Effects of Maternal Protein Supplementation at Mid-Gestation of Cows on Intake, Digestibility, and Feeding Behavior of the Offspring
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Feeding
2.2. Measurements
2.2.1. Body Weight Measurements
2.2.2. Intake, Digestibility Trials, and Feed Efficiency
2.2.3. Chemical Analyses
2.2.4. Feeding Behavior
2.3. Statistical Analysis
3. Results
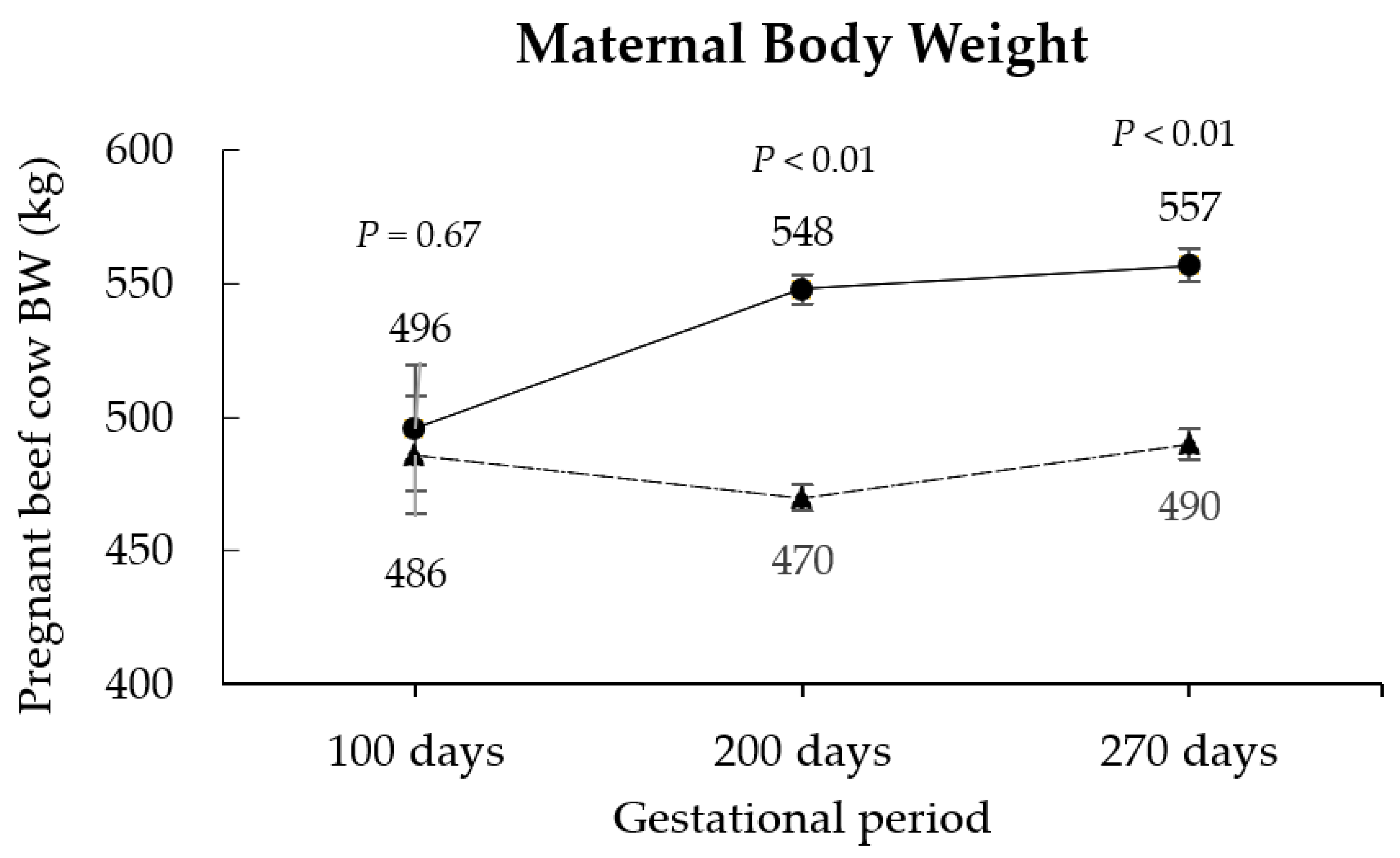
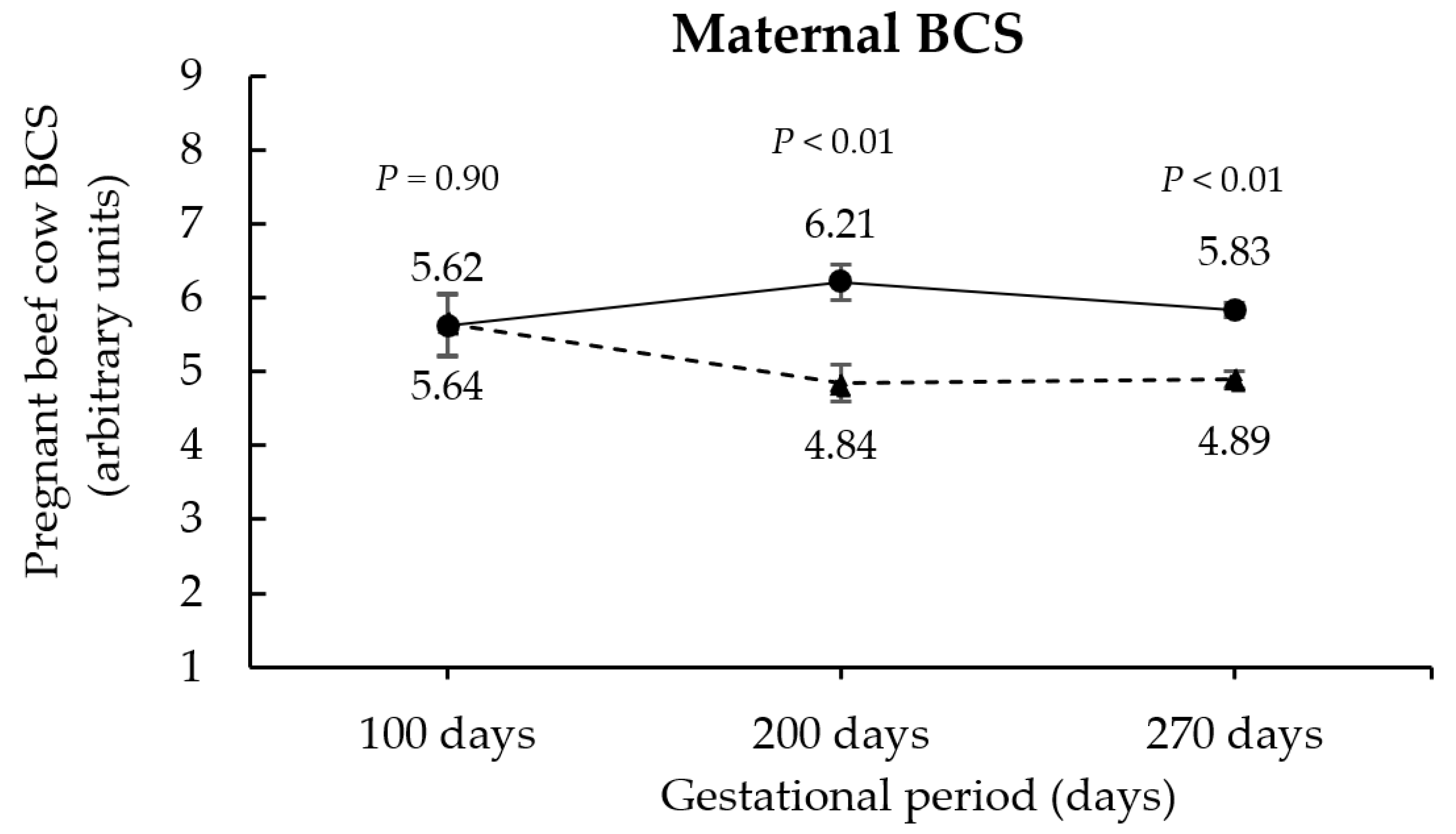
3.1. Maternal Responses
3.2. Offspring Performance
3.3. Milk Yield and Composition
3.4. Dry Matter Intake and Feed Efficiency
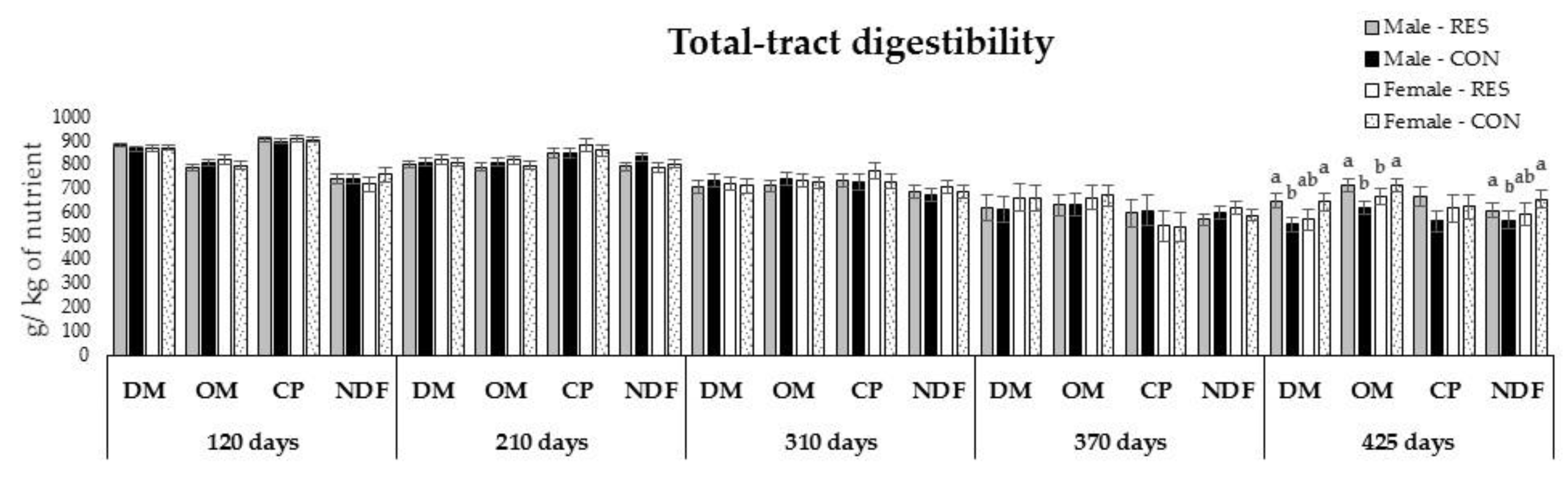
3.5. Components of Diet Intake and Apparent Total-Tract Digestibility
3.6. Feeding Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghotbaldini, H.; Mohammadabadi, M.; Nezamabadi-Pour, H.; Babenko, O.I.; Bushtruk, M.V.; Tkachenko, S.V. Predicting breeding value of body weight at 6-month age using Artificial Neural Networks in Kermani sheep breed. Acta Sci. Anim. Sci. 2018, 41, 45282. [Google Scholar] [CrossRef]
- Shahsavari, M.; Mohammadabadi, M.; Khezri, A.; Fozi, M.A.; Babenko, O.; Kalashnyk, O.; Oleshko, V.; Tkachenko, S. Correlation between insulin-like growth factor 1 gene expression and fennel (Foeniculum vulgare) seed powder consumption in muscle of sheep. Anim. Biotechnol. 2021, 1–11. [Google Scholar] [CrossRef]
- Poppi, D.P.; Quigley, S.P.; da Silva, T.A.C.C.; McLennan, S.R. Challenges of beef cattle production from tropical pastures. Rev. Bras. de Zootec. 2018, 47, e20160419. [Google Scholar] [CrossRef]
- Paulino, M.F.; Zervoudakis, J.T.; Moraes, E.H.B.K.d.; Detmann, E.; Valadares Filho, S. Bovinocultura de ciclo curto em pastagens. Simpósio Produção Gado Corte 2002, 3, 153–196. [Google Scholar]
- Gionbelli, T.; Veloso, C.; Rotta, P.; Valadares Filho, S.C.; Carvalho, B.; Marcondes, M.; S. Cunha, C.; Novaes, M.; Prezotto, L.D.; Duarte, M. Foetal development of skeletal muscle in bovines as a function of maternal nutrition, foetal sex and gestational age. J. Anim. Physiol. Anim. 2018, 102, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Schoonmaker, J.P.; Resende, F.D.; Siqueira, G.R.; Neto, O.R.M.; Gionbelli, M.P.; Gionbelli, T.R.S.; Ladeira, M.M. Effects of protein supplementation on Nellore cows’ reproductive performance, growth, myogenesis, lipogenesis and intestine development of the progeny. Anim. Prod. Sci. 2021, 61, 371. [Google Scholar] [CrossRef]
- Costa, T.; Du, M.; Nascimento, K.; Galvão, M.; Meneses, J.; Schultz, E.; Gionbelli, M.; Duarte, M. Skeletal Muscle Development in Postnatal Beef Cattle Resulting from Maternal Protein Restriction during Mid-Gestation. Animals 2021, 11, 860. [Google Scholar] [CrossRef]
- Marquez, D.C.; Paulino, M.F.; Rennó, L.N.; Villadiego, F.C.; Ortega, R.M.; Moreno, D.S.; Martins, L.; de Almeida, D.M.; Gionbelli, M.P.; Manso, M.R.; et al. Supplementation of grazing beef cows during gestation as a strategy to improve skeletal muscle development of the offspring. Animal 2017, 11, 2184–2192. [Google Scholar] [CrossRef]
