Evaluation of the Effect of Sampling Time on Biomarkers of Stress, Immune System, Redox Status and Other Biochemistry Analytes in Saliva of Finishing Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Stress Biomarkers
2.3. Immune System Biomarkers
2.4. Redox Biomarkers
2.5. Analytes Related with Metabolism and Different Tissues and Organs
2.6. Statistical Analysis
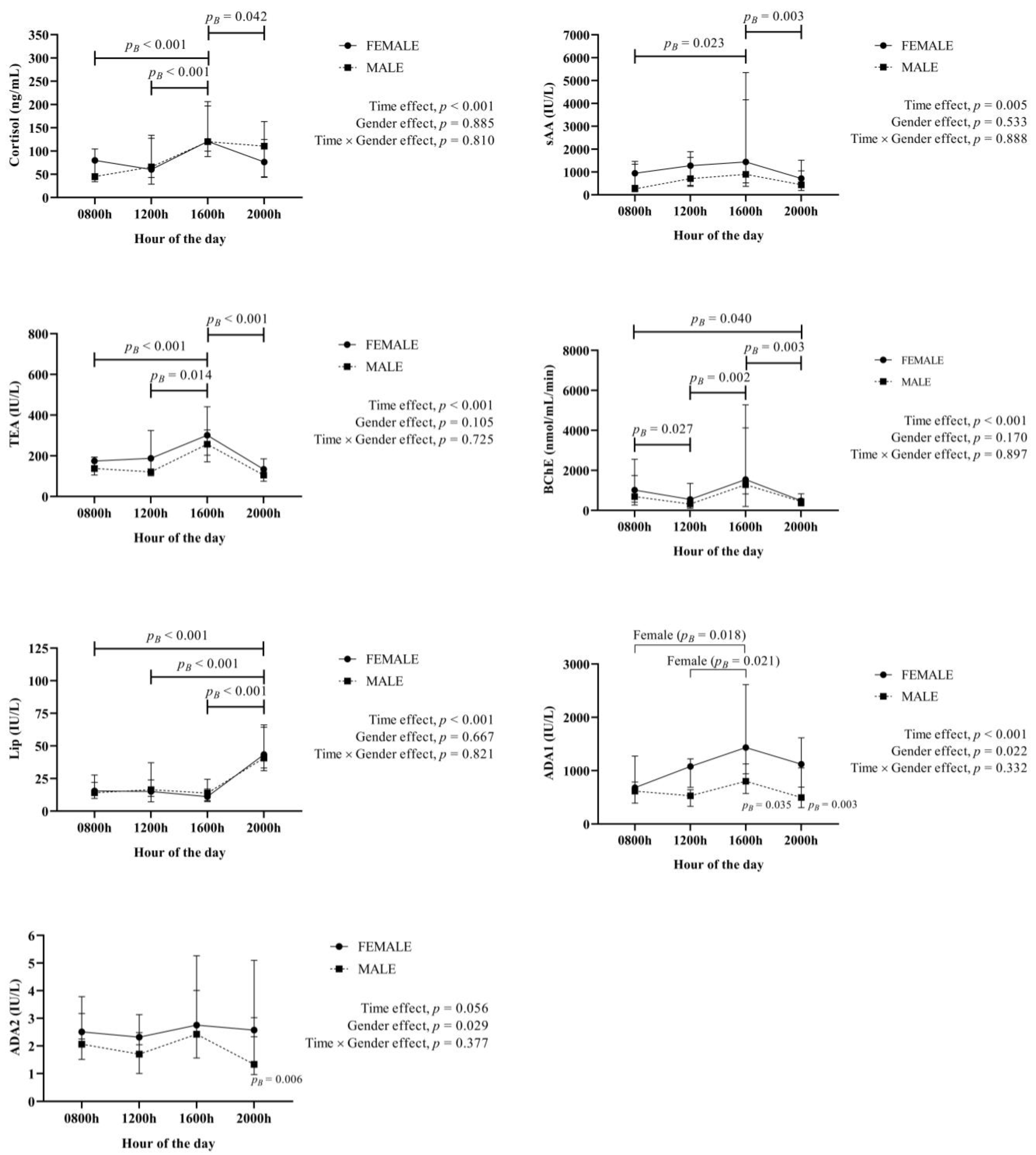
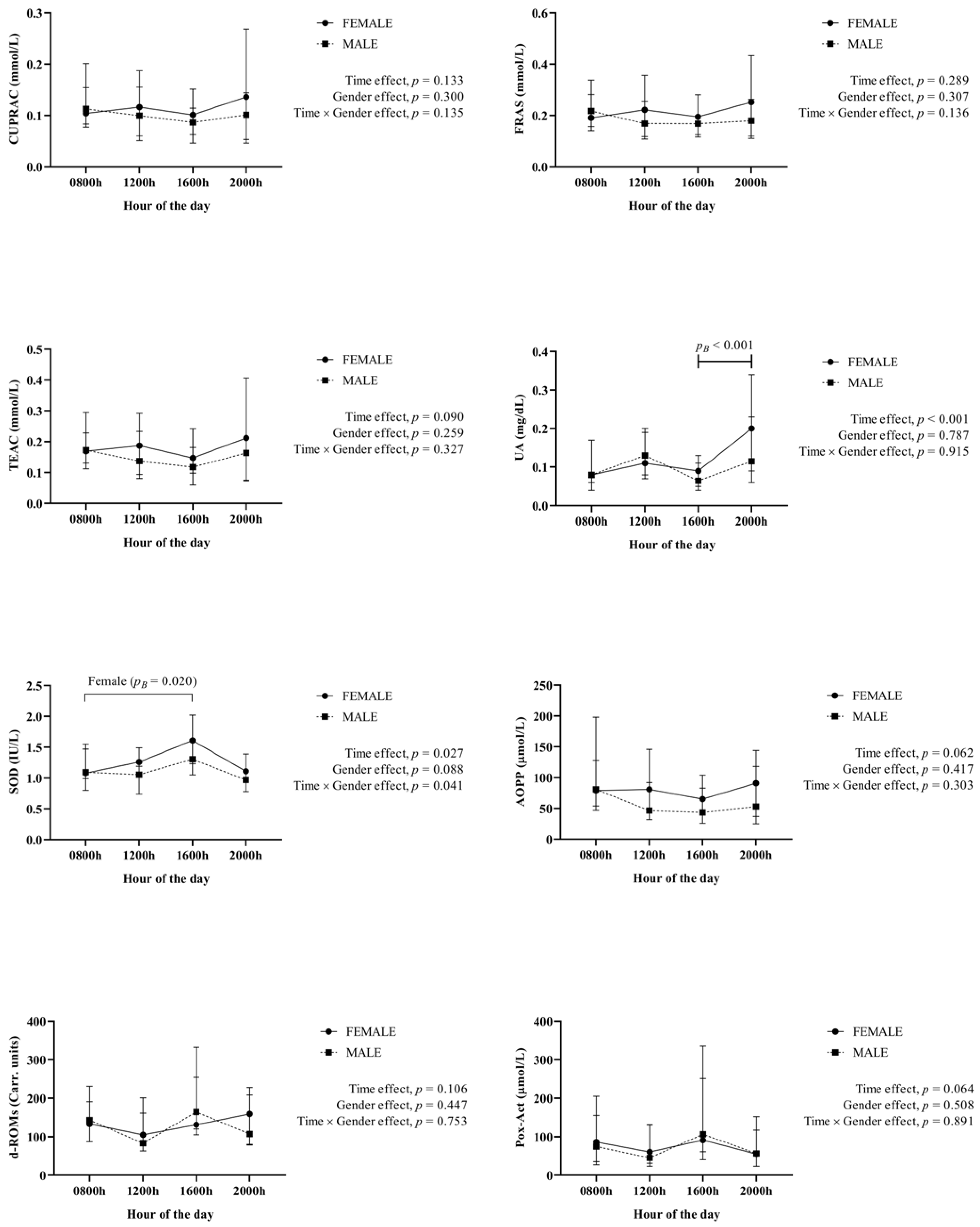
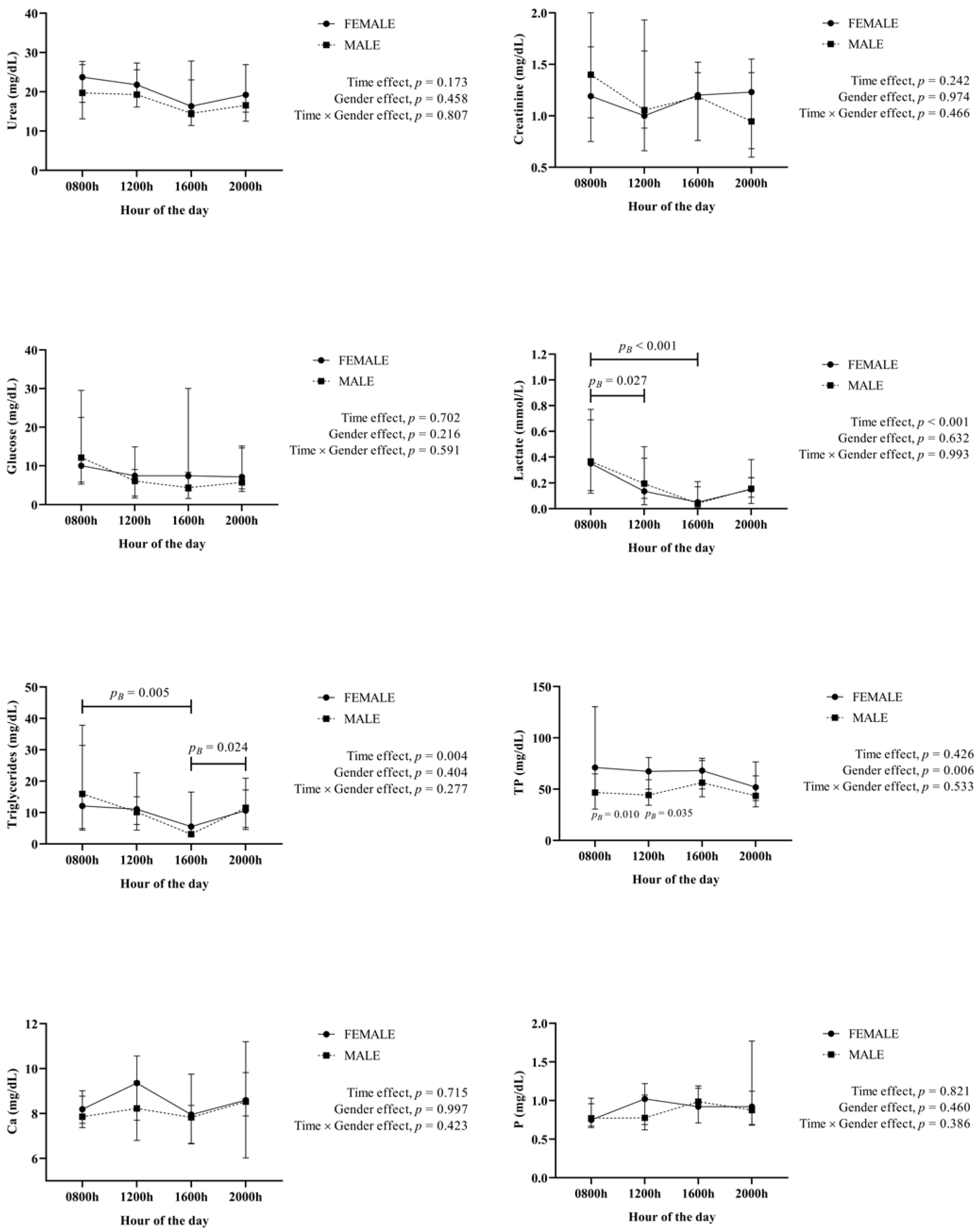
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cerón, J. Acute Phase Proteins, Saliva and Education in Laboratory Science: An Update and Some Reflections. BMC Vet. Res. 2019, 15, 197. [Google Scholar] [CrossRef]
- Cerón, J.; Contreras-Aguilar, M.; Escribano, D.; Martínez-Miró, S.; López-Martínez, M.; Ortín-Bustillo, A.; Franco-Martínez, L.; Rubio, C.; Muñoz-Prieto, A.; Tvarijonaviciute, A.; et al. Basics for the Potential Use of Saliva to Evaluate Stress, Inflammation, Immune System, and Redox Homeostasis in Pigs. BMC Vet. Res. 2022, 18, 81. [Google Scholar] [CrossRef]
- Giri, U.; Nagaraj, V.; Sankaran, A.; Ramesh, R.; Rajaram, S.; Muthanandam, S.; Arumugam, S.; Santhanam, V. Sialochemical Profile in Depressive Individuals under Antidepressant Therapy: An Observational Study. J. Clin. Diagn. Res. 2018, 12, VC06–VC09. [Google Scholar] [CrossRef]
- Miller, B.; Deutsch, O.; Redlich, M.; Konttinen, Y.; Benoliel, R.; Zaks, B.; Davidovich, E.; Palmon, A.; Aframian, D. Sialochemistry and Cortisol Levels in Patients with Sjogren’s Syndrome. Oral Dis. 2012, 18, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.; López-Arjona, M.; Martínez-Miró, S.; Escribano, D.; Hernández-Ruipérez, F.; Cerón, J.; Tecles, F. Changes in Saliva Analytes during Pregnancy, Farrowing and Lactation in Sows: A Sialochemistry Approach. Vet. J. 2021, 273, 105679. [Google Scholar] [CrossRef] [PubMed]
