1. Introduction
Ethiopia has Africa’s largest livestock populations, contributing to approximately 45% of the country’s total value of agricultural production [
1,
2]. Of the livestock species, cattle are considered the most economically significant [
1,
3]. In 2019, the cattle population in Ethiopia was estimated to be 70 million head, comprising almost all local breeds, with most raised in small herds of one to nine head [
4]. Five production systems are used: 76% of cattle are kept in mixed crop–livestock systems, 14% in pastoral/agropastoral systems, 7% in urban/peri-urban systems, 2.5% in commercial dairies, and 0.5% in feedlots [
2]. Overall, the majority (60%) of cattle are 3–10 years of age and are mainly used for breeding (25%) as replacement stock, draught power (22%), and milk (11%) [
4].
Income from livestock accounts for 11% of total income in rural households [
5], and livestock development is considered to be fundamental to the sustainable growth and transformation of Ethiopia [
2,
6]. However, in the last decade, the majority of the growth in the cattle subsector in Ethiopia has been due to increased numbers of animals or farmers rather than improvements in productivity [
3]. To improve the sustainability of livestock growth in Ethiopia, a shift to improved productivity is required.
High rates of morbidity and mortality, particularly in young stock, have constrained progress and limited growth of the cattle sector [
3,
6]. Small-scale studies conducted within the last ten years report dairy calf mortality incidence risks of between 12 and 20% in urban or peri-urban systems [
7,
8,
9] and 31% in mixed crop–livestock systems [
10]. Where reported, calf morbidity incidence risks are higher, ranging between 34 and 67% [
8,
10,
11]. Diarrhea is the most common morbidity reported, followed by respiratory disease and navel ill, with poor colostrum consumption, nutrition, and hygiene management being common risk factors [
7,
8,
9,
10,
11]. Among the available literature, more research has been conducted within the urban/peri-urban/intensive dairy farming sector (including cross-bred cattle) than mixed-crop and pastoral systems, despite the latter two comprising the majority of farms in Ethiopia. Scarce information is available for pastoralist systems.
High mortality leads to not only a loss of income but also a loss of replacement stock and genetic material, making it difficult for farmers to replace their losses or expand their herds [
3,
9]. Environmentally, high mortality rates also contribute to the waste of finite resources and increased emission intensity due to decreased efficiency [
12]. The general recommendations for farmers to improve calf colostrum intake, provide higher quality feed, improve environmental hygiene, and seek veterinary services in order to reduce calf morbidity and mortality are repeated throughout the literature, indicating the persistence of these issues over time and across production systems in Ethiopia.
To contribute to resolving some of these issues, the Young Stock Mortality Reduction Consortium (YSMRC) was formed under the auspices of the Ethiopian Ministry of Agriculture (MoA). Members of the YSMRC have included Addis Ababa University’s College of Veterinary Medicine and Agriculture (AAU-CVMA); Aklilu Lemma Institute of Pathobiology (AAU-ALIPB); the University of Gondar; the National Animal Health Diagnostic and Investigation Centre (NAHDIC); Tufts University; Supporting Evidence-Based Interventions in Livestock (SEBI-Livestock); and the School of Veterinary Medicine, University of California, Davis. An innovative and novel funding mechanism made this project possible, with USAID Feed the Future Innovation Lab for Livestock Systems, the Bill & Melinda Gates Foundation, and the Ethiopian Ministry of Livestock and Fisheries all providing financial support for the project. Through this collaboration, a pilot project was carried out to generate key epidemiological information on the major causes of youngstock morbidity and mortality that hampers the potential productivity of livestock in Ethiopia and to identify and to assess the impact of a package of basic health and husbandry interventions on youngstock mortality within different production systems and ecological zones in Ethiopia. Whereas disease surveillance was conducted in cattle and small ruminants, and health and husbandry interventions were piloted for cattle, small ruminants, and camels, in this paper, we only present the YSMRC findings with respect to cattle findings.
4. Discussion
The results and data presented in this study provide valuable information on livestock production practices, risk factors, and respiratory and diarrheal disease pathogens related to calf morbidity and mortality in Ethiopia. Broad in scope and ambition, the YSMRC generated important insights that can inform livestock extension agents and peri-urban, mixed crop–livestock, and pastoral producers on ways to improve calf health and overall herd production.
