Ovine Neosporosis: The Current Global Situation
Abstract
Simple Summary
Abstract
1. Introduction
2. Life Cycle and Transmission
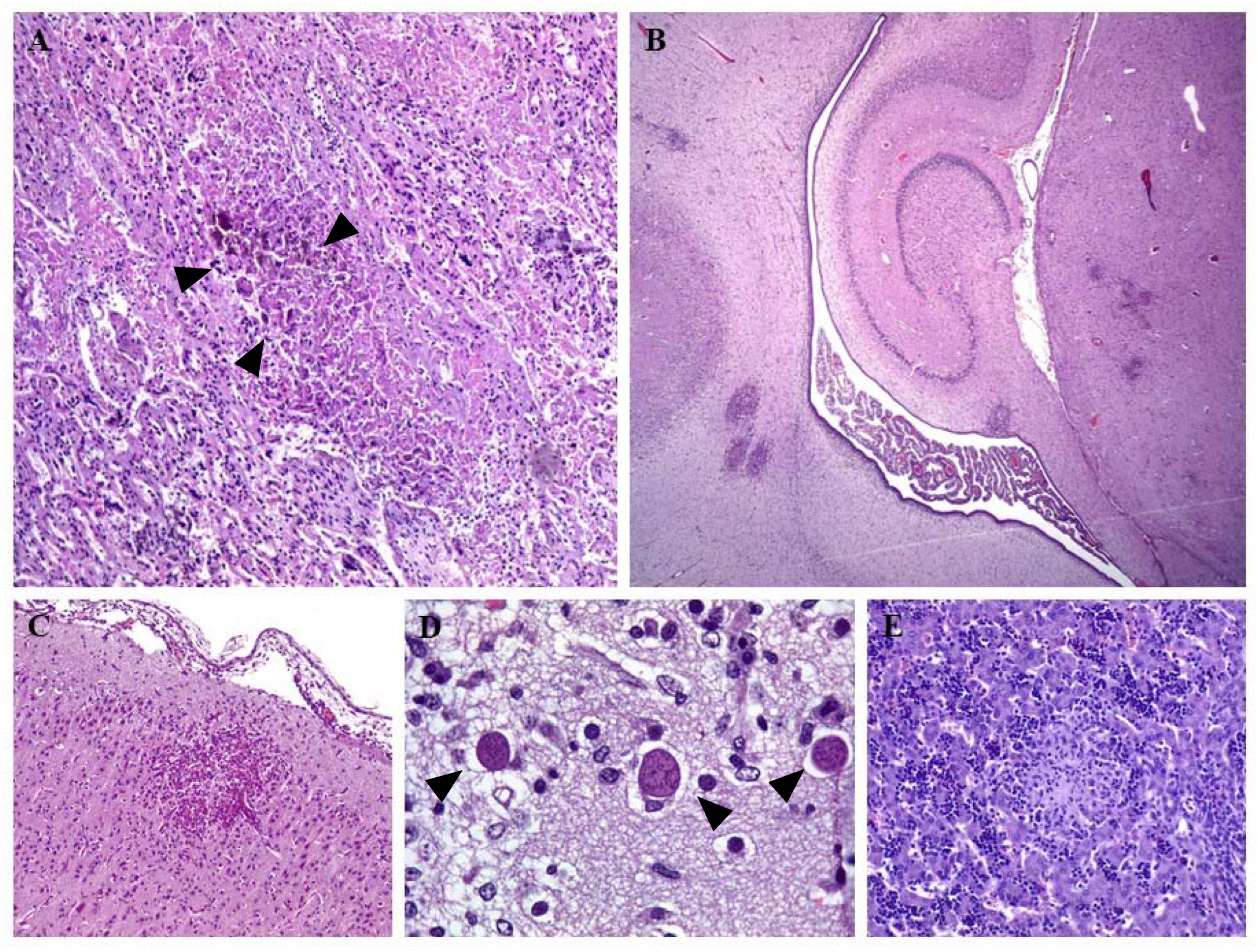
3. Clinical Signs and Lesions
4. Diagnosis
4.1. DNA Detection by PCR
4.2. Serology
| Diagnosis of Neospora Infection | Prevalence (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sampling | Serum Antibodies | Parasite DNA | Serum Antibodies | Parasite DNA | ||||||
| Continent | Country | Animals (N) | Flocks (N) | Technique (Product) | Technique | Animals | Flocks | Animals | Flocks | Reference |
| AFRICA | Egypt | 430 | - | iELISA (IDEXX®) | na | 8.6 | - | - | - | [75] |
| Gabon | 95 | - | iELISA (IDVet®) | na | 42.1 | - | - | - | [76] | |
| Senegal | 174 | - | cELISA (VMRD®) | na | 41.9 | - | - | - | [77] | |
| Tanzania | 412 | - | iELISA (IDVet®) | na | 1.5 | - | - | - | [78] | |
| Tunisia | 198 | - | na | nPCR $ | - | - | 10.6 | - | [27] | |
| AMERICA | Argentina | 704 | 6 | IFAT (IH) | na | 3 | 66.7 | - | - | [79] |
| 130 | - | IFAT (IH) | na | 1.5 | - | - | - | [80] | ||
| Brazil | 141 | 15 | IFAT (IH) | na | 29 | 60 | - | - | [81] | |
| 597 | 30 | IFAT (IH) | na | 9.2 | 86.7 | - | - | [26] | ||
| 62 | - | iELISA (CHEKIT) | na | 3.2 | - | - | - | [82] | ||
| 305 | 9 | IFAT (IH) | na | 9.5 | - | - | - | [30] | ||
| 1028 | 32 | IFAT (IH) | na | 8.8 | 87.50 | - | - | [34] | ||
| 409 | 35 | IFAT (IH) | na | 1.8 | 17.1 | - | - | [83] | ||
| 382 | 8 | IFAT (IH) | na | 12.8 | 87.5 | - | - | [84] | ||
| 381 | 11 | IFAT (IH) | na | 13.91 | 81.8 | - | - | [85] | ||
| 343 | 26 | IFAT (IH) | na | 9.6 | 53.8 | - | - | [86] | ||
| 334 | 12 | IFAT (IH) | na | 8.1 | 83.3 | - | - | [87] | ||
| 81 | 23 | IFAT (IH) | na | 64.2 | ND | - | - | [88] | ||
| 1497 | 16 | IFAT (IH) | na | 8 | 50 | - | - | [89] | ||
| 64 | 5 | IFAT (IH) | na | 4.7 | 40 | - | - | [90] | ||
| 360 | 13 | IFAT (IH) | na | 5.83 | 46.1 | - | - | [91] | ||
| 488 | 63 | IFAT (IH) | na | 13.1 | 49.2 | - | - | [92] | ||
| 795 | 31 | IFAT (IH) | na | 13.2 | - | - | - | [93] | ||
| 596 | - | IFAT (IH) | na | 59.2 | - | - | - | [94] | ||
| 110 | - | iELISA (IH) | na | 33.6 | - | - | - | [62] | ||
| 182 | 8 | IFAT (IH) | na | 13.74 | 75 | - | - | [95] | ||
| 932 | 54 | IFAT (IH) | na | 12.45 | 75.9 | - | - | [96] | ||
| 300 | 10 | IFAT (IH) | na | 16.3 | 90 | - | - | [97] | ||
| 332 | - | IFAT (IH) | na | 10.2 | - | - | - | [98] | ||
| 81 | 7 | IFAT (IH) | na | 3.70 | 42.86 | - | - | [99] | ||
| 1200 | 60 | IFAT (IH) | na | 39.8 | 68.3 | - | - | [100] | ||
| 50 | - | iELISA (IH) | na | 72 | - | - | - | [63] | ||
| 388 | 12 | iELISA (IDEXX®) | na | 6.2 | 50 | - | - | [101] | ||
| 1800 | 705 | IFAT (IH) | na | 18.44 | 19 | - | - | [102] | ||
| 616 | 20 | IFAT (IH) | na | 60.6 | 100 | - | - | [103] | ||
| 1607 | 80 | iELISA (IMUNODOT®) | na | 17.6 | 80 | - | - | [104] | ||
| Costa Rica | 392 | 10 | iELISA (IDVet®) | na | 10.9 | 90 | - | - | [105] | |
| Grenada | 138 | - | iELISA (IDVet®) | na | 13 | - | - | - | [106] | |
| Mexico | 324 | 13 | iELISA (IDEXX®) | nPCR & | 5.5 | 61.5 | 25 | 84.6 | [23] | |
| 368 | 13 | iELISA (IDEXX®) | nPCR & | 13.5 | 92.3 | 27 | 92.3 | [7] | ||
| Uruguay | 184 | 8 | iELISA (IDEXX®) | cPCR & | 15.2 | 75 | 14.1 | 75 | [48] | |
| 1357 | 10 | iELISA (IDEXX®) | na | 1.2 | 30 | - | - | [107] | ||
| ASIA | China | 600 | - | iELISA (IDEXX®) | na | 10.3 | - | - | - | [108] |
| 779 | - | iELISA (IDEXX®) | na | 7.32 | - | - | - | [109] | ||
| 2187 | - | cELISA (IDEXX®) | na | 8.4 | - | - | - | [33] | ||
| 299 | - | MAT (IH) | na | 5.69 | - | - | - | [28] | ||
| Iran | 317 | - | IFAT (IH) cELISA (VMRD®) | na | 2.52 4.1 | - | - | - | [60] | |
| 586 | - | iELISA (IDVet®) cELISA (VMRD®) | na | 1.13 | - | - | - | [110] | ||
| 330 | - | na | nPCR (IH) # | - | - | 3.9 | - | [49] | ||
| 550 | 37 | iELISA (IDVet®) | na | 6.8 | 37.8 | - | - | [31] | ||
| Iraq | 127 | - | iELISA (IDVet®) | na | 4.7 | - | - | - | [111] | |
| Israel | 4804 | - | IFAT (IH) | na | 67.4 | - | - | - | [112] | |
| Jordan | 320 | 38 | iELISA (CHEKIT) | na | 4.3 | 45.8 | - | - | [113] | |
| 339 | 62 | iELISA (BIO-X) | na | 63 | 92 | - | - | [114] | ||
| Malaysia | 317 | 37 | iELISA (IDEXX®) | na | 0 | 0 | - | - | [115] | |
| Pakistan | 128 | - | cELISA (VMRD®) | na | 27.7 | - | - | - | [116] | |
| Turkey | 376 | - | iELISA (CHEKIT) | na | 2.13 | - | - | - | [117] | |
| 180 | - | cELISA VMRD® | na | 7.8 | - | - | - | [118] | ||
| 610 | - | iELISA (IH) | na | 2.1 | - | - | - | [73] | ||
| EUROPE | Czech Republic | 547 | 9 | cELISA (VMRD®) | na | 12 | 100 | - | - | [119] |
| Greece | 458 | 50 | iELISA (IH) | na | 16.8 | 56 | - | - | [120] | |
| 80 | - | iELISA (IDEXX®) | na | 2.5 | - | - | - | [121] | ||
| Italy | 1010 | - | iELISA (CHEKIT) | na | 2 | - | - | - | [122] | |
| 304 | 5 | iELISA (Biotech®) | na | 44.4 | 100 | - | - | [57] | ||
| 428 | 39 | iELISA & WB (IH) | na | 19.3 | 89.4 | - | - | [35] | ||
| 138 | - | na | nPCR ¥ | - | - | 72.5 | - | [50] | ||
| Poland | 64 | - | iELISA (IDVet®) | na | 13 | - | - | - | [123] | |
| Spain | 177 | - | cELISA (VMRD®) | na | 10.1 | - | - | - | [124] | |
| 209 | 12 | iELISA (IDVet®) | na | 1.9 | 25 | - | - | [125] | ||
| 180 | - | cELISA (VMRD®) | na | 3.9 | - | - | - | [126] | ||
| 90 | - | iELISA (IDEXX®) | na | 0.0 | - | - | - | [121] | ||
| 2400 | 44 | iELISA (IDVet®) | na | 5.5 | 72.7 | - | - | [127] | ||
| Switzerland | 117 | - | IFAT (IH) | na | 10.3 | - | - | - | [38] | |
| OCEANIA | Australia | 232 | 5 | iELISA (IDEXX®) cELISA (VMRD®) | na | 0 2.2 | 0 60 | - | - | [42] |
| 558 | 30 | iELISA (IDVet®) | na | 0 | 0 | - | - | [128] | ||
| New Zealand | 640 | - | iELISA (IDEXX®) | na | 0.62 | - | - | - | [129] | |
| 284 | 35 | IFAT (IH) iELISA (IDEXX®) | nPCR & | 41.05 1.3 | - - | 3.5 | - | [47] | ||
5. Prevalence
5.1. America
5.2. Africa
5.3. Asia
5.4. Europe
5.5. Oceania
5.6. Experimental Design Variables and Risk Factors
| Continent | Country | Abortions and/or Perinatal Deaths (N) | Samples | Serum Antibodies | Parasite DNA | IHC | Co-Infection with T. gondii | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Technique | Prevalence (%) | Technique | Prevalence (%) | Prevalence (%) | ||||||
| AMERICA | Argentina | 63 * | P, FT, TF | IFAT | 12.69 | nPCR | 19.04 | 3.17 | Yes (30.7%) | [133] |
| Brazil | 294 | SS | IFAT | 18 | na | - | na | 3.7 | [134] | |
| ASIA | Iran | 70 | SS, P, FT | iELISA (IDEXX®) | 5.7 | cPCR | 8.5 | na | na | [135] |
| 358 | SS | iELISA | 2.2 | na | - | na | na | [136] | ||