- Greenwood, P.L.; Thompson, A.N.; Ford, S.P. Postnatal Consequences of the Maternal Environment and of Growth During Prenatal Life for Productivity of Ruminants; Springer: Dordrecht, The Netherlands, 2009; pp. 3–36. [Google Scholar] [CrossRef]
- Costa, T.C.; Gionbelli, M.P.; Duarte, M.D.S. Fetal programming in ruminant animals: Understanding the skeletal muscle development to improve meat quality. Anim. Front. 2021, 11, 66–73. [Google Scholar] [CrossRef]
- Carvalho, E.B.; Costa, T.C.; Sanglard, L.P.; Nascimento, K.B.; Meneses, J.A.; Galvão, M.C.; Serão, N.V.; Duarte, M.S.; Gionbelli, M.P. Transcriptome profile in the skeletal muscle of cattle progeny as a function of maternal protein supplementation during mid-gestation. Livest. Sci. 2022, 263. [Google Scholar] [CrossRef]
- Barcelos, S.D.S.; Nascimento, K.B.; da Silva, T.E.; Mezzomo, R.; Alves, K.S.; Duarte, M.D.S.; Gionbelli, M.P. The Effects of Prenatal Diet on Calf Performance and Perspectives for Fetal Programming Studies: A Meta-Analytical Investigation. Animals 2022, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.S.; Crouse, M.; Reynolds, L.P.; Neville, T.L.; Dahlen, C.R.; Ward, A.K.; Swanson, K.C. Maternal nutrition and programming of offspring energy requirements1. Transl. Anim. Sci. 2019, 3, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Cafe, L.M.; Greenwood, P.L. Meat Science And Muscle Biology Symposium: Developmental programming in cattle: Consequences for growth, efficiency, carcass, muscle, and beef quality characteristics1,2. J. Anim. Sci. 2013, 91, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.L.; Hunt, A.S.; Hermanson, J.W.; Bell, A.W. Effects of birth weight and postnatal nutrition on neonatal sheep: I. Body growth and composition, and some aspects of energetic efficiency. J. Anim. Sci. 1998, 76, 2354–2367. [Google Scholar] [CrossRef]
- Forbes, J.M. Physiological and Metabolic Aspects of Feed Intake Control. In Farm Animal Metabolism and Nutrition; Felix D’Mello, J.P., Ed.; 2009; pp. 319–333. [Google Scholar] [CrossRef]
- Stevens, A.; Begum, G.; Cook, A.; Connor, K.; Rumball, C.; Oliver, M.; Challis, J.; Bloomfield, F.; White, A. Epigenetic Changes in the Hypothalamic Proopiomelanocortin and Glucocorticoid Receptor Genes in the Ovine Fetus after Periconceptional Undernutrition. Endocrinology 2010, 151, 3652–3664. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Adam, C.L.; Findlay, P.A.; Duffield, J.A.; Mcmillen, I.C. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J. 2006, 20, 1257–1259. [Google Scholar] [CrossRef]
- Prezotto, L.D.; Thorson, J.F.; Borowicz, P.P.; Peine, J.L.; Bedenbaugh, M.; Hileman, S.M.; Lents, C.A.; Caton, J.S.; Swanson, K.C. Influences of maternal nutrient restriction and arginine supplementation on visceral metabolism and hypothalamic circuitry of offspring. Domest. Anim. Endocrinol. 2018, 65, 71–79. [Google Scholar] [CrossRef]
