Supplementation of Boswellia serrata and Salix alba Extracts during the Early Laying Phase: Effects on Serum and Albumen Proteins, Trace Elements, and Yolk Cholesterol
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Study Design, and Sampling
2.2. Management and Diet
2.3. Roche Scale for Yolk Color Evaluation
2.4. Serum Protein Separation Using Agarose Gel Electrophoresis (SPE–AGE)
2.5. Serum and Albumen Protein Separation Using Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.6. Iron and Zinc Determination in Sera
2.7. Cholesterol Quantification in Egg Yolks
2.8. Statistical Analysis
3. Results and Discussion
3.1. Roche Scale
3.2. SPE–AGE
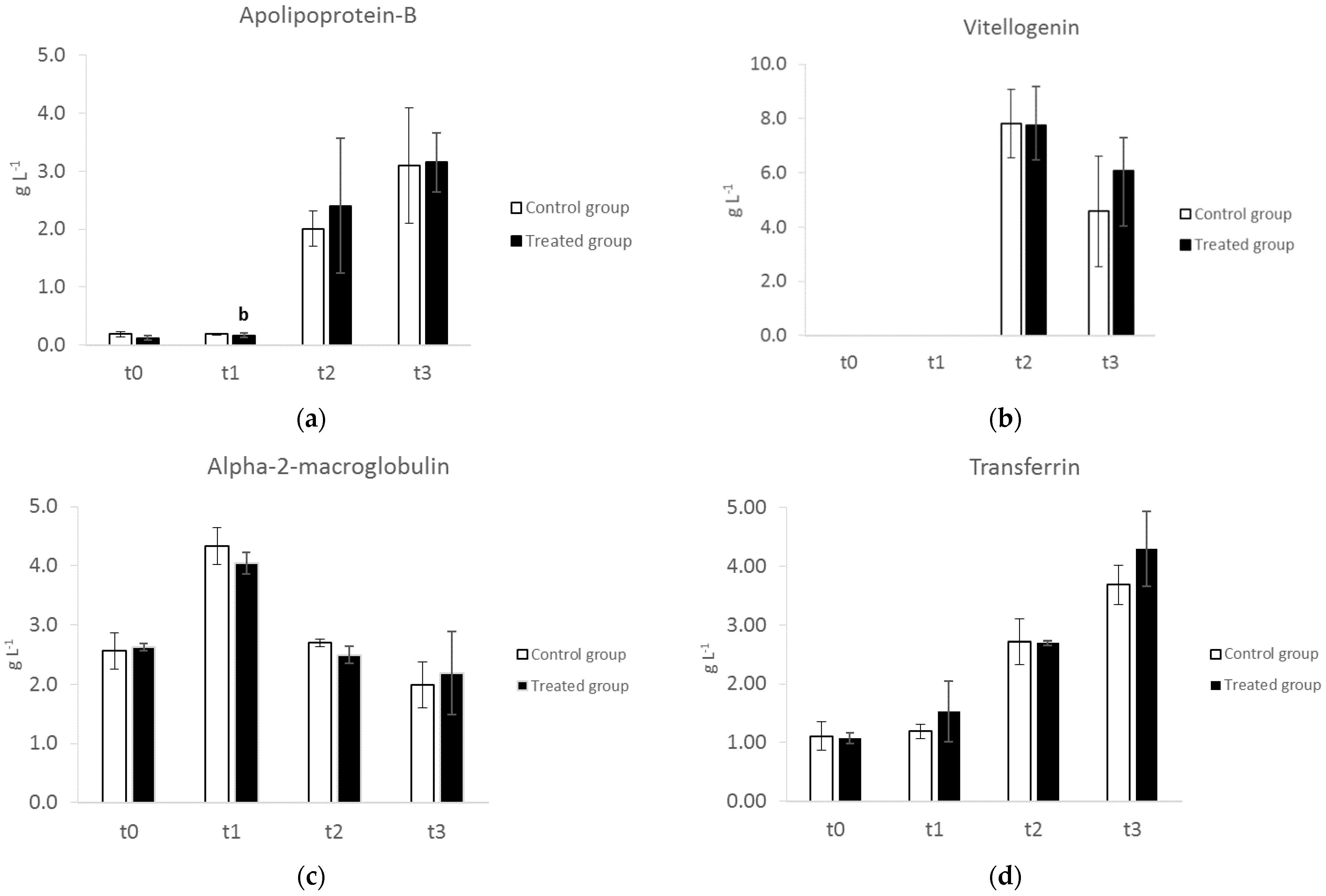
3.3. SDS–PAGE of Serum Proteins
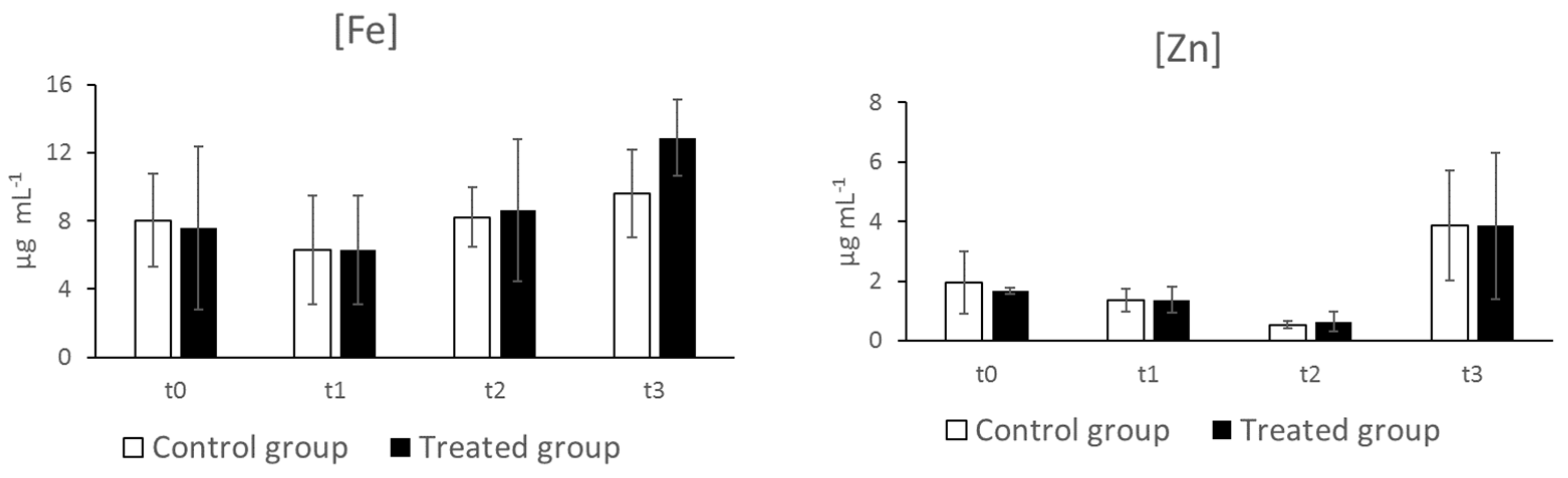
3.4. Iron and Zinc Concentrations in Serum
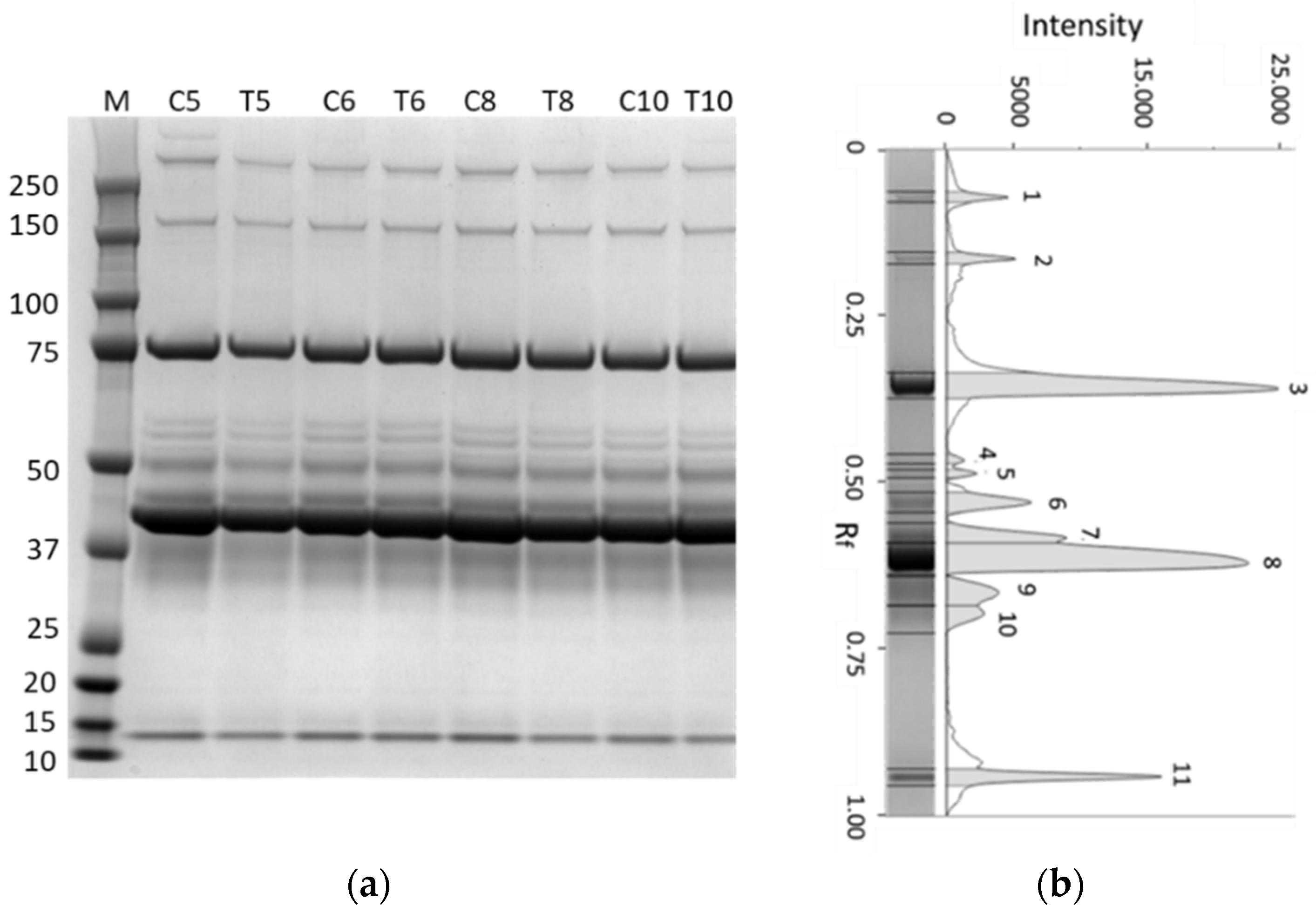
3.5. SDS–PAGE of Albumen Proteins
3.6. Cholesterol Content in Egg Yolk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leinonen, I.; Kyriazakis, I. How can we improve the environmental sustainability of poultry production? Proc. Nutr. Soc. 2016, 75, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeke, W.; Scholderer, J.; Lähteenmäki, L. Consumer appeal of nutrition and health claims in three existing product concepts. Appetite 2009, 52, 684–692. [Google Scholar] [CrossRef]
- Vanhonacker, F.; Verbeke, W. Buying higher welfare poultry products? Profiling flemish consumers who do and do not. Poult. Sci. 2009, 88, 2702–2711. [Google Scholar] [CrossRef]
- Gangnat, I.D.M.; Mueller, S.; Kreuzer, M.; Messikommer, R.E.; Siegrist, M.; Visschers, V.H.M. Swiss consumers’ willingness to pay and attitudes regarding dual-purpose poultry and eggs. Poult. Sci. 2018, 97, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- European Commission. European Union Register of Feed Additives—(EC) No 1831/2003. Appendixes 3e, 4; European Commission: Luxembourg, 2016; ISBN 0195-3842. [Google Scholar]
- Hartmann, R.M.; Fillmann, H.S.; Martins, M.I.M.; Meurer, L.; Marroni, N.P. Boswellia serrata has beneficial anti-inflammatory and antioxidant properties in a model of experimental colitis. Phyther. Res. 2014, 28, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, M.; Isani, G.; Medici, F.; Andreani, G.; Usca, I.T.; Roncada, P.; Forni, M.; Bernardini, C. Anti-inflammatory activity of Boswellia serrata extracts: An in vitro study on porcine aortic endothelial cells. Oxid. Med. Cell. Longev. 2018, 2018, 2504305. [Google Scholar] [CrossRef] [Green Version]