| 109 * | FB | na | - | nPCR | 0.9 | na | na | [137] | ||
| 71 | FB | na | - | cPCR | 9.8 | na | na | [138] | ||
| 57 | FB | na | - | nPCR | 3.5 | na | na | [36] | ||
| 130 | P, FT | na | - | cPCR | 2.3 | na | na | [139] | ||
| 51 | FB | na | - | nPCR | 15.6 | na | na | [140] | ||
| Iraq | 51 * | P | na | - | cPCR | 13.7 | na | na | [37] | |
| Israel | 135 | TF | IFAT | 23 | na | - | na | na | [112] | |
| 245 | SS | IFAT | 64.8 | na | - | na | na | |||
| EUROPE | Germany | 200 * | P | na | - | RT-PCR | 3.5 | na | No | [40] |
| Italy | 292 | FT | na | - | nPCR | 2 | na | Yes (50%) | [44] | |
| Slovakia | 382 | SS | iELISA | 3.7 | na | - | na | Yes (50%) | [141] | |
| Switzerland | 117 | - | IFAT | 10.3 | na | - | na | na | [38] | |
| Spain | 74 * | FT | na | - | nPCR | 6.8 | na | Yes (20%) | [6] | |
| UnitedKingdom | 281 | FT, TF | IFAT | 0 | na | - | 0 | na | [45] | |
| 660 | SS | iELISA/ IFAT | 4.2/0.45 | na | - | na | No | [142] | ||
| 119 | P, FT | na | - | nPCR | 0 | na | na | [143] | ||
| OCEANIA | Australia | 1279 | SS | iELISA | 0.16 | na | - | na | na | [128] |
| New Zealand | 179 | SS | IFAT | 25 | na | - | na | na | [144] | |
| 220 | SS, B | IFAT/ iELISA | 36.4/1.8 | nPCR | 6.9 | na | na | [47] | ||
| 209 * | P, FB | na | - | nPCR | 15.5/13 | na | na | |||
| Country | Cases | Serostatus | Analytical Results in Placenta and Tissues | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sheep | Lamb | Technique | Neospora DNA | Neospora Antigen (IHC) | ||||||||||
| P | CNS | H | Lv | Lu | Technique | P | CNS | T | ||||||
| Argentina | Sheep, lambs and one fetus aged 112 days | + (69/220) | + (15/93) | IFAT | + (1/1) | + (1/1) | + (1/1) | na | + (1/1) | nPCR | + (1/1) | NA | + (1/1) | [41] |
| Australia | Adult sheep with neurological signs | na | na | na | na | + (1/1) | na | na | na | cPCR | na | + (1/1) | na | [42] |
| Brazil | Newborn lamb with neurological signs | na | na | na | na | + (1/1) | na | na | na | cPCR | na | - | na | [46] |
| Stillborn lamb | + (1/1) | na | IFAT | na | + (1/1) | na | na | na | cPCR | na | + (1/1) | na | ||
| 11 fetuses | na | na | na | na | - | + (2/11) | + (4/11) | - | nPCR | - | - | - | [145] | |
| Japan | Aborted sheep 1 and her twin fetuses | + (1/1) | na | IFAT | na | + (1/1) | na | na | na | nPCR | na | + (1/1) | na | [43] |
| New Zealand | 13 fetuses | na | na | na | + (8/13) | + (7/13) | na | na | na | nPCR | na | na | na | [146] |
| Spain | 4 aborted sheep and their 4 fetuses | + (4/4) | na | iELISA | na | + (4/4) | + (1/3) | + (1/3) | - | nPCR | + (1/1) | + (1/1) | na | [21] |
| 15 sheep and their stillborn lambs | + (8/15) | na | iELISA | na | + (7/15) | + (2/5) | + (2/5) | - | nPCR | na | + (1/5) | na | ||
| 2 sheep and their newborn lambs 2 | + (2/2) | na | iELISA | na | + (2/2) | + (1/1) | + (1/1) | + (1/1) | nPCR | na | + (2/2) | na | ||
| Tanzania | 44 aborted sheep * | - | - | iELISA | na | na | na | na | na | cPCR | na | na | na | [78] |
| Switzerland | 21 aborted sheep and their fetuses | + (8/21) | na | IFAT | na | + (4/21) | na | na | na | cPCR | na | + (4/21) | na | [38] |
| UK | 1-week-old Lamb 3 | na | na | na | na | na | na | na | na | na | na | + (1/1) | na | [2] |
| 14 aborted lambs | na | na | na | na | + (14/14) | - | na | na | nPCR | na | na | na | [56] | |
6. Impact of Ovine Neosporosis in Reproductive Failure
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Schares, G.; Ortega-Mora, L.M. Epidemiology and Control of Neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007, 20, 323–367. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Hartley, W.J.; Lindsay, D.S.; Topper, M.J. Fatal Congenital Neospora caninum Infection in a Lamb. J. Parasitol. 1990, 76, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Hartley, W.J.; Bridge, P.S. A Case of Suspected Congenital Toxoplasma Encephalomyelitis in a Lamb Associated with a Spinal Cord Anomaly. Br. Vet. J. 1975, 131, 380–384. [Google Scholar] [CrossRef]
- Buxton, D. Protozoan Infections (Toxoplasma gondii, Neospora caninum and Sarcocystis Spp.) in Sheep and Goats: Recent Advances. Vet. Res. 1998, 29, 289–310. [Google Scholar]
- Buxton, D.; McAllister, M.M.; Dubey, J.P. The Comparative Pathogenesis of Neosporosis. Trends Parasitol. 2002, 18, 546–552. [Google Scholar] [CrossRef]
- Moreno, B.; Collantes-Fernández, E.; Villa, A.; Navarro, A.; Regidor-Cerrillo, J.; Ortega-Mora, L.M. Occurrence of Neospora caninum and Toxoplasma gondii Infections in Ovine and Caprine Abortions. Vet. Parasitol. 2012, 187, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Romo-Gallegos, J.M.; Cruz-Vázquez, C.; Medina-Esparza, L.; Ramos-Parra, M.; Romero-Salas, D. Prevalence and Risk Factors of Neospora caninum Infection in Ovine Flocks of Central-Western Mexico. Acta Vet. Hung. 2019, 67, 51–59. [Google Scholar] [CrossRef]
- Hemphill, A.; Aguado-Martínez, A.; Müller, J. Approaches for the Vaccination and Treatment of Neospora caninum Infections in Mice and Ruminant Models. Parasitology 2016, 143, 245–259. [Google Scholar] [CrossRef]
- McAllister, M.M.; McGuire, A.M.; Jolley, W.R.; Lindsay, D.S.; Trees, A.J.; Stobart, R.H. Experimental Neosporosis in Pregnant Ewes and Their Offspring. Vet. Pathol. 1996, 33, 647–655. [Google Scholar] [CrossRef]
- Syed-Hussain, S.S.; Howe, L.; Pomroy, W.E.; West, D.M.; Hardcastle, M.; Williamson, N.B. Vertical Transmission in Experimentally Infected Sheep despite Previous Inoculation with Neospora caninum NcNZ1 Isolate. Vet. Parasitol. 2015, 208, 150–158. [Google Scholar] [CrossRef]
- Arranz-Solís, D.; Benavides, J.; Regidor-Cerrillo, J.; Horcajo, P.; Castaño, P.; del Carmen Ferreras, M.; Jiménez-Pelayo, L.; Collantes-Fernández, E.; Ferre, I.; Hemphill, A.; et al. Systemic and Local Immune Responses in Sheep after Neospora caninum Experimental Infection at Early, Mid and Late Gestation. Vet. Res. 2016, 47, 2. [Google Scholar] [CrossRef] [PubMed]
- González-Warleta, M.; Castro-Hermida, J.A.; Calvo, C.; Pérez, V.; Gutiérrez-Expósito, D.; Regidor-Cerrillo, J.; Ortega-Mora, L.M.; Mezo, M. Endogenous Transplacental Transmission of Neospora caninum during Successive Pregnancies across Three Generations of Naturally Infected Sheep. Vet. Res. 2018, 49, 106. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Review of Neospora caninum and Neosporosis in Animals. Korean J. Parasitol. 2003, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- O’Handley, R.; Liddell, S.; Parker, C.; Jenkins, M.C.; Dubey, J.P. Experimental Infection of Sheep with Neospora caninum Oocysts. J. Parasitol. 2002, 88, 1120–1123. [Google Scholar] [CrossRef]