- Long, N.M.; Ford, S.P.; Nathanielsz, P. Maternal obesity eliminates the neonatal lamb plasma leptin peak. J. Physiol. 2011, 589, 1455–1462. [Google Scholar] [CrossRef]
- Smith, A.M.; Pankey, C.L.; Odhiambo, J.F.; Ghnenis, A.B.; Nathanielsz, P.W.; Ford, S.P. Rapid Communication: Reduced maternal nutrition during early- to mid-gestation elevates newborn lamb plasma cortisol concentrations and eliminates the neonatal leptin surge. J. Anim. Sci. 2018, 96, 2640–2645. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.W.; Wang, Z.Y.; Deng, M.T.; Zhang, G.M.; Guo, R.H.; Ma, T.W.; Wang, F. Dietary N-carbamylglutamate and rumen-protected L-arginine supplementation ameliorate fetal growth restriction in undernourished ewes1,2. J. Anim. Sci. 2016, 94, 2072–2085. [Google Scholar] [CrossRef]
- Duarte, M.; Gionbelli, M.; Paulino, P.; Serão, N.; Martins, T.; Tótaro, P.; Neves, C.; Filho, S.V.; Dodson, M.; Zhu, M.; et al. Effects of maternal nutrition on development of gastrointestinal tract of bovine fetus at different stages of gestation. Livest. Sci. 2013, 153, 60–65. [Google Scholar] [CrossRef]
- da Cruz, W.F.G.; Schoonmaker, J.P.; de Resende, F.D.; Siqueira, G.R.; Rodrigues, L.M.; Zamudio, G.D.R.; Ladeira, M.M. Effects of maternal protein supplementation and inclusion of rumen-protected fat in the finishing diet on nutrient digestibility and expression of intestinal genes in Nellore steers. Anim. Sci. J. 2019, 90, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Keomanivong, F.E.; Lemley, C.O.; Camacho, L.E.; Yunusova, R.D.; Borowicz, P.P.; Caton, J.S.; Meyer, A.M.; Vonnahme, K.A.; Swanson, K.C. Influence of nutrient restriction and melatonin supplementation of pregnant ewes on maternal and fetal pancreatic digestive enzymes and insulin-containing clusters. Animal 2016, 10, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, H.; Yan, Q.; Ren, A.; Kong, Z.; Tang, S.; Han, X.; Tan, Z.; Salem, A.Z.M. Evidence for liver energy metabolism programming in offspring subjected to intrauterine undernutrition during midgestation. Nutr. Metab. 2019, 16, 1–14. [Google Scholar] [CrossRef]
- Rae, M.; Kyle, C.; Miller, D.; Hammond, A.; Brooks, A.; Rhind, S. The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim. Reprod. Sci. 2002, 72, 63–71. [Google Scholar] [CrossRef]
- Peterson, M.; Gauvin, M.; Pillai, S.; Jones, A.; McFadden, K.; Cameron, K.; Reed, S.; Zinn, S.; Govoni, K. Maternal Under- and Over-Nutrition during Gestation Causes Islet Hypertrophy and Sex-Specific Changes to Pancreas DNA Methylation in Fetal Sheep. Animals 2021, 11, 2531. [Google Scholar] [CrossRef]
- Dobbs, K.B.; Rodriguez, M.; Sudano, M.; Ortega, M.S.; Hansen, P.J. Dynamics of DNA Methylation during Early Development of the Preimplantation Bovine Embryo. PLoS ONE 2013, 8, e66230. [Google Scholar] [CrossRef]
- Dominguez, M.M.; Liptrap, R.M.; Basrur, P.K. Steroidogenesis in fetal bovine gonads. CJVR 1988, 52, 401. [Google Scholar] [CrossRef]
- Trivers, R.L.; Willard, D.E. Natural Selection of Parental Ability to Vary the Sex Ratio of Offspring. Science 1973, 179, 90–92. [Google Scholar] [CrossRef]