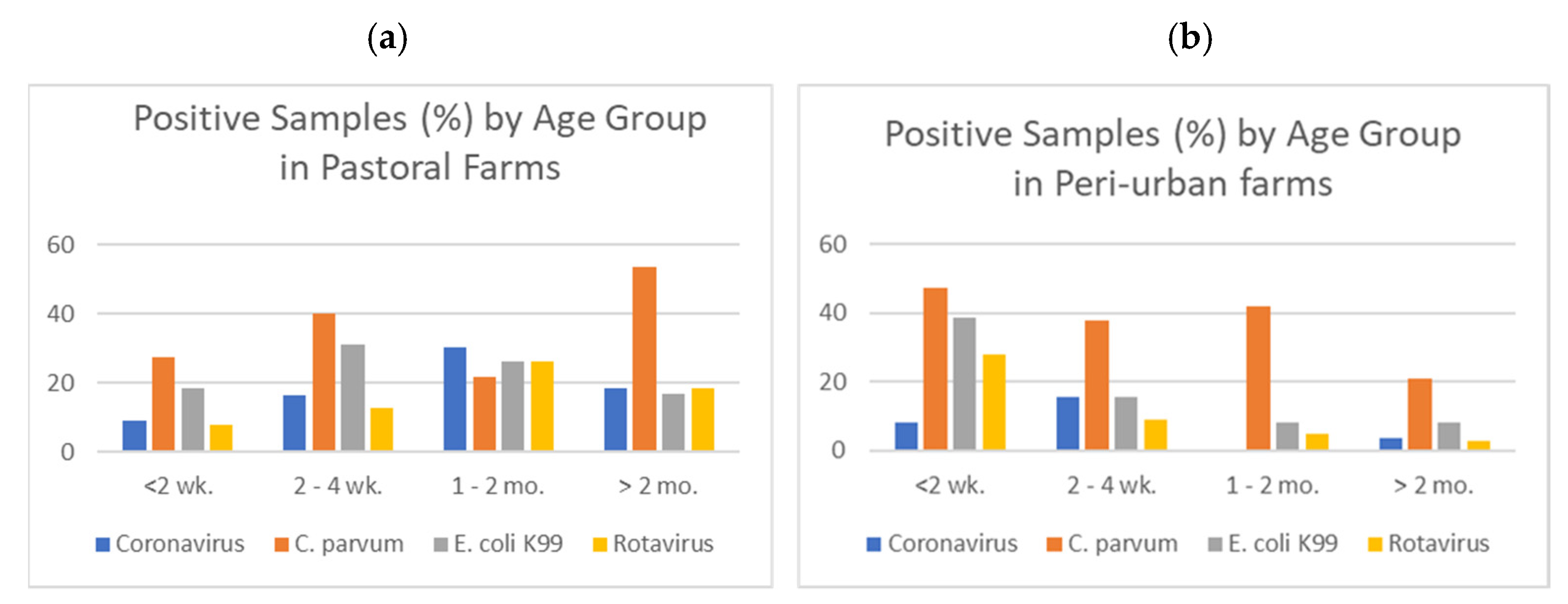
Within the epidemiological arm, the study generated valuable new information regarding the prevalence of specific pathogens that cause diarrhea and respiratory disease in calves. Significant associations between production system and rectal temperature score, body condition score, and fecal score were observed, with the association between fecal score and BCS showing an opposite trend. These findings make sense clinically, in that animals with clinical signs of diarrhea were more likely to show evidence of a fever and/or be in worse physical condition. In both pastoral and peri-urban production systems,
C. parvum was the most common pathogen, followed by
E. Coli K99. Asmare and Kiros (2016) [
8] also identified
Cryptosporidium as the most prevalent pathogen (52.6%) in diarrheic dairy calves, and Ayele et al. (2018) [
19] identified
Cryptosporidium in 18.6% of mixed farm calves. More recent studies observed
Cryptosporidium in 13.8% of calves in a mixed crop–livestock system [
20], with calves under intensive management systems more likely to be infected than calves in extensive systems [
21]. Our findings for
C. parvum in different age groups were slightly unexpected, as
C. parvum most commonly appears in older animals in more developed economies, once animals are kept together in pens. However, this is consistent with the results reported by Asmare and Kiros (2016) [
8], who found
Cryptosporidium in 10 of 19 calves <6 months. Because most of the young stock enrolled in the YSM study were cohoused with animals of all ages, it is possible that is why younger animals on peri-urban farms were as likely to test positive as older animals (>6 mo.). The presence of bovine CoV in younger cattle suggests that it is present on farms, and the longer calves are on a farm, the more likely they may be to be exposed to the virus. The proportion of calves testing positive to CoV in this study is higher than previously reported; Seid et al. (2020) found CoV in only 1 of 83 diarrheic calves [
22]. Bovine CoV can be an important pathogen on farms affecting calves and adults and deserves more investigation. The Pathasure Enteritis 4 diagnostic kit proved a useful and affordable diagnostic tool to employ in the field; additional studies that focus specifically on animals clinically affected by diarrhea paired with more detailed physical exam data could yield more insight into the variations across production systems and inform future preventive care strategies, including vaccination.