- Gutiérrez-Expósito, D.; González-Warleta, M.; Espinosa, J.; Vallejo-García, R.; Castro-Hermida, J.A.; Calvo, C.; Ferreras, M.C.; Pérez, V.; Benavides, J.; Mezo, M. Maternal Immune Response in the Placenta of Sheep during Recrudescence of Natural Congenital Infection of Neospora caninum. Vet. Parasitol. 2020, 285, 109204. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, R.; Vázquez-Calvo, Á.; Fernández-Escobar, M.; Regidor-Cerrillo, J.; Benavides, J.; Gutiérrez, J.; Gutiérrez-Expósito, D.; Crespo-Ramos, F.J.; Ortega-Mora, L.M.; Álvarez-García, G. Dynamics of Neospora caninum-Associated Abortions in a Dairy Sheep Flock and Results of a Test-and-Cull Control Programme. Pathogens 2021, 10, 1518. [Google Scholar] [CrossRef] [PubMed]
- Almería, S.; López-Gatius, F. Bovine Neosporosis: Clinical and Practical Aspects. Res. Vet. Sci. 2013, 95, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Hemphill, A.; Calero-Bernal, R.; Schares, G. Neosporosis in Animals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-5254-1. [Google Scholar]
- Costa, R.C.; Orlando, D.R.; Abreu, C.C.; Nakagaki, K.Y.R.; Mesquita, L.P.; Nascimento, L.C.; Silva, A.C.; Maiorka, P.C.; Peconick, A.P.; Raymundo, D.L.; et al. Histological and Immunohistochemical Characterization of the Inflammatory and Glial Cells in the Central Nervous System of Goat Fetuses and Adult Male Goats Naturally Infected with Neospora caninum. BMC Vet. Res. 2014, 10, 291. [Google Scholar] [CrossRef]
- Mesquita, L.P.; Nogueira, C.I.; Costa, R.C.; Orlando, D.R.; Bruhn, F.R.P.; Lopes, P.F.R.; Nakagaki, K.Y.R.; Peconick, A.P.; Seixas, J.N.; Bezerra, P.S.; et al. Antibody Kinetics in Goats and Conceptuses Naturally Infected with Neospora caninum. Vet. Parasitol 2013, 196, 327–333. [Google Scholar] [CrossRef]
- González-Warleta, M.; Castro-Hermida, J.A.; Regidor-Cerrillo, J.; Benavides, J.; Álvarez-García, G.; Fuertes, M.; Ortega-Mora, L.M.; Mezo, M. Neospora caninum Infection as a Cause of Reproductive Failure in a Sheep Flock. Vet. Res. 2014, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.L.; Hartley, C.S.; Björkman, C.; Trees, A.J. Endogenous and Exogenous Transplacental Transmission of Neospora Caninum—How the Route of Transmission Impacts on Epidemiology and Control of Disease. Parasitology 2009, 136, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Hernández, A.; Cruz-Vázquez, C.; Medina-Esparza, L. Neospora caninum: Seroprevalence and DNA Detection in Blood of Sheep from Aguascalientes, Mexico. Small Rumin. Res. 2014, 119, 182–186. [Google Scholar] [CrossRef]
- Okeoma, C.M.; Stowell, K.M.; Williamson, N.B.; Pomroy, W.E. Neospora caninum: Quantification of DNA in the Blood of Naturally Infected Aborted and Pregnant Cows Using Real-Time PCR. Exp. Parasitol. 2005, 110, 48–55. [Google Scholar] [CrossRef]
- Santana, O.I.; Cruz-Vázquez, C.; Medina-Esparza, L.; Ramos Parra, M.; Castellanos Morales, C.; Quezada Gallardo, D. Neospora caninum: DNA Detection in Blood during First Gestation of Naturally Infected Heifers. Vet. México 2010, 41, 131–137. [Google Scholar]
- Figliuolo, L.P.C.; Kasai, N.; Ragozo, A.M.A.; de Paula, V.S.O.; Dias, R.A.; Souza, S.L.P.; Gennari, S.M. Prevalence of Anti-Toxoplasma gondii and Anti-Neospora caninum Antibodies in Ovine from São Paulo State, Brazil. Vet. Parasitol. 2004, 123, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Amdouni, Y.; Rjeibi, M.R.; Awadi, S.; Rekik, M.; Gharbi, M. First Detection and Molecular Identification of Neospora caninum from Naturally Infected Cattle and Sheep in North Africa. Transbound. Emerg. Dis. 2018, 65, 976–982. [Google Scholar] [CrossRef]
- Sun, L.-X.; Liang, Q.-L.; Nie, L.-B.; Hu, X.-H.; Li, Z.; Yang, J.-F.; Zou, F.-C.; Zhu, X.-Q. Serological Evidence of Toxoplasma gondii and Neospora caninum Infection in Black-Boned Sheep and Goats in Southwest China. Parasitol. Int. 2020, 75, 102041. [Google Scholar] [CrossRef]
- Stelzer, S.; Basso, W.; Benavides Silván, J.; Ortega-Mora, L.M.; Maksimov, P.; Gethmann, J.; Conraths, F.J.; Schares, G. Toxoplasma gondii Infection and Toxoplasmosis in Farm Animals: Risk Factors and Economic Impact. Food Waterborne Parasitol. 2019, 15, e00037. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, P.R.; Freire, R.L.; Vidotto, O.; Marana, E.R.M.; Ogawa, L.; De Paula, V.S.O.; Garcia, J.L.; Navarro, I.T. Prevalence of Neospora caninum and Toxoplasma gondii in Sheep and Dogs from Guarapuava Farms, Paraná State, Brazil. Res. Vet. Sci. 2007, 82, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, J.; Yakhchali, M.; Esmaeilnejad, B.; Mardani, K.; Majidi, G.; Sohrabi, A.; Berahmat, R.; Hazhir Alaei, M. Seroprevalence and Risk Factors of Neospora caninum and Toxoplasma gondii in Small Ruminants in Southwest of Iran. Arch. Razi Inst. 2018, 73, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Conraths, F.J.; Gottstein, B. Protozoal Abortion in Farm Ruminants: Guidelines for Diagnosis and Control, 1st ed.; Ortega-Mora, L.M., Gottstein, B., Conraths, F.J., Buxton, D., Eds.; CABI International: Oxford, UK, 2007. [Google Scholar]
- Nie, L.-B.; Cong, W.; Zou, Y.; Zhou, D.-H.; Liang, Q.-L.; Zheng, W.-B.; Ma, J.-G.; Du, R.; Zhu, X.-Q. First Report of Seroprevalence and Risk Factors of Neospora caninum Infection in Tibetan Sheep in China. Biomed. Res. Int. 2018, 2018, 2098908. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.E.H.; Gonçalves, V.S.P.; Heinemann, M.B.; Dilli, T.L.B.; Akimoto, B.M.; de Souza, S.L.P.; Gennari, S.M.; Soares, R.M. Prevalence of Toxoplasma gondii and Neospora caninum Infections in Sheep from Federal District, Central Region of Brazil. Trop Anim Health Prod. 2009, 41, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Gazzonis, A.L.; Alvarez Garcia, G.; Zanzani, S.A.; Ortega Mora, L.M.; Invernizzi, A.; Manfredi, M.T. Neospora caninum Infection in Sheep and Goats from North-Eastern Italy and Associated Risk Factors. Small Rumin. Res. 2016, 140, 7–12. [Google Scholar] [CrossRef]
- Amouei, A.; Sharif, M.; Sarvi, S.; Bagheri Nejad, R.; Aghayan, S.A.; Hashemi-Soteh, M.B.; Mizani, A.; Hosseini, S.A.; Gholami, S.; Sadeghi, A.; et al. Aetiology of Livestock Fetal Mortality in Mazandaran Province, Iran. PeerJ 2019, 6, e5920. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaeli, S.J.J.; Ethaeb, A.M.; Gharban, H.A.J. Molecular and Histopathological Identification of Ovine Neosporosis (Neospora caninum) in Aborted Ewes in Iraq. Vet. World 2020, 13, 597–603. [Google Scholar] [CrossRef]