- Kiczorowska, B.; Al-Yasiry, A.R.M.; Samolińska, W.; Marek, A.; Pyzik, E. The effect of dietary supplementation of the broiler chicken diet with Boswellia serrata resin on growth performance, digestibility, and gastrointestinal characteristics, morphology, and microbiota. Livest. Sci. 2016, 191, 117–124. [Google Scholar] [CrossRef]
- Tabatabaei, S.N. Effect of Olibanum (Boswellia thurifera) as a feed additive on performance, some blood biochemical and intestinal morphology in broiler chicks. Res. Opin. Anim. Vet. Sci. 2016, 6, 130–134. [Google Scholar]
- Chowdhury, S.; Mandal, G.P.; Patra, A.K. Different essential oils in diets of chickens: Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed Sci. Technol. 2018, 236, 86–97. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolińska, W.; Al-Yasiry, A.; Zając, M. Immunomodulant feed supplement Boswellia serrata to support broiler chickens’ health and dietary and technological meat quality. Poult. Sci. 2020, 99, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Shara, M.; Stohs, S.J. Efficacy and safety of white willow bark (Salix alba) extracts. Phyther. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Saracila, M.; Panaite, T.D.; Soica, C.; Tabuc, C.; Olteanu, M.; Predescu, C.; Rotar, C.M.; Criste, R.D. Use of a hydroalcoholic extract of Salix alba L. bark powder in diets of broilers exposed to high heat stress. S. Afr. J. Anim. Sci. 2019, 49, 942–954. [Google Scholar] [CrossRef]
- Guerrini, A.; Dalmonte, T.; Lupini, C.; Andreani, G.; Salaroli, R.; Quaglia, G.; Zannoni, A.; Scozzoli, M.; Forni, M.; Isani, G. Influence of dietary supplementation with Boswellia serrata and Salix alba on performance and blood biochemistry in free-range leghorn laying hens. Vet. Sci. 2022, 9, 182. [Google Scholar] [CrossRef]
