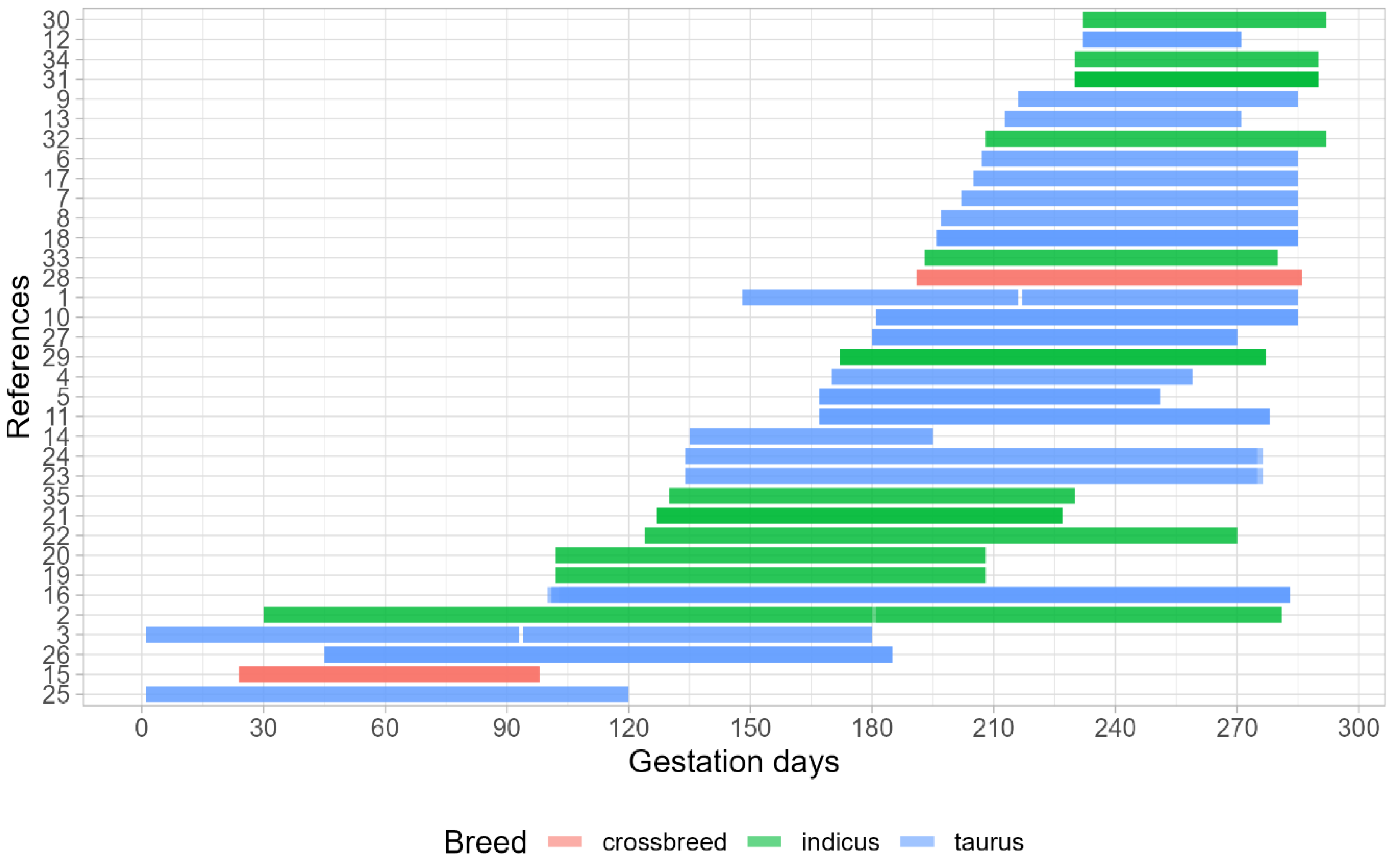
| References | Dams Breed | Description of Maternal Treatments Application during Gestational Period | Gestational Period | Feeding System | Offspring Sex |
|---|---|---|---|---|---|
| [17] | Crossbred | Fed a high- (14% CP) (1) or low- (7% CP) (2) protein diet | Early gestation | Feedlot | Male |
| [13] | Bos taurus | Fed with improved pasture (1) or with a native range (2) | Early and mid-gestation | Pasture | Male |
| [18] | Bos taurus | Fed to provide 100% of NRC requirements (1); limitedly fed to provide 70% of treatment 1 diet (2) or limitedly fed to provide 70% of treatment 1 diet plus a protein supplement to promote an essential AA supply to the small intestine equal to treatment 1 diet (3) | Early and mid-gestation | Feedlot | Mix |
| [11] | Bos taurus | Pasture plus a low (1) or high crude protein supplement (2) | Early and mid-gestation | Pasture | Mix |
| [19] | Bos taurus | Limitedly fed to provide 75% or 250% of CP requirements at early gestation or to provided 228% or 63% of CP requirements | Mid-gestation | Feedlot | Mix |
| [20] | Bos taurus | (1) Unsupplemented from mid to late-gestation or (2) supplemented with protein to late-gestation | Mid-gestation | Pasture | Male |
| [21,22] | Bos indicus | Fed with poor quality forage without (1) or with a CP supplement (2) | Mid-gestation | Feedlot | Mix |
| [23] | Bos indicus | Fed with poor quality forage plus nitrogenous mineral salt (1) or with supplement rich in a non-degradable rumen protein (2) or with other supplements rich in rumen-degradable protein plus ground corn (3) | Mid-gestation | Feedlot | Mix |
| [24] | Bos taurus | (1) Unsupplemented, (2) supplemented with the distiller-based supplement, or (3) supplemented with corn gluten-based supplement | Mid and late gestation | Feedlot | Mix |
| [25] | Bos indicus | (1) Unsupplemented during entire pregnancy; (2) supplemented with protein supplement from early to mid-gestation or (3) supplemented with a protein supplement at late gestation | Mid and late gestation | Pasture | Mix |
| [12,26] | Bos taurus | Fed with low (1) or high (2) protein diets | Mid and late gestation | Feedlot | Mix |
| [27] | Bos taurus | Limitedly fed to provide 102% or 80% of CP requirements during mid to late gestation or during late gestation | Mid and late gestation | Feedlot | Male |
| [14] | Bos indicus | Fed with poor-quality forage plus mineral salt provided ad libitum without (1) or with a crude protein supplement (2) | Mid and late gestation | Pasture | Male |