- Hässig, M.; Sager, H.; Reitt, K.; Ziegler, D.; Strabel, D.; Gottstein, B. Neospora caninum in Sheep: A Herd Case Report. Vet. Parasitol. 2003, 117, 213–220. [Google Scholar] [CrossRef]
- Arranz-Solís, D.; Benavides, J.; Regidor-Cerrillo, J.; Fuertes, M.; Ferre, I.; Ferreras, M.D.C.; Collantes-Fernández, E.; Hemphill, A.; Pérez, V.; Ortega-Mora, L.M. Influence of the Gestational Stage on the Clinical Course, Lesional Development and Parasite Distribution in Experimental Ovine Neosporosis. Vet. Res. 2015, 46, 19. [Google Scholar] [CrossRef] [PubMed]
- Meixner, N.; Sommer, M.F.; Scuda, N.; Matiasek, K.; Müller, M. Comparative Aspects of Laboratory Testing for the Detection of Toxoplasma gondii and Its Differentiation from Neospora caninum as the Etiologic Agent of Ovine Abortion. J. Vet. Diagn Invest. 2020, 32, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Hecker, Y.P.; Morrell, E.L.; Fiorentino, M.A.; Gual, I.; Rivera, E.; Fiorani, F.; Dorsch, M.A.; Gos, M.L.; Pardini, L.L.; Scioli, M.V.; et al. Ovine Abortion by Neospora caninum: First Case Reported in Argentina. Acta Parasit. 2019, 64, 950–955. [Google Scholar] [CrossRef]
- Bishop, S.; King, J.; Windsor, P.; Reichel, M.P.; Ellis, J.; Slapeta, J. The First Report of Ovine Cerebral Neosporosis and Evaluation of Neospora caninum Prevalence in Sheep in New South Wales. Vet. Parasitol 2010, 170, 137–142. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamada, M.; Omata, Y.; Koyama, T.; Saito, A.; Matsuda, T.; Okuyama, K.; Fujimoto, S.; Furuoka, H.; Matsui, T. Naturally-Occurring Neospora caninum Infection in an Adult Sheep and Her Twin Fetuses. J. Parasitol 2001, 87, 434–436. [Google Scholar] [CrossRef]
- Masala, G.; Porcu, R.; Daga, C.; Denti, S.; Canu, G.; Patta, C.; Tola, S. Detection of Pathogens in Ovine and Caprine Abortion Samples from Sardinia, Italy, by PCR. J. Vet. Diagn. Investig. 2007, 19, 96–98. [Google Scholar] [CrossRef]
- Otter, A.; Wilson, B.W.; Scholes, S.F.; Jefrrey, M.; Helmick, B.; Trees, A.J. Results of a Survey to Determine Whether Neospora Is a Significant Cause of Ovine Abortion in England and Wales. Vet. Rec. 1997, 140, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.A.G.; de Sousa, R.S.; Varaschin, M.S.; Becker, A.P.B.B.; Monteiro, A.L.G.; de Oliveira Koch, M.; Costa, R.C.; Laskoski, L.M.; Galindo, C.M.; de Cristo, T.G.; et al. Transplacental Transmission of Neospora caninum to Lambs in Successive Pregnancies of Naturally Infected Sheep in Southern Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100537. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.; Collett, M.G.; Pattison, R.S.; Marshall, J.; West, D.M.; Pomroy, W.E. Potential Involvement of Neospora caninum in Naturally Occurring Ovine Abortions in New Zealand. Vet. Parasitol. 2012, 185, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Alcalá-Gómez, J.; Medina-Esparza, L.; Vitela-Mendoza, I.; Cruz-Vázquez, C.; Quezada-Tristán, T.; Gómez-Leyva, J.F. Prevalence and Risk Factors of Neospora caninum and Toxoplasma gondii Infection in Breeding Ewes from Central Western Mexico. Trop. Anim. Health Prod. 2022, 54, 225. [Google Scholar] [CrossRef]
- Arbabi, M.; Abdoli, A.; Dalimi, A.; Pirestani, M. Identification of Latent Neosporosis in Sheep in Tehran, Iran by Polymerase Chain Reaction Using Primers Specific for the Nc-5 Gene. Onderstepoort J. Vet. Res. 2016, 83, e1–e7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dessì, G.; Tamponi, C.; Pasini, C.; Porcu, F.; Meloni, L.; Cavallo, L.; Sini, M.F.; Knoll, S.; Scala, A.; Varcasia, A. A Survey on Apicomplexa Protozoa in Sheep Slaughtered for Human Consumption. Parasitol. Res. 2022, 121, 1437–1445. [Google Scholar] [CrossRef]
- Santos, S.L.; de Souza Costa, K.; Gondim, L.Q.; da Silva, M.S.A.; Uzêda, R.S.; Abe-Sandes, K.; Gondim, L.F.P. Investigation of Neospora caninum, Hammondia Sp., and Toxoplasma gondii in Tissues from Slaughtered Beef Cattle in Bahia, Brazil. Parasitol. Res. 2010, 106, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Bartley, P.M.; Katzer, F.; Rocchi, M.S.; Maley, S.W.; Benavides, J.; Nath, M.; Pang, Y.; Cantón, G.; Thomson, J.; Chianini, F.; et al. Development of Maternal and Foetal Immune Responses in Cattle Following Experimental Challenge with Neospora caninum at Day 210 of Gestation. Vet. Res. 2013, 44, 91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buxton, D.; Maley, S.W.; Wright, S.; Thomson, K.M.; Rae, A.G.; Innes, E.A. The Pathogenesis of Experimental Neosporosis in Pregnant Sheep. J. Comp. Pathol. 1998, 118, 267–279. [Google Scholar] [CrossRef]
- Yamage, M.; Flechtner, O.; Gottstein, B. Neospora caninum: Specific Oligonucleotide Primers for the Detection of Brain “Cyst” DNA of Experimentally Infected Nude Mice by the Polymerase Chain Reaction (PCR). J. Parasitol. 1996, 82, 272–279. [Google Scholar] [CrossRef]
- Ellis, J.T.; McMillan, D.; Ryce, C.; Payne, S.; Atkinson, R.; Harper, P.A. Development of a Single Tube Nested Polymerase Chain Reaction Assay for the Detection of Neospora caninum DNA. Int. J. Parasitol. 1999, 29, 1589–1596. [Google Scholar] [CrossRef]
- Hughes, J.M.; Williams, R.H.; Morley, E.K.; Cook, D.A.N.; Terry, R.S.; Murphy, R.G.; Smith, J.E.; Hide, G. The Prevalence of Neospora caninum and Co-Infection with Toxoplasma gondii by PCR Analysis in Naturally Occurring Mammal Populations. Parasitology 2006, 132, 29–36. [Google Scholar] [CrossRef]
- Tamponi, C.; Varcasia, A.; Pipia, P.; Zidda, A.; Panzalis, R.; Dore, F.; Dessì, G.; Sanna, G.; Salis, F.; Björkman, C.; et al. ISCOM ELISA in Milk as Screening for Neospora caninum in Dairy Sheep. Large Anim. Rev. 2015, 21, 213–216. [Google Scholar]
- Huertas-López, A.; Martínez-Carrasco, C.; Cerón, J.J.; Sánchez-Sánchez, R.; Vázquez-Calvo, Á.; Álvarez-García, G.; Martínez-Subiela, S. A Time-Resolved Fluorescence Immunoassay for the Detection of Anti- Neospora caninum Antibodies in Sheep. Vet. Parasitol. 2019, 276, 108994. [Google Scholar] [CrossRef]
- Rossi, G.F.; Cabral, D.D.; Ribeiro, D.P.; Pajuaba, A.C.A.M.; Corrêa, R.R.; Moreira, R.Q.; Mineo, T.W.P.; Mineo, J.R.; Silva, D.A.O. Evaluation of Toxoplasma gondii and Neospora caninum Infections in Sheep from Uberlândia, Minas Gerais State, Brazil, by Different Serological Methods. Vet. Parasitol. 2011, 175, 252–259. [Google Scholar] [CrossRef]