- Ferlizza, E.; Dondi, F.; Andreani, G.; Bucci, D.; Archer, J.; Isani, G. Validation of an electrophoretic method to detect albuminuria in cats. J. Feline Med. Surg. 2017, 19, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Crisi, P.E.; Dondi, F.; De Luca, E.; Di Tommaso, M.; Vasylyeva, K.; Ferlizza, E.; Savini, G.; Luciani, A.; Malatesta, D.; Lorusso, A.; et al. Early renal involvement in cats with natural feline morbillivirus infection. Animals 2020, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Isani, G.; Ferlizza, E.; Cuoghi, A.; Bellei, E.; Monari, E.; Bianchin Butina, B.; Castagnetti, C. Identification of the most abundant proteins in equine amniotic fluid by a proteomic approach. Anim. Reprod. Sci. 2016, 174, 150–160. [Google Scholar] [CrossRef]
- Cray, C. Protein electrophoresis of non-traditional species: A review. Vet. Clin. Pathol. 2021, 50, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Kaab, H.; Bain, M.M.; Bartley, K.; Turnbull, F.; Wright, H.W.; Nisbet, A.J.; Birchmore, R.; Eckersall, P.D. Serum and acute phase protein changes in laying hens, infested with poultry red mite. Poult. Sci. 2019, 98, 679–687. [Google Scholar] [CrossRef]
- Pasin, G.; Smith, G.M.; O’Mahony, M. Rapid determination of total cholesterol in egg yolk using commercial diagnostic cholesterol reagent. Food Chem. 1998, 61, 255–259. [Google Scholar] [CrossRef]
- Tóthová, C.; Sesztáková, E.; Bielik, B.; Nagy, O. Changes of total protein and protein fractions in broiler chickens during the fattening period. Vet. World 2019, 12, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Cray, C.; King, E.; Rodriguez, M.; Decker, L.S.; Arheart, K.L. Differences in protein fractions of avian plasma among three commercial electrophoresis systems. J. Avian Med. Surg. 2011, 25, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Gyenis, J.; Sütő, Z.; Romvári, R.; Horn, P. Tracking the development of serum biochemical parameters in two laying hen strains—A comparative study. Arch. Tierz. 2006, 49, 593–606. [Google Scholar] [CrossRef]
- Rehman, A.; Ahsan, H.; Khan, F.H. α-2-Macroglobulin: A physiological guardian. J. Cell. Physiol. 2013, 228, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Mann, K. The chicken egg white proteome. Proteomics 2007, 7, 3558–3568. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.L.; Hansen, R.J.; Williams, D.L.; Hamilton, R.L. Estrogen inducition of VLDL assembly in egg-layng hens. J. Nutr. 1999, 129, 467s–472s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeley, R.G.; Mullinix, K.P.; Wetekam, W.; Kronenberg, H.M.; Meyers, M.; Eldridge, J.D.; Goldberger, R.F. Vitellogenin synthesis in the avian liver. J. Biol. Chem. 1975, 250, 9060–9066. [Google Scholar] [CrossRef]