| [28] | Bos indicus | Fed with high- (1) or low- (2) rumen-undegraded protein | Mid and late gestation | Feedlot | Mix |
| [29] | Bos taurus | A cow managed under different wintering systems: grazing winter range (dormant Sandhills) vs. corn residue; within grazing treatment received or did not receive a protein supplement | Late gestation | Pasture | Male |
| [30] | Bos taurus | Pasture plus a 36% CP supplement provided at the level of 454 g/cow 3 times a week (1); pasture plus a self-fed supplement comprising 50% animal protein sources and 50% trace mineral package (2) or brief and intermittent supplementation using the same supplement of treatment 1 | Late gestation | Pasture | Male |
| [31] | Bos taurus | Not supplemented (1); supplemented with 36% CP supplement provided at the level of 454 g/cow 3 times a week (2) or self-fed supplement of 28% CP supplement | Late gestation | Pasture | Male |
| [32] | Bos taurus | Cows managed to enter the last trimester of gestation with a low (4 points) or high (6 points) body score condition, with each group being fed without (1) and with DDGS supplementation (2) | Late gestation | Pasture | Mix |
| [33] | Bos taurus | Not supplemented (1) or supplemented (2) with dried distillers grains plus solubles | Late gestation | Pasture | Mix |
| [34] | Bos taurus | Limitedly fed (1) with corn co-products and ground cornstalks or (2) ground-mixed, cool-season grass hay to provide 62% or 113% of rumen-degraded protein, respectively | Late gestation | Feedlot | Mix |
| [35] | Bos taurus | Limitedly fed to provided 100% or 129% of CP requirements | Late gestation | Feedlot | Mix |
| [36] | Bos indicus | Fed with pasture without (1) or with a crude protein supplement (2) | Late gestation | Pasture | Mix |
| [37] | Bos indicus | Fed with pasture without (1) or with a crude protein supplement provided at the level of 0.5 kg/day (2), 1.0 kg/day (3), or 1.5 kg/day (4) | Late gestation | Pasture | Male |
| [38] | Bos indicus | Fed with pasture without (1) or with a crude protein supplement (2) | Late gestation | Pasture | Male |
| [39] | Crossbred | Diets provided to promote low (1), medium (2), and high (3) nutritional levels | Late gestation | Pasture | Mix |
| [40] | Bos indicus | Fed with pasture without (1) or with a crude protein supplement (2) | Late gestation | Pasture | Male |