- Vajdi Hokmabad, R.; Khanmohammadi, M.; Sarabim, M. Seroprevalence of Neospora caninum in Miyaneh Sheep (Azerbayejan-E-Shargi Province) by Competitive ELISA and IFAT. Methods 2014, 7, 59–66. [Google Scholar]
- Björkman, C.; Uggla, A. Serological Diagnosis of Neospora caninum Infection. Int. J. Parasitol. 1999, 29, 1497–1507. [Google Scholar] [CrossRef]
- Pinheiro, A.F.; Borsuk, S.; Berne, M.E.A.; da Silva Pinto, L.; Andreotti, R.; Roos, T.; Roloff, B.C.; Leite, F.P.L. Use of ELISA Based on NcSRS2 of Neospora caninum Expressed in Pichia Pastoris for Diagnosing Neosporosis in Sheep and Dogs. Rev. Bras. Parasitol. Vet. 2015, 24, 148–154. [Google Scholar] [CrossRef]
- Filho, P.C.G.A.; Oliveira, J.M.B.; Andrade, M.R.; Silva, J.G.; Kim, P.C.P.; Almeida, J.C.; Porto, W.J.N.; Mota, R.A. Incidence and Vertical Transmission Rate of Neospora caninum in Sheep. Comp. Immunol. Microbiol. Infect. Dis. 2017, 52, 19–22. [Google Scholar] [CrossRef]
- Alvarez-García, G.; García-Culebras, A.; Gutiérrez-Expósito, D.; Navarro-Lozano, V.; Pastor-Fernández, I.; Ortega-Mora, L.M. Serological Diagnosis of Bovine Neosporosis: A Comparative Study of Commercially Available ELISA Tests. Vet. Parasitol. 2013, 198, 85–95. [Google Scholar] [CrossRef]
- Jacobson, R.H. Validation of Serological Assays for Diagnosis of Infectious Diseases. Rev. Sci. Tech. 1998, 17, 469–526. [Google Scholar] [CrossRef]
- Wapenaar, W.; Barkema, H.W.; Vanleeuwen, J.A.; McClure, J.T.; O’Handley, R.M.; Kwok, O.C.H.; Thulliez, P.; Dubey, J.P.; Jenkins, M.C. Comparison of Serological Methods for the Diagnosis of Neospora caninum Infection in Cattle. Vet. Parasitol. 2007, 143, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.O.; Lobato, J.; Mineo, T.W.P.; Mineo, J.R. Evaluation of Serological Tests for the Diagnosis of Neospora caninum Infection in Dogs: Optimization of Cut off Titers and Inhibition Studies of Cross-Reactivity with Toxoplasma gondii. Vet. Parasitol. 2007, 143, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Gondim, L.F.P.; Mineo, J.R.; Schares, G. Importance of Serological Cross-Reactivity among Toxoplasma gondii, Hammondia Spp., Neospora Spp., Sarcocystis Spp. and Besnoitia besnoiti. Parasitology 2017, 144, 851–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lee, E.-G.; Yu, L.; Kawano, S.; Huang, P.; Liao, M.; Kawase, O.; Zhang, G.; Zhou, J.; Fujisaki, K.; et al. Identification of the Cross-Reactive and Species-Specific Antigens between Neospora caninum and Toxoplasma gondii Tachyzoites by a Proteomics Approach. Parasitol. Res. 2011, 109, 899–911. [Google Scholar] [CrossRef]
- Regidor-Cerrillo, J.; García-Lunar, P.; Pastor-Fernández, I.; Álvarez-García, G.; Collantes-Fernández, E.; Gómez-Bautista, M.; Ortega-Mora, L.M. Neospora caninum Tachyzoite Immunome Study Reveals Differences among Three Biologically Different Isolates. Vet. Parasitol. 2015, 212, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Claveria, F.G.; Fujisaki, K.; Nagasawa, H. Studies on Serological Cross-Reaction of Neospora caninum with Toxoplasma gondii and Hammondia heydorni. J. Vet. Med. Sci. 2002, 64, 161–164. [Google Scholar] [CrossRef]
- Andreotti, R.; de Fátima Cepa Matos, M.; Gonçalves, K.N.; Oshiro, L.M.; da Costa Lima-Junior, M.S.; Paiva, F.; Leite, F.L. Comparison of Indirect ELISA Based on Recombinant Protein NcSRS2 and IFAT for Detection of Neospora caninum Antibodies in Sheep. Rev. Bras. Parasitol. Vet. 2009, 18, 19–22. [Google Scholar] [CrossRef]
- Zhou, M.; Cao, S.; Sevinc, F.; Sevinc, M.; Ceylan, O.; Liu, M.; Wang, G.; Moumouni, P.F.A.; Jirapattharasate, C.; Suzuki, H.; et al. Enzyme-Linked Immunosorbent Assays Using Recombinant TgSAG2 and NcSAG1 to Detect Toxoplasma gondii and Neospora caninum -Specific Antibodies in Domestic Animals in Turkey. J. Vet. Med. Sci. 2017, 78, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Novoa, M.B.; Aguirre, N.P.; Ormaechea, N.; Palmero, S.; Rouzic, L.; Valentini, B.S.; Sarli, M.; Orcellet, V.M.; Marengo, R.; Vanzini, V.R.; et al. Validation and Field Evaluation of a Competitive Inhibition ELISA Based on the Recombinant Protein TSAG1 to Detect Anti-Neospora caninum Antibodies in Sheep and Goats. Vet. Parasitol. 2020, 284, 109201. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Khater, H.; Almohammed, H.I. A Recent Update about Seroprevalence of Ovine Neosporosis in Northern Egypt and Its Associated Risk Factors. Sci. Rep. 2021, 11, 14043. [Google Scholar] [CrossRef] [PubMed]
- Maganga, G.D.; Abessolo, A.L.; Mikala Okouyi, C.S.; Labouba, I.; Mbeang Beyeme, A.M.; Mavoungou, J.F.; Agossou, E.; Cossic, B.; Akue, J.-P. Seroprevalence and Risk Factors of Two Abortive Diseases, Toxoplasmosis and Neosporosis, in Small Ruminants of the Mongo County, Southern Gabon. Small Rumin. Res. 2016, 144, 56–61. [Google Scholar] [CrossRef]
- Dahourou, L.D.; Gbati, O.B.; Savadogo, M.; Yougbare, B.; Dicko, A.; Combari, A.H.B.; Kamga-Waladjo, A.R. Prevalence of Toxoplasma gondii and Neospora caninum Infections in Households Sheep “Elevage En Case” in Dakar, Senegal. Vet. World 2019, 12, 1028–1032. [Google Scholar] [CrossRef]
- Thomas, K.M.; Kibona, T.; Claxton, J.R.; de Glanville, W.A.; Lankester, F.; Amani, N.; Buza, J.J.; Carter, R.W.; Chapman, G.E.; Crump, J.A.; et al. Prospective Cohort Study Reveals Unexpected Aetiologies of Livestock Abortion in Northern Tanzania. Sci Rep. 2022, 12, 11669. [Google Scholar] [CrossRef]
- Hecker, Y.P.; Moore, D.P.; Manazza, J.A.; Unzaga, J.M.; Späth, E.J.A.; Pardini, L.L.; Venturini, M.C.; Roberi, J.L.; Campero, C.M. First Report of Seroprevalence of Toxoplasma gondii and Neospora caninum in Dairy Sheep from Humid Pampa, Argentina. Trop Anim Health Prod. 2013, 45, 1645–1647. [Google Scholar] [CrossRef]
- Hecker, Y.P.; Masson, F.M.; Armendano, J.I.; Cora, J.; Olivares, C.F.; Gual, I.; Pardini, L.; Moore, D.P.; Moré, G.; Cantón, G.J. Evaluation of Frequency of Antibodies against Toxoplasma gondii, Neospora caninum and Sarcocystis Spp. and Transmission Routes in Sheep from Humid Pampa, Argentina. Acta Parasitol. 2018, 63, 416–421. [Google Scholar] [CrossRef]
- Aguiar, D.; Chiebao, D.; Rodrigues, A.; Cavalcante, G.; Labruna, M.; Gennari, S. Prevalência de Anticorpos Anti-Neospora caninum Em Ovinos Do Município de Monte Negro, RO, Amazônia Ocidental Brasileira. Arq. Do Inst. Biológico 2004, 71, 616–618. [Google Scholar]
- Vogel, F.S.F.; Arenhart, S.; Bauermann, F.V. Anticorpos anti- Neospora caninum em bovinos, ovinos e bubalinos no Estado do Rio Grande do Sul. Ciência Rural. 2006, 36, 1948–1951. [Google Scholar] [CrossRef]