C. parvum is a zoonotic pathogen capable of causing diarrhea in children and adults and is associated with environmental enteropathy, malnutrition, and stunting in young children in settings with poor water, sanitation, and hygiene [
23,
24]. Given the proximity of human and livestock housing on some farms in Ethiopia and the impact improved hygiene could have on both calf and human health, an increased focus on common zoonotic pathogens and their relationship with environmental enteropathy is an area worthy of substantially more scientific inquiry. Applying advanced molecular diagnostic techniques in tandem with concurrent sampling of livestock, children, other household members, other domestic animals, and environmental samples in households is an approach that has the potential to yield important information about how infections are spread (i.e., from animals to humans or between humans), the importance of coinfections and comorbidities, and the importance of symptomatic vs. asymptomatic cases with regard to pathogen shedding.
A variety of fecal parasites were identified, with the most common in both mixed crop–livestock and peri-urban farms being
Strongyloides spp. and significantly more likely in mixed crop–livestock systems. Within the epidemiological arm, only 1% of peri-urban producers and no mixed crop–livestock producers reported regular use of anthelmintics in young calves; however, anecdotal experience of several authors of this paper indicate that regular deworming of yearling and adult cattle is common. There are likely several reasons that account for the difference in prevalence rates, including differences in deworming practices, housing, and/or grazing practices in the different production systems, as well as potential anthelmintic resistance. Future intervention packages could consider adopting targeted deworming strategies informed by clinical examination and fecal egg count reduction tests [
25].
The most commonly identified respiratory pathogens in the tested serum samples tested were bovine adenovirus, parainfluenza virus-3 (PIV-3), and bovine respiratory syncytial virus (BRSV). We found no differences in respiratory infection prevalence by age group in peri-urban calves (due to several limitations, in the epidemiological arm, we were only able to collect respiratory pathogen seroprevalence data in peri-urban calves). Viral respiratory pathogens in Ethiopian calves are generally poorly described in the literature. A comparison across a wider geography identified PIV-3 in 20.1% of calves in mixed crop–livestock systems in Kenya [
26], whereas in a recent Belgian study of dairy, dairy-mixed, and beef calves, BRSV was the most commonly isolated respiratory pathogen (29.4%), and PIV-3 was isolated in 8.1% of outbreaks [
27]. A study of Finnish dairy calves reported BRSV in 19% (serum samples) and PIV-3 in 16% of calves [
28]. The majority of the respiratory disease surveillance data relied on serological assays, which can be complicated to interpret in young calves <3 months of age due to maternal antibody interference. This means that a positive test in a young calf may mean the dam was either previously infected (or vaccinated, which is extremely unlikely in this context) and then passed on antibodies to the calf when the calf consumed colostrum or that the calf produced its own antibodies in response to a naturally occurring infection. Because the herders whose animals were sampled do not regularly vaccinate their dams for any respiratory pathogens, a positive test result indicates that, at the very least, these pathogens are circulating in the herd and have the potential to cause disease in both young and adult animals. As calves age, maternal antibodies wane, so the lack of a decrease in prevalence in the older calves supports the fact that calves were infected by these common respiratory pathogens on peri-urban farms in Gondar. Future research to further explore respiratory infections would require longitudinal sampling and paired titers the use of nucleic acid testing assays, such as reverse transcriptase polymerase chain reaction (RT-PCR), for confirmation of infection and/or pathogen identification.