| References | Dams Breed | Description of Maternal Treatments Application during Gestational Period | Gestational Period | Feeding System | Offspring Sex |
|---|---|---|---|---|---|
| [41] | Bos taurus | Three primary energy sources: (1) Fed ad libitum with grass hay (high-fiber concentration), (2) limitedly fed with corn (high-starch concentration), and (3) limitedly fed with dried corn distillers grains with solubles (high fiber, protein, and fat concentrations). | Mid and late gestation | Feedlot | Mix |
| [42] | Bos taurus | Fed for promoting a positive (1) or negative energy balance (1) | Mid and late gestation | Pasture and Feedlot | Male |
| [43] | Bos taurus | Limitedly fed to promote a severe restriction (50% of requirements) (1), a moderate restriction (75% of requirements) (2), or to meet 100% of requirements (3) | Mid and late gestation | Feedlot | Male |
| [44] | Bos taurus | Limitedly fed to provided 100% (1) or 125% of TDN requirements (2) | Late gestation | Feedlot | Mix |
| [45] | Bos taurus | Not supplemented (1) or fed with a bunk supplement at the level of 2.16 kg/ cow/day (2) or at 8.61 kg/cow/day (3) | Pasture | Male |
| References | Dams Breed | Description of Maternal Treatments Application during Gestational Period | Gestational Period | Feeding System | Offspring Sex |
|---|---|---|---|---|---|
| [46] | Bos indicus | Pasture (1); pasture plus a daily (2) or infrequent energy-protein supplementation (3) | Late gestation | Pasture | Mix |
| [47] | Bos indicus | Fed with pasture without (1) or with an energy-protein supplementation (2) | Late gestation | Pasture | Mix |
| Variables | n ¹ | Number of Means ² | Minimum | Mean | Median | Maximum | SD ³ |
|---|---|---|---|---|---|---|---|
| Cow | |||||||
| Days on treatment | 3854 | 88 | 39 | 95.2 | 89.0 | 251 | 35.2 |
| Stage of gestation 4, day | 3854 | 88 | 1 | 154 | 172.0 | 232 | 66.8 |
| Metabolizable protein supplied, % | 3854 | 88 | 30 | 109 | 102.0 | 298 | 44.8 |
| Metabolizable energy supplied, % | 3854 | 88 | 50 | 108 | 100.0 | 243 | 32.4 |
| Body weight, kg | 3337 | 75 | 362 | 523 | 516 | 710 | 78.5 |
| Average daily gain, kg/d | 3122 | 74 | −0.8 | 0.23 | 0.22 | 1.19 | 0.44 |
| Offspring | |||||||
| Birth weight, kg | 3121 | 71 | 26.6 | 35.3 | 35.8 | 44.0 | 3.53 |
| Weaning weight 210 d, kg | 2386 | 46 | 176 | 249 | 232 | 345 | 43.4 |
| Weaning age, day | 2462 | 53 | 82 | 178 | 185 | 245 | 39.9 |
| Average daily gain cow-calf phase, kg/d | 2182 | 44 | 0.68 | 0.93 | 0.94 | 1.15 | 0.10 |
| Total gain cow-calf phase, kg | 2386 | 46 | 142 | 214 | 200 | 310 | 41.7 |