- Ortín-Bustillo, A.; Escribano, D.; López-Arjona, M.; Botia, M.; Fuentes, P.; Martínez-Miró, S.; Rubio, C.P.; García-Manzanilla, E.; Franco-Martínez, L.; Pardo-Marín, L.; et al. Changes in a Comprehensive Profile of Saliva Analytes in Fattening Pigs during a Complete Productive Cycle: A Longitudinal Study. Animals 2022, 12, 1865. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.; Escribano, D.; Martínez-Subiela, S.; Martín-Cuervo, M.; Lamy, E.; Tecles, F.; Cerón, J. Changes in Saliva Analytes in Equine Acute Abdominal Disease: A Sialochemistry Approach. BMC Vet. Res. 2019, 15, 187. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.; Vallejo-Mateo, P.; Želvytė, R.; Tecles, F.; Rubio, C. Changes in Saliva Analytes Associated with Lameness in Cows: A Pilot Study. Animals 2020, 10, 2078. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A Review of Saliva: Normal Composition, Flow, and Function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Lamy, E.; Escribano, D.; Cerón, J.J.; Tecles, F.; Quiles, A.J.; Hevia, M.L. Changes in Salivary Analytes of Horses Due to Circadian Rhythm and Season: A Pilot Study. Animals 2020, 10, 1486. [Google Scholar] [CrossRef]
- Muneta, Y.; Yoshikawa, T.; Minagawa, Y.; Shibahara, T.; Maeda, R.; Omata, Y. Salivary IgA as a Useful Non-Invasive Marker for Restraint Stress in Pigs. J. Vet. Med. Sci. 2010, 72, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, E.; Schrader, L.; Mayer, C.; Gygax, L. Effects of Weight, Temperature and Behaviour on the Circadian Rhythm of Salivary Cortisol in Growing Pigs. Animal 2008, 2, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, N.L.; Giles, L.R.; Wynn, P.C. The Development of a Circadian Pattern of Salivary Cortisol Secretion in the Neonatal Piglet. Biol. Neonate 2002, 81, 113–118. [Google Scholar] [CrossRef]
- Escribano, D.; Gutiérrez, A.; Fuentes-Rubio, M.; Cerón, J. Saliva Chromogranin A in Growing Pigs: A Study of Circadian Patterns during Daytime and Stability under Different Storage Conditions. Vet. J. 2014, 199, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.M.; Escribano, D.; Fuentes, M.; Cerón, J.J. Circadian Pattern of Acute Phase Proteins in the Saliva of Growing Pigs. Vet. J. 2013, 196, 167–170. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.; Tvarijonaviciute, A.; Monkeviciene, I.; Martín-Cuervo, M.; González-Arostegui, L.; Franco-Martínez, L.; Cerón, J.; Tecles, F.; Escribano, D. Characterization of Total Adenosine Deaminase Activity (ADA) and Its Isoenzymes in Saliva and Serum in Health and Inflammatory Conditions in Four Different Species: An Analytical and Clinical Validation Pilot Study. BMC Vet. Res. 2020, 16, 384. [Google Scholar] [CrossRef]
- Rubio, C.; Mainau, E.; Cerón, J.; Contreras-Aguilar, M.; Martínez-Subiela, S.; Navarro, E.; Tecles, F.; Manteca, X.; Escribano, D. Biomarkers of Oxidative Stress in Saliva in Pigs: Analytical Validation and Changes in Lactation. BMC Vet. Res. 2019, 15, 144. [Google Scholar] [CrossRef]
- López-Arjona, M.; Escribano, D.; Mateo, S.V.; Contreras-Aguilar, M.D.; Rubio, C.P.; Tecles, F.; Cerón, J.J.; Martínez-Subiela, S. Changes in Oxytocin Concentrations in Saliva of Pigs after a Transport and during Lairage at Slaughterhouse. Res. Vet. Sci. 2020, 133, 26–30. [Google Scholar] [CrossRef]
- Fuentes, M.; Tecles, F.; Gutiérrez, A.; Otal, J.; Martínez-Subiela, S.; Cerón, J. Validation of an Automated Method for Salivary Alpha-Amylase Measurements in Pigs (Sus Scrofa Domesticus) and Its Application as a Stress Biomarker. J. Vet. Diagn. Investig. 2011, 23, 282–287. [Google Scholar] [CrossRef]
- Tecles, F.; Contreras-Aguilar, M.D.; Martínez-Miró, S.; Tvarijonaviciute, A.; Martínez-Subiela, S.; Escribano, D.; Cerón, J.J. Total Esterase Measurement in Saliva of Pigs: Validation of an Automated Assay, Characterization and Changes in Stress and Disease Conditions. Res. Vet. Sci. 2017, 114, 170–176. [Google Scholar] [CrossRef]
- Tecles, F.; Escribano, D.; Martínez-Miró, S.; Hernández, F.; Contreras, M.D.; Cerón, J.J. Cholinesterase in Porcine Saliva: Analytical Characterization and Behavior after Experimental Stress. Res. Vet. Sci. 2016, 106, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tecles, F.; Rubio, C.; Contreras-Aguilar, M.; López-Arjona, M.; Martínez-Miró, S.; Martínez-Subiela, S.; Cerón, J. Adenosine Deaminase Activity in Pig Saliva: Analytical Validation of Two Spectrophotometric Assays. J. Vet. Diagn. Investig. 