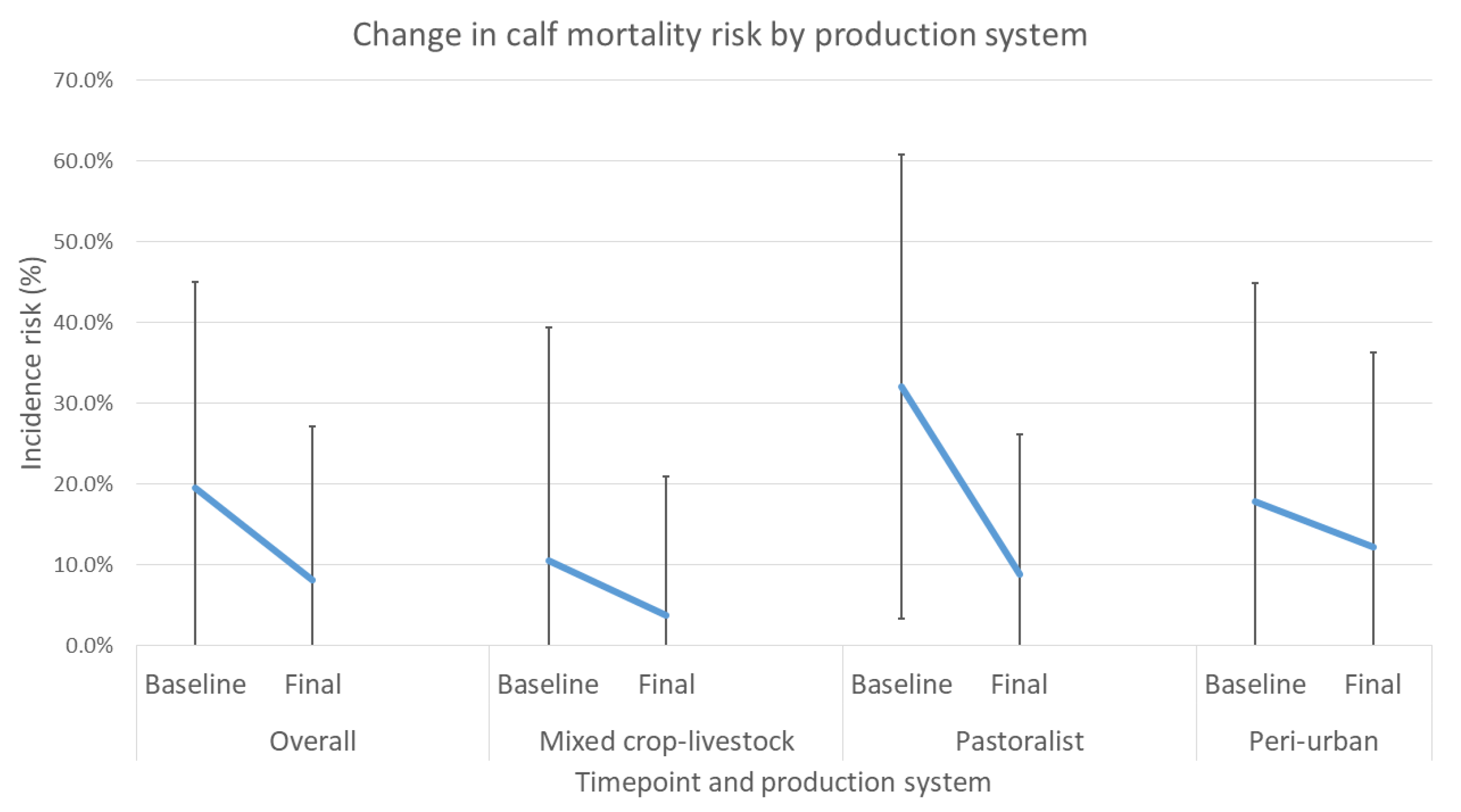
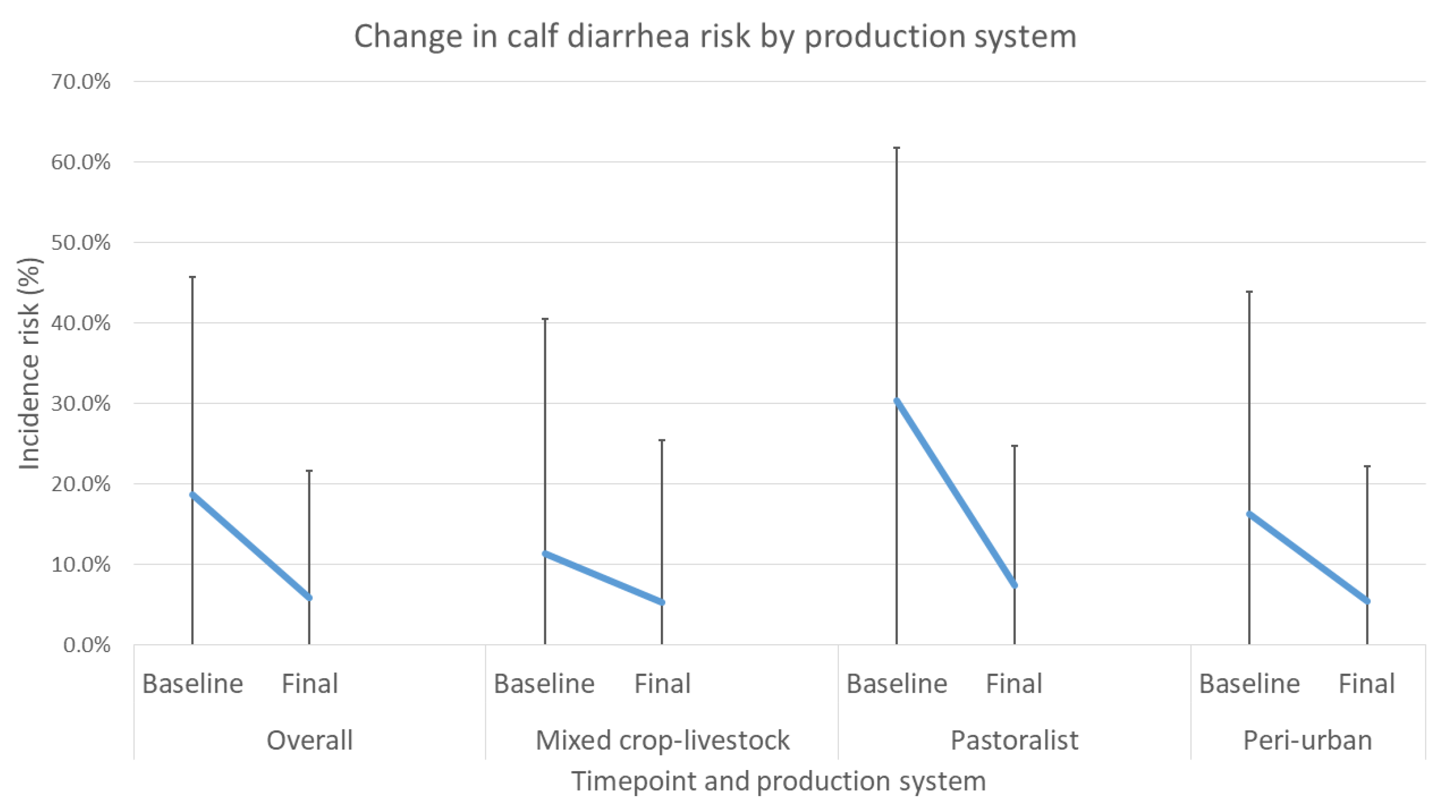
In the intervention arm, baseline levels of mortality in the peri-urban system were similar to those reported in recent studies by Romha (2014) [
7], Asmare and Kiros (2016) [
8], and Fentie et al. (2020) [
9] but lower in the mixed crop–livestock group compared to the results reported by Ferede et al. (2014) [
10]. As delivered, the targeted interventions were shown to definitively decrease calf mortality and reduce the incidence of diarrhea and respiratory disease in calves. Overall, households enrolled in the study showed significant reductions in incidence risk of mortality, diarrhea and respiratory disease. All production systems showed reduced mortality, with 64.2%, 72.4%, and 31.4% reductions for mixed crop–livestock, pastoralist, and peri-urban systems, respectively.
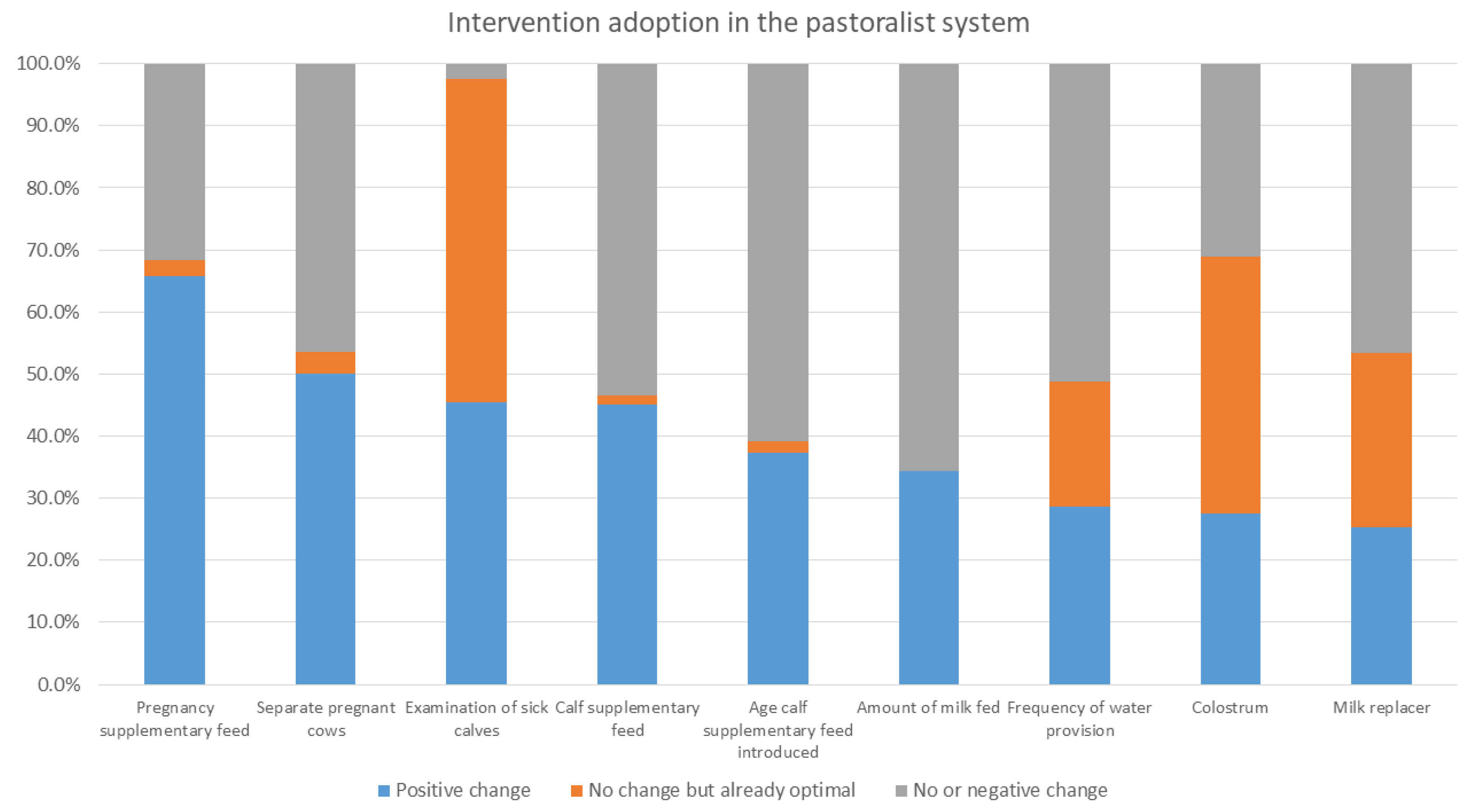
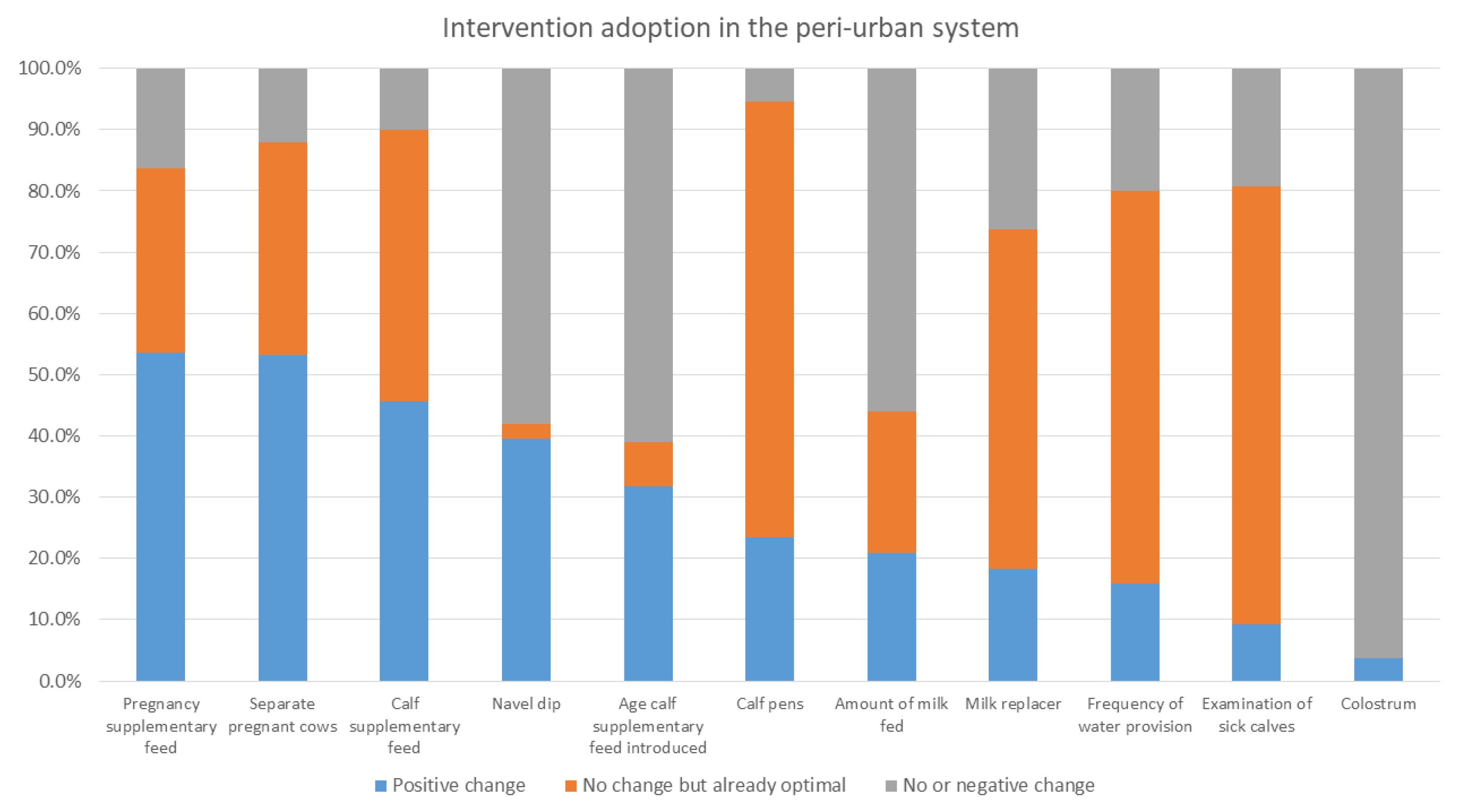
In the intervention arm, overall (i.e., for all systems in all areas), the interventions that had the greatest level of uptake were the provision of supplementary feed during pregnancy and for calves, navel dipping of calves, and separating pregnant cows. As stated, provision of