- Soares, H.S.; Ahid, S.M.M.; Bezerra, A.C.D.S.; Pena, H.F.J.; Dias, R.A.; Gennari, S.M. Prevalence of Anti-Toxoplasma gondii and Anti- Neospora caninum Antibodies in Sheep from Mossoró, Rio Grande Do Norte, Brazil. Vet. Parasitol. 2009, 160, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Langoni, H.; Greca, H.; Guimarães, F.F.; Ullmann, L.S.; Gaio, F.C.; Uehara, R.S.; Rosa, E.P.; Amorim, R.M.; Da Silva, R.C. Serological Profile of Toxoplasma gondii and Neospora caninum Infection in Commercial Sheep from São Paulo State, Brazil. Vet. Parasitol. 2011, 177, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Munhóz, K.F.; de Luca Neto, M.; de Almeida Santos, S.M.; Garcia, J.L.; Da Silva Guimarães Junior, J.; Vidotto, O.; Headley, S.A.; Yamamura, M.H. Occurrence of Anti-Neospora caninum Antibodies in Sheep from Farms Located in Northern Parana, Brazil. Semin. Ciências Agrárias 2010, 31, 1031. [Google Scholar] [CrossRef][Green Version]
- Faria, E.B.; Cavalcanti, E.F.T.S.F.; Medeiros, E.S.; Pinheiro, J.W.; Azevedo, S.S.; Athayde, A.C.R.; Mota, R.A. Risk Factors Associated with Neospora caninum Seropositivity in Sheep from the State of Alagoas, in the Northeast Region of Brazil. J. Parasitol. 2010, 96, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Salaberry, S.R.S.; Okuda, L.H.; de Castro Nassar, A.F.; de Castro, J.R.; Lima-Ribeiro, A.M.C. Prevalence of Neospora caninum Antibodies in Sheep Flocks of Uberlândia County, MG. Rev. Bras. Parasitol. Vet. 2010, 19, 148–151. [Google Scholar] [CrossRef]
- Tembue, A.A.S.M.; de Nascimento Ramos, R.A.; de Sousa, T.R.; Albuquerque, A.R.; da Costa, A.J.; Meunier, I.M.J.; da Gloria Faustino, M.A.; Alves, L.C. Serological Survey of Neospora caninum in Small Ruminants from Pernambuco State, Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 246–248. [Google Scholar] [CrossRef]
- Machado, G.P.; Kikuti, M.; Langoni, H.; Paes, A.C. Seroprevalence and Risk Factors Associated with Neosporosis in Sheep and Dogs from Farms. Vet. Parasitol. 2011, 182, 356–358. [Google Scholar] [CrossRef]
- de Brito Moraes, L.M.; Raimundo, J.M.; Guimarães, A.; Santos, H.A.; de Lima Macedo, G.; Massard, C.L.; Machado, R.Z.; Baldani, C.D. Occurrence of Anti-Neospora caninum and Anti-Toxoplasma gondii IgG Antibodies in Goats and Sheep in Western Maranhão, Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 312–317. [Google Scholar] [CrossRef]
- dalla Rosa, L.; de Moura, A.B.; Güths, M.F.; Bellato, V.; Sartor, A.A.; de Souza, A.P. Prevalence and risk factors for infection of Neospora caninum in sheep from Lages county, Santa Catarina State, Brazil. Rev. De Ciências Agroveterinárias 2011, 10, 127–137. [Google Scholar]
- da Silva Andrade, G.; Bruhn, F.R.P.; Rocha, C.M.B.M.; de Sá Guimarães, A.; Gouveia, A.M.G.; Guimarães, A.M. Seroprevalence and Risk Factors for Neospora caninum in Sheep in the State Minas Gerais, Southeastern Brazil. Vet. Parasitol. 2012, 188, 168–171. [Google Scholar] [CrossRef][Green Version]
- de Santana Rocha, D.; Guimarães, L.A.; Bezerra, R.A.; Mendonça, C.E.D.; Dórea, T.G.; Munhoz, A.D.; Albuquerque, G.R. Soroprevalência e fatores associados à infecção de Neospora caninum em ovinos no sudeste da Bahia, Brasil. Braz. J. Vet. Med. 2014, 36, 443–447. [Google Scholar]
- Paiz, L.M.; da Silva, R.C.; Menozzi, B.D.; Langoni, H. Antibodies to Neospora caninum in Sheep from Slaughterhouses in the State of São Paulo, Brazil. Rev. Bras. Parasitol. Vet. 2015, 24, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Raimundo, J.M.; Moraes, L.M.B.; Silva, A.T.; Santos, H.A.; Pires, M.S.; Machado, R.Z.; Baldani, C.D. Occurrences of Anti-Toxoplasma Gondii and Anti-Neospora caninum Antibodies in Sheep from Four Districts of Tocantins State, Brazilian Legal Amazon Region. Pesq. Vet. Bras. 2015, 35, 110–114. [Google Scholar] [CrossRef]
- Mendonça, C.E.D.; Munhoz, A.D.; de Santana Rocha, D.; Guimarães, L.A.; Bezerra, R.A.; Albuquerque, G.R.; de Melo, C.B. Factors Associated with the Seroprevalence of Neospora caninum (Apicomplexa: Toxoplasmatinae) in Sheep from the State of Sergipe, Brazil. Braz. J. Vet. Med. 2019, 41, e002819. [Google Scholar] [CrossRef]
- Ferreira, M.S.T.; Vogel, F.S.F.; Sangioni, L.A.; Cezar, A.S.; de Menezes, F.R. Neospora Spp. and Toxoplasma gondii Infection in Sheep Flocks from Rio Grande Do Sul, Brazil. Semin. Ciências Agrárias 2016, 37, 1397–1406. [Google Scholar] [CrossRef]
- Arraes-Santos, A.I.; Araújo, A.C.; Guimarães, M.F.; Santos, J.R.; Pena, H.F.J.; Gennari, S.M.; Azevedo, S.S.; Labruna, M.B.; Horta, M.C. Seroprevalence of Anti-Toxoplasma gondii and Anti-Neospora caninum Antibodies in Domestic Mammals from Two Distinct Regions in the Semi-Arid Region of Northeastern Brazil. Vet. Parasitol. Reg. Stud. Reports 2016, 5, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Gheller, J.M.; Carniel, R.; de Carrasco, A.O.T.; Seki, M.C. Occurrence and Risk Factors for Toxoplasma gondii and Neospora caninum in Sheep of the Guarapuava Region, Paraná, Brazil. Braz. J. Vet. Res. Anim. Sci. 2016, 53, 177–181. [Google Scholar] [CrossRef][Green Version]
- Rizzo, H.; de Jesus, T.K.S.; Gaeta, N.C.; Carvalho, J.S.; Pinheiro Júnior, J.W.; Gregory, L.; Gennari, S.M.; Villalobos, E.M.C. Neospora caninum IgG Antibody Research and Evaluation of the Risk Factors Associated to the Infection in Ovine, State of Sergipe, Brazil. Pesqui. Veterinária Bras. 2017, 37, 813–819. [Google Scholar] [CrossRef]
- Cosendey, R.I.J.; de Oliveira, F.C.R.; Frazão-Teixeira, E.; de Souza, G.N.; Brandão, F.Z.; Ferreira, A.M.R.; Lilenbaum, W. Seroprevalence of Anti-Neospora caninum Antibodies in Sheep from the Rapidly Expanding Flock of Riod.Janeiro, Brazil. Vet. Parasitol. Reg. Stud. Reports 2018, 14, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, P.R.; de Matos, A.M.R.N.; Pinto-Ferreira, F.; Caldart, E.T.; Mareze, M.; Matos, R.L.N.; Freire, R.L.; Mitsuka-Breganó, R.; Headley, S.A.; Minho, A.P.; et al. Seroepidemiology of Ovine Toxoplasmosis and Neosporosis in Breeding Rams from Rio Grande Do Sul, Brazil. Transbound Emerg Dis. 2020, 67 (Suppl. 2), 208–211. [Google Scholar] [CrossRef]
- Maia, M.O.; Maia, M.O.; de Silva, A.R.S.; Gomes, A.A.D.; de Aguiar, D.M.; Pacheco, R.C.; de Costa, A.J.; Santos-Doni, T.R. dos Seroprevalence of Toxoplasma gondii and Neospora caninum in Sheep Intended for Human Consumption in the Rondônia State, Western Brazilian Amazon. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101599. [Google Scholar] [CrossRef]