2018, 30, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, A. Evaluation of the Copper(II) Reduction Assay Using Bathocuproinedisulfonic Acid Disodium Salt for the Total Antioxidant Capacity Assessment: The CUPRAC-BCS Assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef]
- Arnao, M.; Cano, A.; Hernández-Ruiz, J.; García-Cánovas, F.; Acosta, M. Inhibition by L-Ascorbic Acid and Other Antioxidants of the 2.2’-Azino-Bis(3-Ethylbenzthiazoline-6-Sulfonic Acid) Oxidation Catalyzed by Peroxidase: A New Approach for Determining Total Antioxidant Status of Foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tatzber, F.; Griebenow, S.; Wonisch, W.; Winkler, R. Dual Method for the Determination of Peroxidase Activity and Total Peroxides-Iodide Leads to a Significant Increase of Peroxidase Activity in Human Sera. Anal. Biochem. 2003, 316, 147–153. [Google Scholar] [CrossRef]
- Cesarone, M.; Belcaro, G.; Carratelli, M.; Cornelli, U.; De Sanctis, M.; Incandela, L.; Barsotti, A.; Terranova, R.; Nicolaides, A. A Simple Test to Monitor Oxidative Stress. Int. Angiol. 1999, 18, 127–130. [Google Scholar]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Ruis, M.; Te Brake, J.; Engel, B.; Ekkel, E.; Buist, W.; Blokhuis, H.; Koolhaas, J. The Circadian Rhythm of Salivary Cortisol in Growing Pigs: Effects of Age, Gender, and Stress. Physiol. Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
- Cornélissen, G.; Touitou, Y.; Tritsch, G.; Bogdan, A.; Auzéby, A.; Reinberg, A.; Halberg, F. Circadian Rhythms of Adenosine Deaminase Activity in Human Erythrocytes: A Transverse Study on Young, Elderly and Senile Demented Subjects. Ric. Clin. Lab. 1985, 15, 365–374. [Google Scholar] [CrossRef]
- Macdermott, R.P.; Tritsch, G.L.; Formeister, J.F.; York, N. Adenosine Deaminase and Nucleoside Phosphorylase Activities in Normal Human Blood Mononuclear Cell Subpopulations. Clin. Exp. Immunol. 1980, 42, 303–307. [Google Scholar] [PubMed]