- Ithurralde, J.; Pérez-Clariget, R.; Corrales, F.; Fila, D.; López-Pérez, .; Marichal, M.D.J.; Saadoun, A.; Bielli, A. Sex-dependent effects of maternal undernutrition on growth performance, carcass characteristics and meat quality of lambs. Livest. Sci. 2019, 221, 105–114. [Google Scholar] [CrossRef]
- Copping, K.J.; Hoare, A.; Callaghan, M.; McMillen, I.C.; Rodgers, R.J.; Perry, V.E.A. Fetal programming in 2-year-old calving heifers: Peri-conception and first trimester protein restriction alters fetal growth in a gender-specific manner. Anim. Prod. Sci. 2014, 54, 1333–1337. [Google Scholar] [CrossRef]
- Valente, T.N.P.; Detmann, E.; De Queiroz, A.C.; Filho, S.V.; Gomes, D.I.; Figueiras, J.F. Evaluation of ruminal degradation profiles of forages using bags made from different textiles. Rev. Bras. de Zootec. 2011, 40, 2565–2573. [Google Scholar] [CrossRef]
- Ferreira, M.A.; valadares Filho, S.C.; Marcondes, M.I.; Paixão, M.L.; Paulino, M.F.; Valadares, R.F.D. Avaliação de indicadores em estudos com ruminantes: Digestibilidade. Rev. Bras. de Zootec. 2009, 38, 1568–1573. [Google Scholar] [CrossRef]
- Detmann, E.; Paulino, M.F.; Zervoudakis, J.T.; Valadares Filho, S.d.C.; Euclydes, R.F.; Lana, R.d.P.; Queiroz, D.S.d. Cromo e indicadores internos na determinação do consumo de novilhos mestiços, suplementados, a pasto Rev. Bras. de Zootec. 2001, 30, 1600–1609. [Google Scholar] [CrossRef]
- Ferreira, M.d.A.; Valadares Filho, S.d.C.; Silva, L.F.C.; Nascimento, F.B.; Detmann, E.; Valadares, R.F.D. Avaliação de indicadores em estudos com ruminantes: Estimativa de consumos de concentrado e de silagem de milho por vacas em lactação. Rev. Bras. de Zootec. 2009, 38, 1574–1580. [Google Scholar] [CrossRef]
- Kimura, F.T.; Miller, V.L. Chromic Oxide Measurement, Improved Determination of Chromic Oxide in Cow Feed and Feces. J. Agric. Food Chem. 1957, 5, 216. [Google Scholar] [CrossRef]
- Myers, W.D.; Ludden, P.A.; Nayigihugu, V.; Hess, B.W. Technical Note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide1. J. Anim. Sci. 2004, 82, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Titgemeyer, E.C.; Armendariz, C.K.; Bindel, D.J.; Greenwood, R.H.; Loest, C. Evaluation of titanium dioxide as a digestibility marker for cattle. J. Anim. Sci. 2001, 79, 1059–1063. [Google Scholar] [CrossRef]
- Galvão, M.C. Long-Term Effects of the Use of Creep-Feeding for Beef Calves under Tropical Conditions. Master’s Thesis. Universidade Federal de Lavras, Lavras, Brazil, 2018. [Google Scholar]
- AOAC. Official methods of analysis., 15th ed.; Association of Official Analysis Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Detmann, E.; Filho, S.V. On the Estimation of Non-fibrous Carbohydrates in Feeds and Diets; Universidade Federal de Viçosa: Viçosa, Brazil, 2010; Volume 62, pp. 980–984. [Google Scholar] [CrossRef]