- Li, J.; Leghari, I.H.; He, B.; Zeng, W.; Mi, Y.; Zhang, C. Estrogen stimulates expression of chicken hepatic vitellogenin II and very low-density apolipoprotein II through ER-α. Theriogenology 2014, 82, 517–524. [Google Scholar] [CrossRef]
- Giansanti, F.; Leboffe, L.; Pitari, G.; Ippoliti, R.; Antonini, G. Physiological roles of ovotransferrin. Biochim. Biophys. Acta 2012, 1820, 218–225. [Google Scholar] [CrossRef]
- Watanabe, S.; Iwasaki, S. Developmental changes in serum albumin, transferrin, iron, and iron binding capacity of growing chickens and egg-laying hens. Jpn. J. Zootech. Sci. 1985, 56, 221–225. [Google Scholar]
- Richards, M.P. Influence of egg production on zinc, copper and iron metabolism in the turkey hen (Meleagris gallopavo). Comp. Biochem. Physiol.—Part A Physiol. 1989, 93, 811–817. [Google Scholar] [CrossRef]
- Sarlak, S.; Tabeidian, S.A.; Toghyani, M.; Shahraki, A.D.F.; Goli, M.; Habibian, M. Effects of replacing inorganic with organic iron on performance, egg quality, serum and egg yolk lipids, antioxidant status, and iron accumulation in eggs of laying hens. Biol. Trace Elem. Res. 2021, 199, 1986–1999. [Google Scholar] [CrossRef]
- Bartlett, J.R.; Smith, M.O. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 2003, 82, 1580–1588. [Google Scholar] [CrossRef]
- Olukosi, O.A.; Van Kuijk, S.; Han, Y. Copper and zinc sources and levels of zinc inclusion influence growth performance, tissue trace mineral content, and carcass yield of broiler chickens. Poult. Sci. 2018, 97, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Rahman, Z.U.; Ijaz, A.; Yousaf, M.S.; Ashraf, K.; Yaqub, T.; Zaneb, H.; Anwar, H.; Rehman, H. Single or combined effects of mannan-oligosaccharides and probiotic supplements on the total oxidants, total antioxidants, enzymatic antioxidants, liver enzymes, and serum trace minerals in cyclic heat-stressed broilers. Poult. Sci. 2011, 90, 2573–2577. [Google Scholar] [CrossRef]