colostrum, use of calf pens, and examination of sick calves were already reported to be practiced by a high percent of households at baseline. Within each production system, different interventions were taken up; producers in the mixed crop–livestock system demonstrated greater uptake of health and hygiene interventions, whereas pastoralist and peri-urban producers had greater uptake for interventions providing nutritional support to pregnant cows and calves. The uptake of interventions may reflect pre-existing knowledge of farmers; accessibility of inputs, including those provided through YSMRC funding; what farmers consider most important; or what was most efficient for farmers to incorporate into their current practices. A repeat evaluation to investigate the sustainability of interventions after conclusion of the YSMRC support and assess long-term impact, as well as further research as to why some interventions were more successful than others, may support the refinement of future intervention packages.
In both the epidemiological intervention arms, the need for adequate nutrition for dams and calves was highlighted. In particular, the importance of adequate colostrum intake within the first few hours after birth cannot be overstated. Other studies in Ethiopia have reported similar findings, with higher mortality rates associated with inadequate colostrum and delayed colostrum [
7,
8,
29]. Neonatal ruminants are dependent on the ingestion of colostrum to obtain crucial antibodies from the dam that support their immune system. Failure of passive transfer of colostral antibodies means that a calf is more susceptible to infectious disease early in life and is a known risk factor for future morbidity and mortality, particularly from respiratory and diarrheal diseases. Many of the enrolled farms in the Intervention arm of the study reported high levels of practicing colostrum management. Despite a high reported level of feeding colostrum, 20.7% of pastoral and 28.6% peri-urban calves exhibited partial or complete failure of passive antibody transfer. This difference highlights an important nuance. Just because a farmer reports a practice does not always mean they are effective at or knowledgeable about the practice, and ensuring a calf suckles after birth does not always translate into a calf consuming enough high-quality colostrum from the dam. Importantly, in this study, we also identified that in general, calves and cows did not receive enough milk or supplemental food in the evaluated population. Underfeeding, particularly of milk in the first few weeks of a calf’s life, likely contributes to poor health outcomes. Market and household economic pressures to sell milk (rather than feeding it to calves), unavailability or prohibitively high cost of milk replacer and other supplemental feeds, farmer knowledge of how much milk a young calf requires, and other factors are all possible causes of the underfeeding observed in this study. However, after receiving training, farmers often readily adopted supplemental feeding as a new practice. Identifying misconceptions, barriers, and facilitators of both colostrum and supplemental feeding practices warrants further investigation.
This study is subject to several limitations and lessons learned. Due to a series of implementation challenges, although there was substantial overlap of the households in the four regions where both the epidemiological and intervention arms were conducted, we were not able to aggregate household or animal data across both arms. Furthermore, a large number of households were lost to follow-up or during the data-cleaning process in both arms of the study.