- Azevêdo, J.A.G.; Valadares Filho, S.d.C.; Pina, D.d.S.; Detmann, E.; Valadares, R.F.D.; Pereira, L.G.R.; Souza, N.K.d.P.; Silva, L.F.C. Consumo, digestibilidade total, produção de proteína microbiana e balanço de nitrogênio em dietas com subprodutos de frutas para ruminantes. Rev. Bras. de Zootec. 2011, 40, 1052–1060. [Google Scholar] [CrossRef][Green Version]
- Gionbelli, M.P.; Valadares Filho, S.C.; Duarte, M.S. Nutritional requirements for pregnant and non-pregnant beef cows. In Nutrient Requirements of Zebu and Crossbred Cattle, 3rd ed.; Valadares Filho, S.C., Costa e Silva, L.F., Gionbelli, M.P., Rotta, P.P., Marcondes, M.I., Chizzotti, M.L., Prados, L.F., Eds.; Suprema Gráfica Ltda: Viçosa, Brazil, 2016; pp. 259–282. [Google Scholar]
- Hales, C.N.; Barker, D.J.P. The thrifty phenotype hypothesis. Br. Med Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Ginane, C.; Bonnet, M.; Baumont, R.; Revell, D.K. Feeding behaviour in ruminants: A consequence of interactions between a reward system and the regulation of metabolic homeostasis. Anim. Prod. Sci. 2015, 55, 247–260. [Google Scholar] [CrossRef]
- George, L.A.; Zhang, L.; Tuersunjiang, N.; Ma, Y.; Long, N.M.; Uthlaut, A.B.; Smith, D.T.; Nathanielsz, P.; Ford, S.P. Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function in aged female offspring. Am. J. Physiol. Integr. Comp. Physiol. 2012, 302, R795–R804. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Lourenço, P.; Souza, R.; Lopes, M.; Araújo, R.; Santos, M.; Luciano, L.; Massensini, J.; Chalfun, L.; Rennó, L.; et al. Ruminal undegradable protein enriched diet during late gestation of beef cows affects maternal metabolism and offspring’s skeletal muscle development. Anim. Feed Sci. Technol. 2022, 291. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal programming of skeletal muscle development in ruminant animals1. J. Anim. Sci. 2010, 88, E51–E60. [Google Scholar] [CrossRef]
- Summers, A.F.; Blair, A.D.; Funston, R.N. Impact of supplemental protein source offered to primiparous heifers during gestation on II. Progeny performance and carcass characteristics. J. Anim. Sci. 2015, 93, 1871–1880. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.; Peng, A.; Dong, L.; Wang, M.; Yu, L.; Loor, J.J.; Wang, H. Effects of Dietary l-Arginine and N-Carbamylglutamate Supplementation on Intestinal Integrity, Immune Function, and Oxidative Status in Intrauterine-Growth-Retarded Suckling Lambs. J. Agric. Food Chem. 2018, 66, 4145–4154. [Google Scholar] [CrossRef]
- de Vargas Junior, F.M.; Wechsler, F.S.; Rossi, P.; de Oliveira, M.V.M.; Schmidt, P. Ingestive behavior of Nellore cows and their straightbred or crossbred calves1. R. Bras. Zootec. 2010, 39, 648–655. [Google Scholar] [CrossRef]
- da Costa, M.J.R.P.; Albuquerque, L.G.; Eler, J.P.; de Vasconcelos Silva, J.A.I. Suckling behaviour of Nelore, Gir and Caracu calves and their crosses. Appl. Anim. Behav. Sci. 2006, 101, 276–287. [Google Scholar] [CrossRef]
| Mid-Gestation (Day 100 to Day 200, Period of Treatment Application) | Late Gestation (Day 100 to Parturition) | ||