- Desert, C.; Guérin-Dubiard, C.; Nau, F.; Jan, G.; Val, F.; Mallard, J. Comparison of different electrophoretic separations of hen egg white proteins. J. Agric. Food Chem. 2001, 49, 4553–4561. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents-A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Uysal, R.S.; Sumnu, G.; Boyaci, I.H. Effects of heat-treated liquid whole egg on cake batter rheology and the quality of baked cake. J. Food Process Eng. 2019, 42, e12977. [Google Scholar] [CrossRef]
- Mann, K.; Mann, M. In-depth analysis of the chicken egg white proteome using an LTQ Orbitrap Velos. Proteome Sci. 2011, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liang, Y.; Omana, D.A.; Kav, N.N.V.; Wu, J. Proteomics analysis of egg white proteins from different egg varieties. J. Agric. Food Chem. 2012, 60, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.; Nakai, S. Biochemical basis for the properties of egg white. Crit. Rev. Poult. Biol. 1991, 2, 21–59. [Google Scholar]
- Giosafto, C.V.L.; Rigby, N.; Sorrentino, A.; Mulholland, F.; Mills, C.; Mackie, A.R. Optimization of in vitro N-deglycosylation of ovomucoid protein. MOJ Food Process. Technol. 2016, 2, 205–212. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Selim, S.; Hussein, E. Effect of supplementing layer hen diet with phytogenic feed additives on laying performance, egg quality, egg lipid peroxidation and blood biochemical constituents. Anim. Nutr. 2018, 4, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Reddy, M.R.; Rama Rao, S.V.; Praharaj, N.K. Production performance, serum/yolk cholesterol and immune competence of White Leghorn layers as influenced by dietary supplementation with probiotic. Trop. Anim. Health Prod. 2003, 35, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Zita, L.; Krunt, O.; Härtlová, H.; Chmelíková, E. Determination of selected biochemical parameters in blood serum and egg quality of Czech and Slovak native hens depending on the housing system and hen age. Poult. Sci. 2021, 100, 1142–1153. [Google Scholar] [CrossRef]
- Pandey, R.S.; Singh, B.K.; Tripathi, Y.B. Extract of gum resins of Boswellia serrata L. inhibits lipopolysaccharide induced nitric oxide production in rat macrophages along with hypolipidemic property. Indian J. Exp. Biol. 2005, 43, 509–516. [Google Scholar]