| Response Variables | Candidate Variables ¹ | Equation Number | Model Selected | Model Statistics | |||
|---|---|---|---|---|---|---|---|
| Full ² | Reduced ³ | R-Squared 4 | |||||
| Conditional | Marginal | ||||||
| Cow ADG, kg | CBW, MPSup, MESup, MPSup × MESup, breed, and system | Equation (1) | −1.93 + 0.004 × CBW + 0.002 × MPSup | AIC 5: 34.3; RSD 6: 0.782 | AIC: 33.5; RSD: 0.790 | 0.32 | 0.10 |
| Calf birth BW, kg | CBW, CADG, MPSup, MESup, MPSup × MESup, breed, sex, and system | Equation (2) | 16.09 + 0.029 × CBW + 0.025 × MPSup + 0.0187 × MESup − 0.0001 × MPSup × MESup | AIC: 207.9; RSD: 3.92 | AIC: 204.6; RSD: 3.92 | 0.53 | 0.21 |
| Cow-calf ADG, kg | BBW, CBW, CADG, MPSup, MESup, MPSup × MESup, breed, sex, and system | Equation (3) | 0.623 + 0.0075 × BBW + 0.0005 × MESup | AIC: −77.1; RSD: 0.18 | AIC: −83.4; RSD: 0.19 | 0.22 | 0.03 |
| BW at Weaning 210 adj., kg | BBW, CBW, CADG, MPSup, MESup, MPSup × MESup, breed, sex, and system | Equation (4) | 165.73 + 4.34 × BBW + 0.226 × MESup − 87.33 × sex | AIC: 237.2; RSD: 24.8 | AIC: 235.9; RSD: 23.2 | 0.85 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcelos, S.d.S.; Nascimento, K.B.; Silva, T.E.d.; Mezzomo, R.; Alves, K.S.; de Souza Duarte, M.; Gionbelli, M.P. The Effects of Prenatal Diet on Calf Performance and Perspectives for Fetal Programming Studies: A Meta-Analytical Investigation. Animals 2022, 12, 2145. https://doi.org/10.3390/ani12162145
Barcelos SdS, Nascimento KB, Silva TEd, Mezzomo R, Alves KS, de Souza Duarte M, Gionbelli MP. The Effects of Prenatal Diet on Calf Performance and Perspectives for Fetal Programming Studies: A Meta-Analytical Investigation. Animals. 2022; 12(16):2145. https://doi.org/10.3390/ani12162145
Chicago/Turabian StyleBarcelos, Sandra de Sousa, Karolina Batista Nascimento, Tadeu Eder da Silva, Rafael Mezzomo, Kaliandra Souza Alves, Márcio de Souza Duarte, and Mateus Pies Gionbelli. 2022. "The Effects of Prenatal Diet on Calf Performance and Perspectives for Fetal Programming Studies: A Meta-Analytical Investigation" Animals 12, no. 16: 2145. https://doi.org/10.3390/ani12162145
APA StyleBarcelos, S. d. S., Nascimento, K. B., Silva, T. E. d., Mezzomo, R., Alves, K. S., de Souza Duarte, M., & Gionbelli, M. P. (2022). The Effects of Prenatal Diet on Calf Performance and Perspectives for Fetal Programming Studies: A Meta-Analytical Investigation. Animals, 12(16), 2145. https://doi.org/10.3390/ani12162145