Within the intervention arm, more pastoralist participants (61.5% of households lost to follow-up) did not participate in the final evaluation compared to mixed-crop (11.5%) and peri-urban households (27%). The difficulties of enrolling pastoralist households included similarities in names across households and the lack of fixed addresses to definitively identify households or to follow-up. The difficulties in reaching pastoralist communities likely contribute to both the dearth of data available and the level of support pastoralists are able to access and is an area that deserves more dedicated attention and research. Additionally, many households in the mixed crop–livestock production system were excluded from the intervention-arm analysis due to no calves being born in the 12 months prior to the baseline or final evaluations. A larger sample size and/or longer research period is recommended for future studies, and reproductive management should be considered as an area for farmer training. Finally, the number of data entries censured for incompleteness, inaccuracy, or implausibility could be improved by enhanced enumerator training, checks and balances built into the questionnaires, or with the use of electronic data collection systems that can automatically flag missing or aberrant data, whereas smaller degrees of change could be captured with improved questionnaire response options.
Another important limitation of the intervention arm arose through the quantity of data it was feasible to collect, as well as the use of a package of interventions, which, combined, did not allow the analysis to identify the intervention(s) that contributed most significantly to change. Understanding the contribution of individual practices could be useful in terms of prioritizing interventions and analyzing the return on investment for producers, particularly when maintaining certain practices requires significant producer or government expenditure.
We also experienced challenges in the epidemiological arm with respect to data depth, quality, and consistency. Due to financial and logistical constraints, it was not possible to test each animal enrolled for the full range of pathogens. Procuring the appropriate testing supplies, kits, and laboratory reagents in Ethiopia was incredibly time- and resource-intensive. A portion of the funding was focused on capacity development, and Ethiopian graduate and veterinary students were trained to assist in data collection and laboratory testing on this project. Variability in the level of student engagement, competency, and proficiency in animal handling and physical examination, as well as attention to detail (especially with record keeping and data entry) and faculty oversight, all contributed to variations in the quality and consistency of data collected in the epidemiological arm. The study team worked to address these challenges by providing standardized data collection protocols, several in-person trainings, and remote mentoring with the students that worked with the project. A useful lesson was that placing students together in groups of three or four dramatically improved the quality of data collected in the field, as they could work together while performing household surveys and clinical exams, collecting specimens, and performing laboratory diagnostics.
Despite the limitations and challenges encountered, we believe that the information gleaned through this effort is useful for Ethiopian farmers, livestock extension workers, and policy makers. Additionally, the working relationships of the YSMRC formed an invaluable foundation for future collaborative work and set a new precedent for a collaborative funding model for livestock development work in Ethiopia. Throughout the study period, the YSMRC was committed to developing human and institutional capacity through dedicated trainings, mentorship, and oversight, working with faculty professors, graduate students, and laboratory staff, along with extension officers and livestock keepers, through the YSMRC intervention activities. Specifically, 28 students from 7 universities. including 15 veterinary students and 13 graduate students, were supported by the project. All gained valuable field and laboratory experience to supplement their education and, importantly, helped contribute to the national food security, nutrition, and economic development objectives of the Ethiopian veterinary and livestock research sectors.
At the time of publication, we are aware that the activities piloted under the intervention arm are currently being scaled-up by the Ethiopian government based on the findings and recommendations of the YSMRC study. The epidemiological arm revealed that certain behaviors and practices associated with improved health outcomes on farms were associated with education, gender, education, use of AI for breeding, and/or production system. This included use of a separate birthing area, use of a close-up pen, use of bedding, record keeping, feeding practices, and quarantining new animals. Several reasons or explanations for this may exist, and confounders, such as household income and training or supervision received by private AI technicians, may be responsible for some of these associations. However, as the intervention packages are scaled by the MoA, recognizing the importance of prior or repetitive training, making packages gender-sensitive, identifying practices most likely to be adopted by producers, and considering which require the most external support to be sustained are all important points to consider. Training for farmers on how to identify at-risk calves and how to prioritize treatment of the sickest animals may represent a second level of training that could be delivered. Furthermore, improving the understanding of the nuanced differences that affect behaviors across the different production systems, particularly those surrounding gender and culture, might be especially important to refine the intervention packages and overcome long-held beliefs and practices associated with livestock ownership. Additionally, interventions could be evaluated from a perspective of what is most economically worthwhile while simultaneously evaluating the time benefit and tradeoff in time and effort at the household level, particularly though a gender lens.