- Ahangarpour, A.; Heidari, H.; Fatemeh, R.A.A.; Pakmehr, M.; Shahbazian, H.; Ahmadi, I.; Mombeini, Z.; Mehrangiz, B.H. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type2 diabetic patients. J. Diabetes Metab. Disord. 2014, 13, 29. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.H.; Attia, A.I.; Reda, F.M.; Abd El-Hack, M.E.; Ismail, I.E. Impacts of dietary supplementation of Boswellia serrata on growth, nutrients digestibility, immunity, antioxidant status, carcase traits and caecum microbiota of broilers. Ital. J. Anim. Sci. 2021, 20, 205–214. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic feed additives in poultry: Achievements, prospective and challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]


| Composition | Values of Nutrients (%/kg of Finisher Diet) | Additives (mg/kg; IU; OTU/kg) | Complementary Feed Composition (%) |
| Corn; Corn gluten flour; Soybeans meal (* CP 43%); Calcium carbonate; Roasted soybeans; Rice husk; Corn gluten; Wheat bran; Soybean oil. | CP, 17%; ** CF, 5%; *** CF, 3.51%; ! Cash, 13.27%; ǂ Ca, 4.02%; ǂǂ P, 0.58%; ≠ NaCl, 0.15%; + Ly, 0.85%; $ Met: 0.33%. | Vitamin A, 9950 IU; Vitamin D3, 2701 IU; Vitamin E, 38 mg; Vitamin K3, 2 mg; Vitamin B1, 1.5 mg; Vitamin B2, 4.5 mg; Vitamin B6, 2.5 mg; Vitamin B12, 0.008 mg; Niacin, 35 mg; Ca-D-pantothenate, 10 mg; Folic acid, 1 mg; Biotin, 0.1 mg; Betaine hydrochloride, 250 mg; Cu, 5 mg; Anhydrous calcium iodate, 0.50 mg; Mn, 50 mg; Se, 0.075 mg; Zn, 40 mg; Cantaxantine, 2 mg; Promoters of digestion 6-phytase, 213 OTU; DL-methionine, 627 mg. | Calcium carbonate, 74.5%; Colloidal silica, 15%; Salix alba & DE, 5%; Boswellia serrata & DE, 5%; Sodium chloride, 0.35%; Magnesium carbonate, 0.15%. |
| Proteins | C | T | ||||||
|---|---|---|---|---|---|---|---|---|
| t0 | t1 | t2 | t3 | t0 | t1 | t2 | t3 | |
| Total proteins (g L−1) | 42.7 ± 3.13 (1,2) | 48.4 ± 3.62 (1) | 48.7 ± 4.85 (2) | 45.9 ± 3.40 | 39.3 ± 2.92 (1,2,3) | 46.2 ± 4.11 (1) | 46.8 ± 4.30 (2) | 48.3 ± 4.10 (3) |
| Prealbumin (g L−1) | 0.70 ± 0.20 | 0.80 ± 0.40 | 0.70 ± 0.20 | 0.80 ± 0.20 | 0.80 ± 0.20 | 0.80 ± 0.10 | 0.60 ± 0.20 | 0.80 ± 0.30 |
| Albumin (g L−1) | 15.0 ± 1.6 (1,2) | 19.5 ± 1.36 § | 22.2 ± 1.40 (1) | 21.9 ± 0.50 (2) | 14.2 ± 3.00 (1,2) | 14.9 ± 1.69 (3,4),§ | 20.9 ± 1.70 (1,3) | 23.5 ± 1.10 (2,4) |