- Romanelli, P.R.; de Matos, A.M.R.N.; Pinto-Ferreira, F.; Caldart, E.T.; de Carmo, J.L.M.; Santos, N.G.D.; de Silva, N.R.; Loeffler, B.B.; Sanches, J.F.Z.; Francisquini, L.S.; et al. Anti-Toxoplasma gondii and Anti-Neospora caninum Antibodies in Sheep from Paraná State, South Brazil: Prevalence and Associated Factors. Rev. Bras. Parasitol. Vet. 2021, 30, e023220. [Google Scholar] [CrossRef]
- Villagra-Blanco, R.; Barrantes-Granados, O.; Montero-Caballero, D.; Romero-Zúñiga, J.J.; Dolz, G. Seroprevalence of Toxoplasma gondii and Neospora caninum Infections and Associated Factors in Sheep from Costa Rica. Parasite. Epidemiol. Control. 2019, 4, e00085. [Google Scholar] [CrossRef]
- Sharma, R.N.; Bush, J.; Tiwari, K.; Chikweto, A.; Bhaiyat, M.I. Seroprevalence of Neospora caninum in Sheep and Goats from Grenada, West Indies. Open J. Vet. Med. 2015, 5, 219–223. [Google Scholar] [CrossRef][Green Version]
- Suzuki, K.; Corva, S.G.; Travería, G.E.; Cattáneo, M.; Puentes, R.; Martinicorena, M.; Moreno, J.; Furtado, A.; Freyre, A.; Satragno, D.; et al. Seroprevalence of Toxoplasma Gondii and Neospora Caninum in Sheep in Uruguay. Seroprevalencia de Toxoplasma gondii y Neospora caninum en ovinos de Uruguay. Analecta Vet 2011, 31, 28–32. [Google Scholar]
- Liu, Z.-K.; Li, J.-Y.; Pan, H. Seroprevalence and Risk Factors of Toxoplasma gondii and Neospora caninum Infections in Small Ruminants in China. Prev. Vet. Med. 2015, 118, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Lu, Y.; Zhang, H.; Xie, Q.; Zhang, Z. Seroprevalence and Risk Factors of Neospora caninum Infection among Domestic Sheep in Henan Province, Central China. Parasite 2018, 25, 15. [Google Scholar] [CrossRef]
- Ezatpour, B.; Alirezaei, M.; Hassanvand, A.; Zibaei, M.; Azadpour, M.; Ebrahimzadeh, F. The First Report of Neospora caninum Prevalence in Aborted and Healthy Sheep from West of Iran. Comp. Clin. Pathol. 2015, 24, 19–22. [Google Scholar] [CrossRef]
- Al-Jomaily, A.I.A.; Al-Rubaie, H.M.a.S. Study the Prevalence of Neospora caninum in Serum and Milk of Sheep in Al-Fallujah City. Al-Anbar J. Vet. Sci. 2013, 6, 114–120. [Google Scholar]
- Tirosh-Levy, S.; Savitsky, I.; Blinder, E.; Mazuz, M.L. The Involvement of Protozoan Parasites in Sheep Abortions—A Ten-Year Review of Diagnostic Results. Vet. Parasitol. 2022, 303, 109664. [Google Scholar] [CrossRef] [PubMed]
- Al-Majali, A.; Jawasreh, K.; Talafha, H.A.; Talafha, A. Neosporosis in Sheep and Different Breeds of Goats from Southern Jordan: Prevalence and Risk Factors Analysis. Am. J. Anim. Vet. Sci. 2008, 3, 47–52. [Google Scholar] [CrossRef]
- Abo-Shehada, M.N.; Abu-Halaweh, M.M. Flock-Level Seroprevalence of and Risk Factors for Neospora caninum among Sheep and Goats in Northern Jordan. Prev. Vet. Med. 2010, 93, 25–32. [Google Scholar] [CrossRef]
- Kyaw, T.; Mokhtar, A.M.; Ong, B.; Hoe, C.H.; Aziz, A.R.; Aklilu, E.; Kamarudin, S. Seroprevalence of Neospora caninum in Sheep and Goats of Gua Musang District in Kelantan, Malaysia. Pertanika J. Trop. Agric. Sci. 2018, 41, 477–483. [Google Scholar]
- Nasir, A.; Ashraf, M.; Khan, M.S.; Javeed, A.; Yaqub, T.; Avais, M.; Reichel, M.P. Prevalence of Neospora caninum Antibodies in Sheep and Goats in Pakistan. J. Parasitol. 2012, 98, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Gökce, G.; Mor, N.; Kırmızıgül, A.H.; Bozukluhan, K.; ERKILIC, E. The First Report of Seropositivity for Neospora caninum in Sheep from Turkey. Isr. J. Vet. Med. 2015, 70, 40–44. [Google Scholar]
- Karatepe, M.; Karatepe, B. Prevalence of Anti-Neospora caninum Antibodies in Sheep in Nevşehir Province, Turkey. Isr. J. Vet. Med. 2020, 75, 3. [Google Scholar]
- Bártová, E.; Sedlák, K.; Literák, I. Toxoplasma gondii and Neospora caninum Antibodies in Sheep in the Czech Republic. Vet. Parasitol. 2009, 161, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Diakoua, A.; Anastasia, D.; Papadopoulos, E.; Elias, P.; Panousis, N.; Nikolaos, P.; Karatzias, C.; Charilaos, K.; Giadinis, N.; Nektarios, G. Toxoplasma gondii and Neospora caninum Seroprevalence in Dairy Sheep and Goats Mixed Stock Farming. Vet. Parasitol. 2013, 198, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Kouam, M.; Cabezón, O.; Nogareda, C.; Almeria, S.; Theodoropoulos, G. Comparative Cross-Sectional Study of Neospora caninum and Toxoplasma gondii: Seroprevalence in Sheep of Greece and North-Eastern Spain. Sustain. Dev. Cult. Tradit. J. Spec. Vol. Honor. Profr. Georg. I. 2019, 1–7. [Google Scholar] [CrossRef]
- Gaffuri, A.; Giacometti, M.; Tranquillo, V.M.; Magnino, S.; Cordioli, P.; Lanfranchi, P. Serosurvey of Roe Deer, Chamois and Domestic Sheep in the Central Italian Alps. J. Wildl. Dis. 2006, 42, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, B.; Kornacka, A.; Cybulska, A.; Cabaj, W.; Reiterova, K.; Bogdaszewski, M.; Steiner-Bogdaszewska, Z.; Bien, J. Seroprevalence of Toxoplasma gondii and Neospora caninum Infection in Sheep, Goats, and Fallow Deer Farmed on the Same Area. J. Anim. Sci. 2018, 96, 2468–2473. [Google Scholar] [CrossRef]
- Panadero, R.; Painceira, A.; López, C.; Vázquez, L.; Paz, A.; Díaz, P.; Dacal, V.; Cienfuegos, S.; Fernández, G.; Lago, N.; et al. Seroprevalence of Toxoplasma gondii and Neospora caninum in Wild and Domestic Ruminants Sharing Pastures in Galicia (Northwest Spain). Res. Vet. Sci. 2010, 88, 111–115. [Google Scholar] [CrossRef]
- Astorga, R.J.; Reguillo, L.; Hernández, M.; Cardoso-Toset, F.; Tarradas, C.; Maldonado, A.; Gómez-Laguna, J. Serosurvey on Schmallenberg Virus and Selected Ovine Reproductive Pathogens in Culled Ewes from Southern Spain. Transbound Emerg Dis. 2014, 61, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F.; González-Barrio, D.; Aguilar-Ríos, F.; Soler, A.J.; Garde, J.J.; Gortázar, C.; Fernández-Santos, M.D.R. Infectious Pathogens Potentially Transmitted by Semen of the Black Variety of the Manchega Sheep Breed: Health Constraints for Conservation Purposes. Anim. Reprod Sci. 2014, 149, 152–157. [Google Scholar] [CrossRef]