- Bertouch, J.V.; Roberts Thomson, P.J.; Bradley, J. Diurnal Variation of Lymphocyte Subsets Identified by Monoclonal Antibodies. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, M.; Cabras, T.; Denotti, G.; Fadda, M.B.; Gambarini, G.; Lupi, A.; Manca, I.; Onnis, G.; Piras, V.; Soro, V.; et al. Circadian Rhythms of Histatin 1, Histatin 3, Histatin 5, Statherin and Uric Acid in Whole Human Saliva Secretion. Biol. Rhythm Res. 2010, 33, 213–222. [Google Scholar] [CrossRef]
- Ferguson, D.B.; Fort, A. Circadian Variations in Human Resting Submandibular Saliva Flow Rate and Composition. Arch. Oral Biol. 1974, 19, 47–55. [Google Scholar] [CrossRef]
- Ferguson, D.B.; Botchway, C.A. Circadian Variations in the Flow Rate and Composition of Whole Saliva Stimulated by Mastication. Arch. Oral Biol. 1979, 24, 877–881. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Pardo-Marin, L.; Tecles, F.; Carrillo, J.; Garcia-Martinez, J.; Bernal, L.; Pastor, J.; Cerón, J.; Martinez-Subiela, S. Measurement of Urea and Creatinine in Saliva of Dogs: A Pilot Study. BMC Vet. Res. 2018, 14, 223. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Barranco, T.; Rubio, M.; Carrillo, J.; Martinez-Subiela, S.; Tecles, F.; Carrillo, J.; Cerón, J. Measurement of Creatine Kinase and Aspartate Aminotransferase in Saliva of Dogs: A Pilot Study. BMC Vet. Res. 2017, 13, 168. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.; Escribano, D.; Martínez-Miró, S.; López-Arjona, L.; Rubio, C.; Martínez-Subiela, S.; Cerón, J.; Tecles, F. Application of a Score for Evaluation of Pain, Distress and Discomfort in Pigs with Lameness and Prolapses: Correlation with Saliva Biomarkers and Severity of the Disease. Res. Vet. Sci. 2019, 126, 155–163. [Google Scholar] [CrossRef]
- Kaiser, M.; Jacobson, M.; Andersen, P.; Bækbo, P.; Cerón, J.; Dahl, J.; Escribano, D.; Jacobsen, S. Inflammatory Markers before and after Farrowing in Healthy Sows and in Sows Affected with Postpartum Dysgalactia Syndrome. BMC Vet. Res. 2018, 14, 83. [Google Scholar] [CrossRef]