| α1 Globulins (g L−1) | 2.30 ± 0.30 (1) | 2.50 ± 0.70 (2,3) | 1.60 ± 0.30 (1,2) | 1.90 ± 0.30 (3) | 2.50 ± 0.70 (1,2) | 2.10 ± 0.20 | 1.80 ± 0.30 (1) | 1.50 ± 0.30 (2) |
| α2 Globulins (g L−1) | 7.10 ± 0.90 (1,2) | 8.40 ± 1.40 (3,4) | 5.00 ± 0.20 (1,3) | 5.20 ± 0.50 (2,4) | 6.40 ± 0.80 (1) | 7.80 ± 0.50 (2,3) | 4.80 ± 0.09 (1,2) | 5.30 ± 0.40 (3) |
| β Globulins (g L−1) | 4.80 ± 1.5 | 6.60 ± 1.60 | - | - | 3.40 ± 1.20 | 7.80 ± 1.10 | - | - |
| γ Globulins (g L−1) | 12.3 ± 2.2 | 11.5 ± 2.10 | - | - | 11.5 ± 2.10 | 12.9 ± 1.90 | - | - |
| β-γ Globulins (g L−1) | - | - | 18.1 ± 4.30 | 16.2 ± 3.50 | - | - | 18.6 ± 3.50 | 17.2 ± 3.70 |
| Laying Week | C (Control Hens) | T (Supplemented Hens) |
|---|---|---|
| 1st | 12.8 ± 1.49 | 10.8 ± 0.81 |
| 5th | 11.9 ± 1.50 | 11.0 ± 1.30 |
| 8th | 13.4 ± 2.12 | 10.8 ± 1.05 |
| 10th | 13.2 ± 3.12 | 10.1 ± 1.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreani, G.; Dalmonte, T.; Guerrini, A.; Lupini, C.; Fabbri, M.; Ferlizza, E.; Isani, G. Supplementation of Boswellia serrata and Salix alba Extracts during the Early Laying Phase: Effects on Serum and Albumen Proteins, Trace Elements, and Yolk Cholesterol. Animals 2022, 12, 2014. https://doi.org/10.3390/ani12162014
Andreani G, Dalmonte T, Guerrini A, Lupini C, Fabbri M, Ferlizza E, Isani G. Supplementation of Boswellia serrata and Salix alba Extracts during the Early Laying Phase: Effects on Serum and Albumen Proteins, Trace Elements, and Yolk Cholesterol. Animals. 2022; 12(16):2014. https://doi.org/10.3390/ani12162014
Chicago/Turabian StyleAndreani, Giulia, Thomas Dalmonte, Alessandro Guerrini, Caterina Lupini, Micaela Fabbri, Enea Ferlizza, and Gloria Isani. 2022. "Supplementation of Boswellia serrata and Salix alba Extracts during the Early Laying Phase: Effects on Serum and Albumen Proteins, Trace Elements, and Yolk Cholesterol" Animals 12, no. 16: 2014. https://doi.org/10.3390/ani12162014
APA StyleAndreani, G., Dalmonte, T., Guerrini, A., Lupini, C., Fabbri, M., Ferlizza, E., & Isani, G. (2022). Supplementation of Boswellia serrata and Salix alba Extracts during the Early Laying Phase: Effects on Serum and Albumen Proteins, Trace Elements, and Yolk Cholesterol. Animals, 12(16), 2014. https://doi.org/10.3390/ani12162014