- Díaz, J.M.; Fernández, G.; Prieto, A.; Valverde, S.; Lago, N.; Díaz, P.; Panadero, R.; López, C.; Morrondo, P.; Díez-Baños, P. Epidemiology of Reproductive Pathogens in Semi-Intensive Lamb-Producing Flocks in North-West Spain: A Comparative Serological Study. Vet. J. 2014, 200, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Clune, T.; Lockwood, A.; Hancock, S.; Bruce, M.; Thmpson, A.N.; Beetson, S.; Campbell, A.J.; Glanville, E.; Brookes, D.; Trengove, C.; et al. Neospora caninum Is Not an Important Contributor to Poor Reproductive Performance of Primiparous Ewes from Southern Australia: Evidence from a Cross-Sectional Study. Parasitol. Res. 2021, 120, 3875–3882. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.P.; Ross, G.P.; McAllister, M.M. Evaluation of an Enzyme-Linked Immunosorbent Assay for the Serological Diagnosis of Neospora caninum Infection in Sheep and Determination of the Apparent Prevalence of Infection in New Zealand. Vet. Parasitol. 2008, 151, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Blaizot, S.; Herzog, S.A.; Abrams, S.; Theeten, H.; Litzroth, A.; Hens, N. Sample Size Calculation for Estimating Key Epidemiological Parameters Using Serological Data and Mathematical Modelling. BMC Med. Res. Methodol. 2019, 19, 51. [Google Scholar] [CrossRef]
- Corbellini, L.G.; Smith, D.R.; Pescador, C.A.; Schmitz, M.; Correa, A.; Steffen, D.J.; Driemeier, D. Herd-Level Risk Factors for Neospora caninum Seroprevalence in Dairy Farms in Southern Brazil. Prev. Vet. Med. 2006, 74, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Schares, G. Neosporosis in Animals—The Last Five Years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Della Rosa, P.; Fiorentino, M.A.; Morrell, E.L.; Scioli, M.V.; Paolicchi, F.A.; Moore, D.P.; Cantón, G.J.; Hecker, Y.P. Neospora caninum and Toxoplasma gondii as Causes of Reproductive Losses in Commercial Sheep Flocks from Argentina. Curr Res. Parasitol. Vector Borne Dis. 2021, 1, 100057. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, H.; Villalobos, E.M.C.; Meira Júnior, E.B.S.; Marques, E.C.; Beraldi, F.; Gregory, L. Occurrence of Antibodies Anti-Toxoplasma Gondii and Anti-Neospora Caninum in Sheep with History of Reproductive Disorders and Risk Factors. Pesq. Vet. Bras. 2018, 38, 1317–1326. [Google Scholar] [CrossRef]
- Asadpour, R.; Jafari-Joozani, R.; Salehi, N. Detection of Neospora caninum in Ovine Abortion in Iran. J. Parasit. Dis. 2013, 37, 105–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gharekhani, J.; Tavoosidana, G.; Zandieh, M. Seroprevalence of Neospora caninum in Sheep from Western Iran. Vet. World 2013, 6, 709–710. [Google Scholar] [CrossRef]
- Sasani, F.; Javanbakht, J.; Seifori, P.; Hassan, M. Neospora caninum as Causative Agent of Ovine Encephalitis in Iran. Pathol. Discov. 2013, 1, 5. [Google Scholar] [CrossRef]
- Razmi, G.; Naseri, Z. Molecular Detection of Neospora caninum Infection in Ovine Aborted Foetuses in the Mashhad Area, Iran. Ann. Parasitol. 2017, 63, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, A.; Malekifard, F.; Batavani, R.A. Investigation of Toxoplasma gondii and Neospora caninum as Cause of Ovine Abortion in Affected Flocks of Urmia, Northwest of Iran. Bulg. J. Vet. Med. 2022, 25, 308–317. [Google Scholar] [CrossRef]
- Salehi, B.; Amouei, A.; Dodangeh, S.; Daryani, A.; Sarvi, S.; Safari-Kharyeki, M.R.; Salehi, S.; Hosseini, S.A.; Hosseininejad, Z. Molecular Identification of Neospora caninum Infection in Aborted Fetuses of Sheep, Cattle, and Goats in Mazandaran Province, Northern Iran. Iran. J. Parasitol. 2021, 16, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Spilovská, S.; Reiterová, K.; Kovácová, D.; Bobáková, M.; Dubinský, P. The First Finding of Neospora caninum and the Occurrence of Other Abortifacient Agents in Sheep in Slovakia. Vet. Parasitol. 2009, 164, 320–323. [Google Scholar] [CrossRef]
- Helmick, B.; Otter, A.; McGarry, J.; Buxton, D. Serological Investigation of Aborted Sheep and Pigs for Infection by Neospora caninum. Res. Vet. Sci. 2002, 73, 187–189. [Google Scholar] [CrossRef]
- Bartley, P.M.; Guido, S.; Mason, C.; Stevenson, H.; Chianini, F.; Carty, H.; Innes, E.A.; Katzer, F. Detection of Neospora caninum DNA in Cases of Bovine and Ovine Abortion in the South-West of Scotland. Parasitology 2019, 146, 979–982. [Google Scholar] [CrossRef] [PubMed]
- West, D.M.; Pomroy, W.E.; Collett, M.G.; Hill, F.I.; Ridler, A.L.; Kenyon, P.R.; Morris, S.T.; Pattison, R.S. A Possible Role for Neospora caninum in Ovine Abortion in New Zealand. Small Rumin. Res. 2006, 62, 135–138. [Google Scholar] [CrossRef]
- Nunes, A.C.B.T.; Yamasaki, E.M.; Kim, P.C.P.; Melo, R.P.B.; Ribeiro-Andrade, M.; Porto, W.J.N.; Mota, R.A. Transplacental Transmission of Neospora Caninum in Naturally Infected Small Ruminants from Northeastern Brazil. Pesq. Vet. Bras. 2017, 37, 921–925. [Google Scholar] [CrossRef]
- Howe, L.; West, D.M.; Collett, M.G.; Tattersfield, G.; Pattison, R.S.; Pomroy, W.E.; Kenyon, P.R.; Morris, S.T.; Williamson, N.B. The Role of Neospora caninum in Three Cases of Unexplained Ewe Abortions in the Southern North Island of New Zealand. Small Rumin. Res. 2008, 75, 115–122. [Google Scholar] [CrossRef]
- Koyama, T.; Kobayashi, Y.; Omata, Y.; Yamada, M.; Furuoka, H.; Maeda, R.; Matsui, T.; Saito, A.; Mikami, T. Isolation of Neospora caninum from the Brain of a Pregnant Sheep. J. Parasitol. 2001, 87, 1486–1488. [Google Scholar] [CrossRef]
- Pena, H.F.J.; Soares, R.M.; Ragozo, A.M.A.; Monteiro, R.M.; Yai, L.E.O.; Nishi, S.M.; Gennari, S.M. Isolation and Molecular Detection of Neospora caninum from Naturally Infected Sheep from Brazil. Vet. Parasitol. 2007, 147, 61–66. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Moreno-Gonzalo, J.; González-Warleta, M.; Mezo, M.; Ortega-Mora, L.M.; Regidor-Cerrillo, J. Isolation and Genetic Characterization of Neospora caninum from Naturally Infected Sheep. Vet. Parasitol. 2020, 280, 109091. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavides, J.; González-Warleta, M.; Arteche-Villasol, N.; Pérez, V.; Mezo, M.; Gutiérrez-Expósito, D. Ovine Neosporosis: The Current Global Situation. Animals 2022, 12, 2074. https://doi.org/10.3390/ani12162074
Benavides J, González-Warleta M, Arteche-Villasol N, Pérez V, Mezo M, Gutiérrez-Expósito D. Ovine Neosporosis: The Current Global Situation. Animals. 2022; 12(16):2074. https://doi.org/10.3390/ani12162074
Chicago/Turabian StyleBenavides, Julio, Marta González-Warleta, Noive Arteche-Villasol, Valentín Pérez, Mercedes Mezo, and Daniel Gutiérrez-Expósito. 2022. "Ovine Neosporosis: The Current Global Situation" Animals 12, no. 16: 2074. https://doi.org/10.3390/ani12162074
APA StyleBenavides, J., González-Warleta, M., Arteche-Villasol, N., Pérez, V., Mezo, M., & Gutiérrez-Expósito, D. (2022). Ovine Neosporosis: The Current Global Situation. Animals, 12(16), 2074. https://doi.org/10.3390/ani12162074