|---|---|---|---|
| Chemical Composition of Feedstuffs, g/kg of DM | Corn silage + Sugarcane Bagasse | Supplement | Corn Silage |
| Dry matter | 418 ± 5.82 | 881 ± 0.70 | 330 ± 2.88 |
| Organic matter | 951 ± 2.67 | 958 ± 0.90 | 941± 1.37 |
| Crude protein | 53.3 ± 2.30 | 400 ± 1.44 | 72.2 ± 0.42 |
| Ash and protein-free neutral detergent fiber | 631 ± 10.60 | 213 ± 0.18 | 549 ± 3.62 |
| Non-fibrous carbohydrates | 242 ± 5.61 | 342 ± 2.20 | 291 ± 2.14 |
| Ether extract | 24.1 ± 1.10 | 41.2 ± 0.32 | 29.2 ± 0.40 |
| Backgrounding 1 | Growing 1 2 | Growing 2 3 | |||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Ingredients, g/kg of DM | |||||
| Corn Silage | 717 | 717 | 650 | 650 | 309 |
| Ground corn | 129 | 152 | 204 | 232 | 577 |
| Soybean meal | 116 | 92.5 | 106 | 77.0 | 101 |
| Urea | 6.75 | 6.75 | 8.10 | 8.10 | 2.88 |
| Ammonium sulfate | 0.75 | 0.75 | 0.90 | 0.90 | 0.32 |
| Mineral nucleus 4 | 30.0 | 30.0 | 30.0 | 30.0 | 10.0 |
| Chemical composition of experimental diet, g/kg of DM | |||||
| Concentrate | |||||
| Dry matter | 910 ± 10 | 900 ± 7 | 900 ± 16 | 896 ± 19 | 918 ± 18 |
| Organic matter | 829 ± 73 | 865.9 ± 21 | 850 ± 18 | 890 ± 26 | 930 ± 24 |
| Crude protein | 402 ± 107 | 401 ± 89 | 315 ± 22 | 243 ± 31 | 220 ± 4 |
| Ash and protein-free neutral detergent fiber | 144 ± 24 | 181 ± 82 | 151 ± 16 | 231 ± 24 | 253 ± 27 |
| Non-fibrous carbohydrate | 238 ± 173 | 263 ± 35 | 363 ± 50 | 398 ± 39 | 427 ± 60 |
| Ether extract | 16.4 ± 4 | 21.3 ± 7 | 20.6 ± 6 | 17.9 ± 6 | 30.9 ± 6 |
| Corn Silage | |||||
| Dry matter | 322 ± 94 | 347 ± 138 | 329 ± 92 | ||
| Organic matter | 949 ± 7 | 883 ± 84 | 905 ± 80 | ||
| Crude protein | 92.7 ± 14 | 72.0 ± 18 | 80.3 ± 20 | ||
| Ash and protein-free neutral detergent fiber | 558 ± 77 | 547 ± 81 | 554 ± 77 | ||
| Non-fibrous carbohydrates | 275 ± 79 | 245 ± 141 | 249 ± 105 | ||
| Ether extract | 25.4 ± 7 | 20.0 ± 4 | 24.9 ± 6 | ||
| Item | Males | Females | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RES | CON | RES | CON | PS | OS | PS × OS | ||||
| Males | Females | General | ||||||||
| Intake, kg of DM/day | ||||||||||
| 120 days | ||||||||||
| Total DMI | 2.47 | 2.19 | 1.72 | 1.74 | 0.35 | - | - | 0.45 | 0.02 | 0.35 |
| Milk intake | 1.44 | 1.21 | 1.20 | 1.22 | 0.11 | - | - | 0.32 | 0.25 | 0.18 |
| Pasture intake | 0.91 | 0.91 | 0.61 | 0.74 | 0.15 | - | - | 0.61 | 0.11 | 0.58 |
| Supplement intake | 0.10 | 0.23 | 0.20 | 0.12 | 0.07 | - | - | - | - | 0.10 |
| 210 days | ||||||||||
| Total DMI | 4.13 | 4.65 | 3.80 | 4.18 | 0.45 | - | - | 0.06 | 0.10 | 0.77 |
| Milk intake | 0.85 | 0.71 | 0.90 | 0.91 | 0.15 | - | - | 0.53 | 0.24 | 0.43 |
| Pasture intake | 2.88 | 3.07 | 2.40 | 2.86 | 0.38 | - | - | 0.10 | 0.09 | 0.49 |
| Supplement intake | 0.40 | 0.72 | 0.42 | 0.43 | 0.12 | - | - | 0.14 | 0.22 | 0.19 |
| Backgrounding | 5.63 | 5.50 | 5.41 | 5.67 | 0.77 | 0.83 | 0.91 | - | - | - |
| Growing 1 | 7.25 | 6.96 | 7.15 | 7.60 | 1.36 | 0.55 | 0.12 | - | - | - |