- López-Martínez, M.J.; Escribano, D.; Ortín-Bustillo, A.; Franco-Martínez, L.; González-Arostegui, L.G.; Cerón, J.J.; Rubio, C.P. Changes in Biomarkers of Redox Status in Saliva of Pigs after an Experimental Sepsis Induction. Antioxidants 2022, 11, 1380. [Google Scholar] [CrossRef]
- Horn, P.S.; Pesce, A.J. Reference Intervals: An Update. Clin. Chim. Acta 2003, 334, 5–23. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortín-Bustillo, A.; Contreras-Aguilar, M.D.; Rubio, C.P.; Botia, M.; Cerón, J.J.; López-Arjona, M.; Martínez-Subiela, S.; Escribano, D.; Tecles, F. Evaluation of the Effect of Sampling Time on Biomarkers of Stress, Immune System, Redox Status and Other Biochemistry Analytes in Saliva of Finishing Pigs. Animals 2022, 12, 2127. https://doi.org/10.3390/ani12162127
Ortín-Bustillo A, Contreras-Aguilar MD, Rubio CP, Botia M, Cerón JJ, López-Arjona M, Martínez-Subiela S, Escribano D, Tecles F. Evaluation of the Effect of Sampling Time on Biomarkers of Stress, Immune System, Redox Status and Other Biochemistry Analytes in Saliva of Finishing Pigs. Animals. 2022; 12(16):2127. https://doi.org/10.3390/ani12162127
Chicago/Turabian StyleOrtín-Bustillo, Alba, María D. Contreras-Aguilar, Camila P. Rubio, María Botia, José J. Cerón, Marina López-Arjona, Silvia Martínez-Subiela, Damián Escribano, and Fernando Tecles. 2022. "Evaluation of the Effect of Sampling Time on Biomarkers of Stress, Immune System, Redox Status and Other Biochemistry Analytes in Saliva of Finishing Pigs" Animals 12, no. 16: 2127. https://doi.org/10.3390/ani12162127
APA StyleOrtín-Bustillo, A., Contreras-Aguilar, M. D., Rubio, C. P., Botia, M., Cerón, J. J., López-Arjona, M., Martínez-Subiela, S., Escribano, D., & Tecles, F. (2022). Evaluation of the Effect of Sampling Time on Biomarkers of Stress, Immune System, Redox Status and Other Biochemistry Analytes in Saliva of Finishing Pigs. Animals, 12(16), 2127. https://doi.org/10.3390/ani12162127