| Growing 2 | 8.43 | 8.53 | 7.86 | 8.11 | 0.39 | - | - | 0.53 | 0.16 | 0.79 |
| DMI/BW, g of DM/kg of BW | ||||||||||
| 120 days | 19.9 | 18.2 | 14.93 | 15.5 | 1.34 | - | - | 0.59 | 0.01 | 0.30 |
| 210 days | 19.9 | 21.5 | 17.8 | 20.5 | 2.02 | - | - | 0.08 | 0.22 | 0.64 |
| Backgrounding | 22.8 | 22.5 | 22.1 | 22.9 | 2.99 | 0.78 | 0.88 | - | - | - |
| Growing 1 | 22.8 | 22.2 | 23.1 | 24.6 | 4.16 | 0.21 | <0.01 | - | - | - |
| Growing 2 | 23.2 | 23.8 | 22.1 | 22.9 | 1.04 | - | - | 0.34 | 0.24 | 0.92 |
| Feed efficiency for gain, g/day of BW per kg of DMI/day | ||||||||||
| 120 days | 0.323 | 0.360 | 0.388 | 0.397 | 0.03 | - | - | 0.34 | 0.12 | 0.55 |
| 210 days | 0.235 | 0.239 | 0.269 | 0.228 | 0.02 | - | - | 0.33 | 0.22 | 0.56 |
| Backgrounding | 0.210 | 0.214 | 0.182 | 0.174 | 0.01 | 0.05 | 0.01 | - | - | - |
| Growing 1 | 0.151 | 0.153 | 0.141 | 0.138 | 0.03 | 0.21 | 0.11 | - | - | - |
| Growing 2 | 0.130 | 0.134 | 0.132 | 0.125 | 0.02 | - | - | 0.91 | 0.77 | 0.61 |
| Item | Males | Females | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PS | OS | PS × OS | ||||||||
| RES | CON | RES | CON | Males | Females | General | ||||
| 100 days, min/day | ||||||||||
| Suckling | 24.5 | 26.7 | 15.2 | 22.6 | 6.57 | - | - | 0.20 | 0.09 | 0.48 |
| Eating supplement | 20.3 | 29.9 | 12.8 | 20.5 | 4.98 | - | - | 0.05 | 0.06 | 0.83 |
| Grazing | 240 | 234 | 244 | 233 | 28.1 | - | - | 0.34 | 0.79 | 0.85 |
| Ruminating | 231 | 219 | 196 | 243 | 32.6 | - | - | 0.28 | 0.75 | 0.07 |
| Idleness | 773 | 782 | 862 | 815 | 58.3 | - | - | 0.45 | 0.03 | 0.26 |
| Other activities | 143 | 159 | 107 | 113 | 29.6 | - | - | 0.58 | 0.04 | 0.81 |
| 210 days, min/day | ||||||||||
| Suckling | 26.4 | 23.6 | 25.3 | 25.0 | 4.36 | - | - | 0.68 | 0.97 | 0.74 |
| Eating supplement | 37.1 | 41.6 | 29.7 | 27.5 | 8.05 | - | - | 0.87 | 0.13 | 0.63 |
| Grazing | 368 | 386 | 400 | 454 | 67.0 | - | - | 0.29 | 0.16 | 0.59 |
| Ruminating | 239 | 234 | 187 | 238 | 28.7 | - | - | 0.21 | 0.21 | 0.15 |
| Idleness | 698 | 646 | 748 | 591 | 45.5 | - | - | <0.01 | 0.95 | 0.13 |
| Other activities | 136 | 171 | 115 | 220 | 57.7 | - | - | 0.02 | 0.65 | 0.21 |
| 360 days, min/day | ||||||||||
| Eating | 198 | 186 | 206 | 199 | 11.6 | 0.23 | 0.94 | - | - | - |
| Ruminating | 395 | 410 | 342 | 420 | 28.9 | 0.01 | 0.27 | - | - | - |
| Idleness | 739 | 709 | 729 | 728 | 74.1 | 0.68 | 0.77 | - | - | - |
| Other activities | 106 | 119 | 126 | 91 | 78.5 | 0.80 | 0.50 | - | - | - |
| Item | Males | Females | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PS | OS | PS × OS | ||||||||
| RES | CON | RES | CON | Males | Females | General | ||||
| 120 days | ||||||||||
| OM | 1.07 | 1.24 | 0.93 | 1.06 | 0.18 | - | - | 0.34 | 0.42 | 0.91 |
| CP | 0.54 | 0.53 | 0.47 | 0.45 | 0.03 | - | - | 0.63 | 0.05 | 0.89 |
| NDF | 0.70 | 0.71 | 0.51 | 0.59 | 0.13 | - | - | 0.68 | 0.24 | 0.68 |
| 210 days | ||||||||||
| OM | 2.64 | 3.04 | 2.03 | 2.46 | 0.24 | - | - | 0.08 | 0.01 | 0.95 |
| CP | 0.69 | 0.78 | 0.63 | 0.73 | 0.05 | - | - | 0.04 | 0.18 | 0.85 |
| NDF | 2.32 | 2.51 | 1.96 | 2.32 | 0.37 | - | - | 0.14 | 0.16 | 0.66 |
| 310 days—Backgrounding Phase | ||||||||||
| DM | 6.08 | 6.30 | 6.06 | 6.11 | 1.00 | 0.96 | 0.67 | - | - | - |
| OM | 6.09 | 5.96 | 5.94 | 6.09 | 0.94 | 0.97 | 0.99 | - | - | - |
| CP | 1.21 | 1.12 | 1.11 | 1.16 | 0.30 | 0.94 | 0.63 | - | - | - |
| NDF | 2.63 | 2.53 | 2.66 | 2.68 | 0.18 | 0.55 | 0.73 | - | - | - |
| TDN | 4.24 | 4.25 | 4.37 | 4.22 | 0.89 | 0.81 | 0.93 | - | - | - |
| 370 days—Growing 1 Phase | ||||||||||
| DM | 7.55 | 7.76 | 7.40 | 7.88 | 1.68 | 0.43 | 0.29 | - | - | - |
| OM | 7.28 | 7.52 | 7.21 | 7.85 | 1.62 | 0.50 | 0.08 | - | - | - |
| CP | 1.19 | 1.23 | 1.02 | 1.04 | 0.26 | <0.01 | <0.01 | - | - | - |
| NDF | 3.05 | 3.13 | 3.15 | 3.33 | 0.39 | 0.91 | 0.04 | - | - | - |
| TDN | 4.49 | 4.68 | 4.41 | 4.91 | 1.65 | 0.63 | 0.24 | - | - | - |
| 425 days—Growing 2 Phase | ||||||||||
| DM | 9.57 | 9.61 | 9.06 | 9.51 | 0.83 | - | - | 0.62 | 0.62 | 0.65 |
| OM | 9.22 | 9.19 | 8.65 | 8.45 | 0.55 | - | - | 0.72 | 0.05 | 0.79 |
| CP | 1.81 | 1.86 | 1.70 | 1.72 | 0.16 | - | - | 0.71 | 0.16 | 0.83 |
| EE | 0.28 | 0.28 | 0.29 | 0.29 | 0.02 | - | - | 0.97 | 0.55 | 0.88 |
| NDF | 3.35 | 3.44 | 3.09 | 3.24 | 0.28 | - | - | 0.38 | 0.12 | 0.85 |
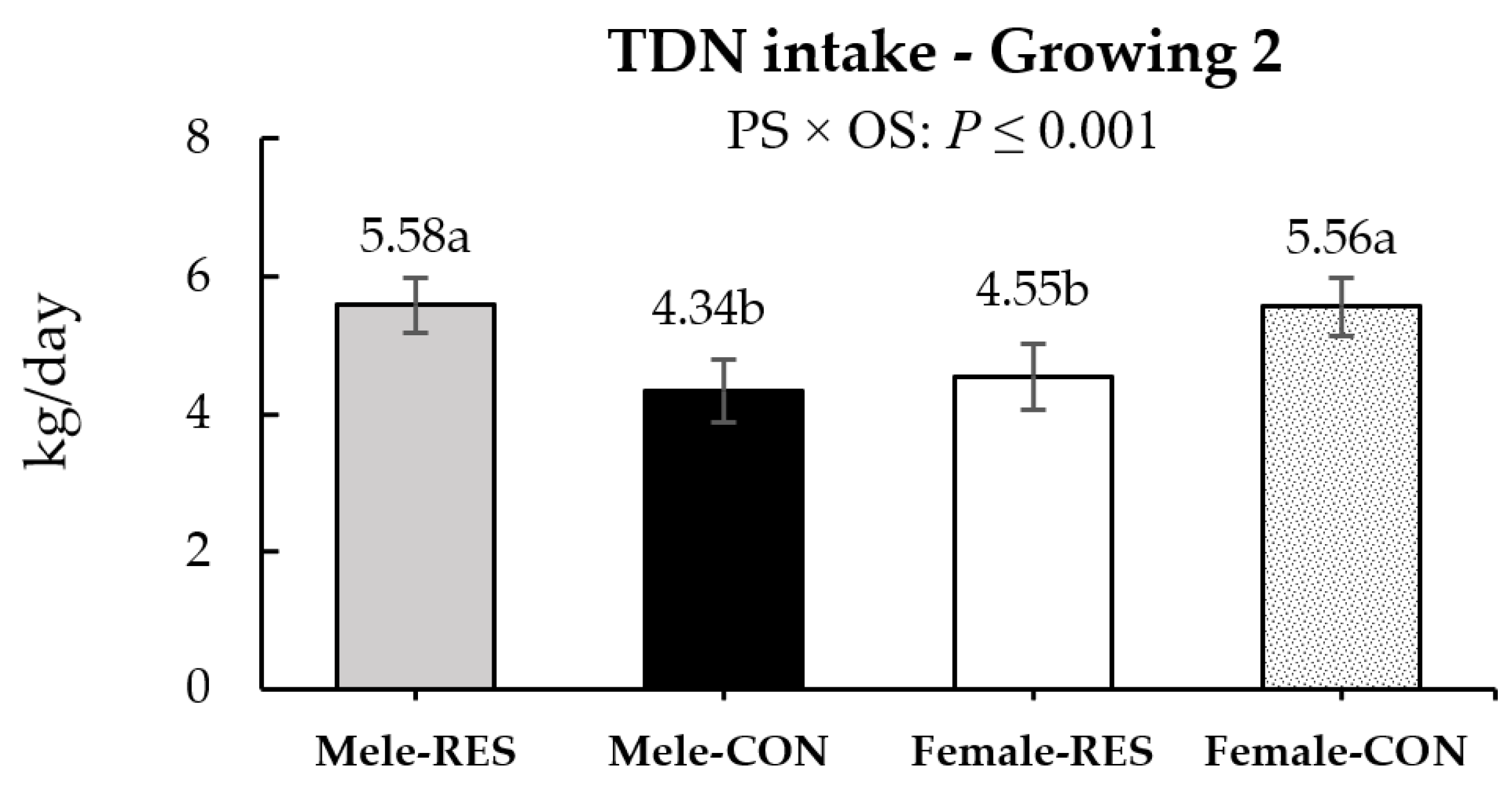
| TDN | 5.58 | 4.35 | 4.55 | 5.56 | 0.48 | - | - | - | - | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Moreira, G.M.; Ramírez-Zamudio, G.D.; Souza, S.P.d.; Prezotto, L.D.; Chalfun, L.H.L.; Duarte, M.d.S.; Casagrande, D.R.; et al. Effects of Maternal Protein Supplementation at Mid-Gestation of Cows on Intake, Digestibility, and Feeding Behavior of the Offspring. Animals 2022, 12, 2865. https://doi.org/10.3390/ani12202865
Nascimento KB, Galvão MC, Meneses JAM, Moreira GM, Ramírez-Zamudio GD, Souza SPd, Prezotto LD, Chalfun LHL, Duarte MdS, Casagrande DR, et al. Effects of Maternal Protein Supplementation at Mid-Gestation of Cows on Intake, Digestibility, and Feeding Behavior of the Offspring. Animals. 2022; 12(20):2865. https://doi.org/10.3390/ani12202865
Chicago/Turabian StyleNascimento, Karolina Batista, Matheus Castilho Galvão, Javier Andrés Moreno Meneses, Gabriel Miranda Moreira, German Darío Ramírez-Zamudio, Stefania Priscilla de Souza, Ligia Dias Prezotto, Luthesco Haddad Lima Chalfun, Marcio de Souza Duarte, Daniel Rume Casagrande, and et al. 2022. "Effects of Maternal Protein Supplementation at Mid-Gestation of Cows on Intake, Digestibility, and Feeding Behavior of the Offspring" Animals 12, no. 20: 2865. https://doi.org/10.3390/ani12202865
APA StyleNascimento, K. B., Galvão, M. C., Meneses, J. A. M., Moreira, G. M., Ramírez-Zamudio, G. D., Souza, S. P. d., Prezotto, L. D., Chalfun, L. H. L., Duarte, M. d. S., Casagrande, D. R., & Gionbelli, M. P. (2022). Effects of Maternal Protein Supplementation at Mid-Gestation of Cows on Intake, Digestibility, and Feeding Behavior of the Offspring. Animals, 12(20), 2865. https://doi.org/10.3390/ani12202865










