Harbour Porpoise Abundance in Portugal over a 5-Year Period and Estimates of Potential Distribution
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
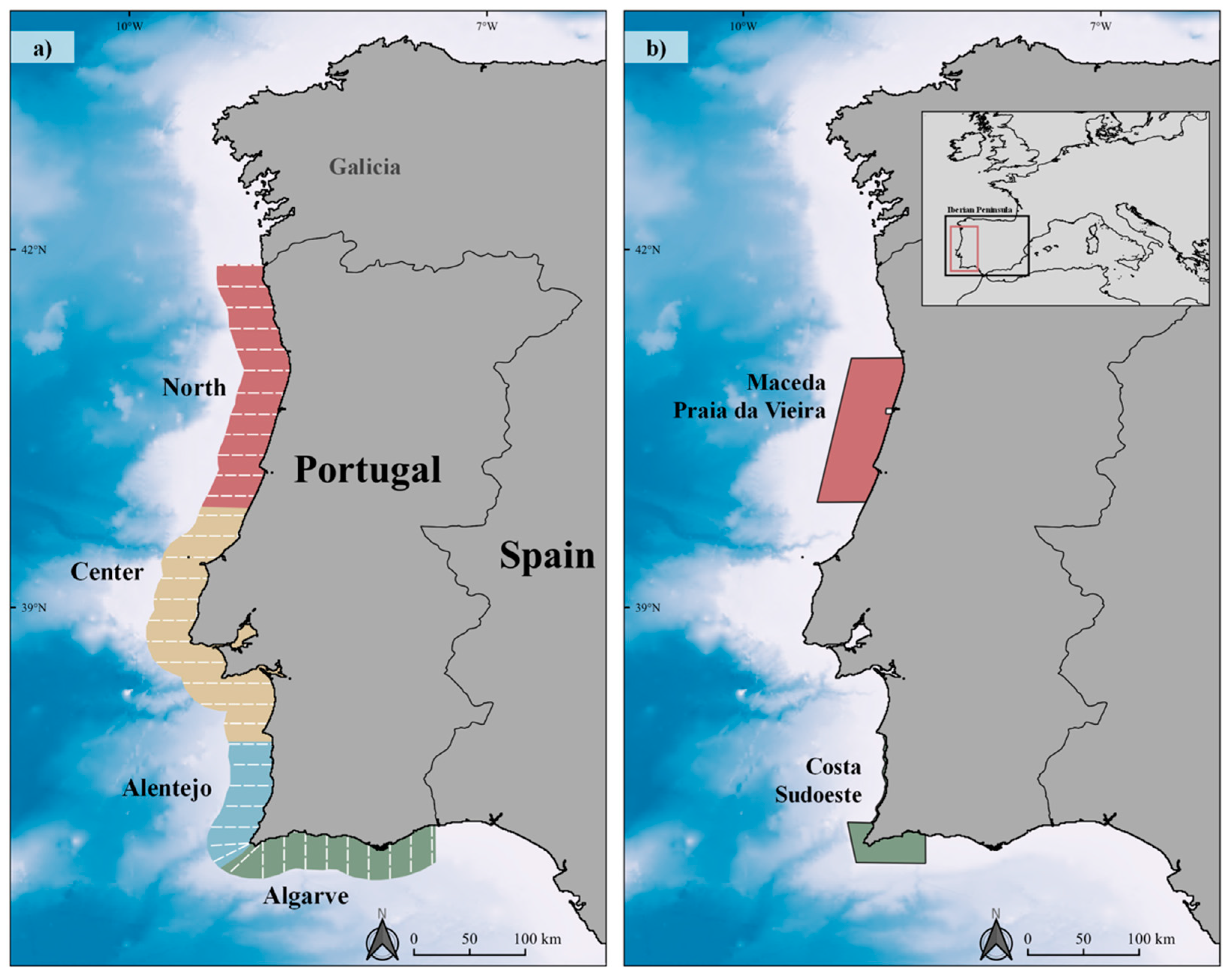
2.1. Study Area
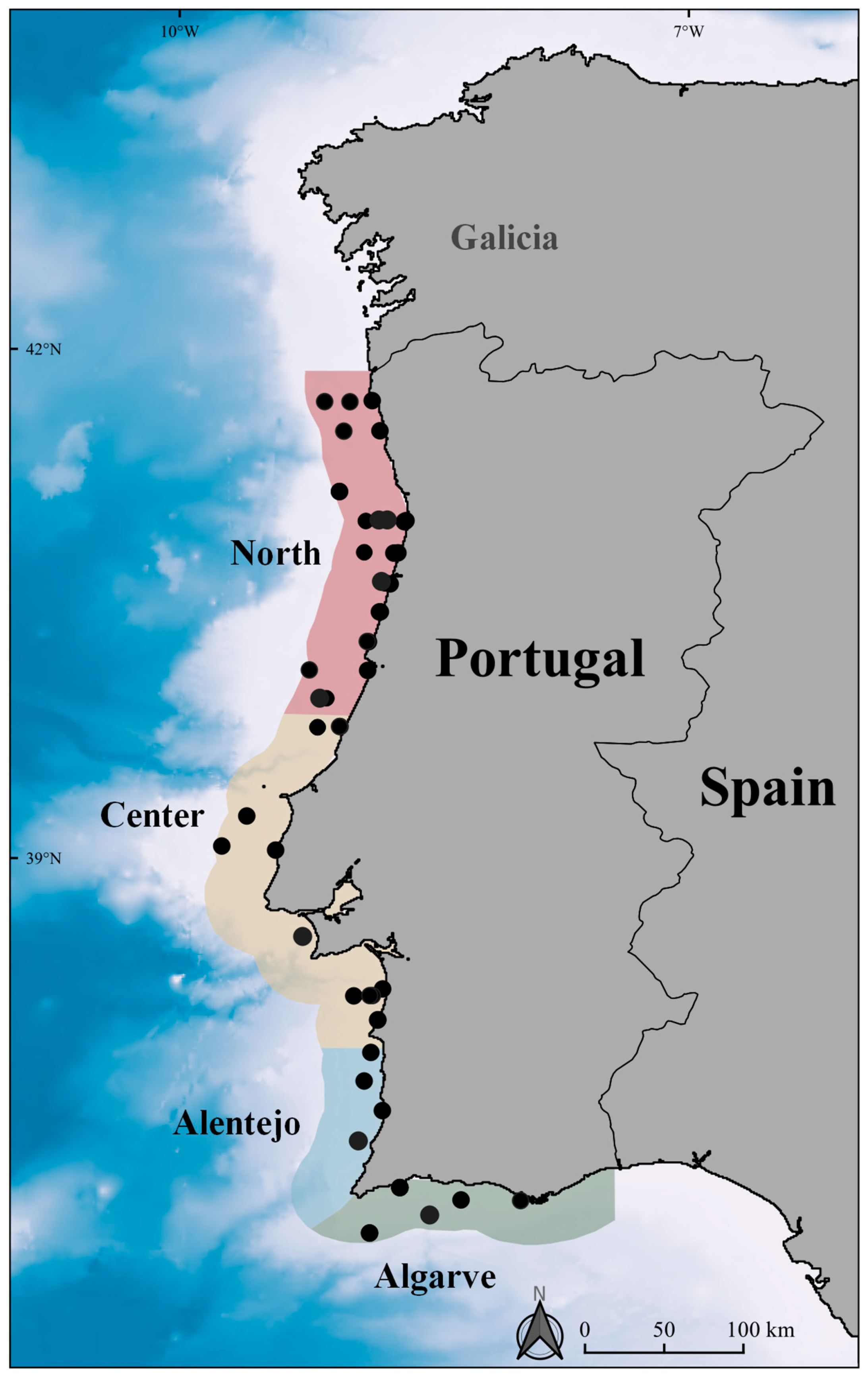
2.2. Survey Design and Data Collection
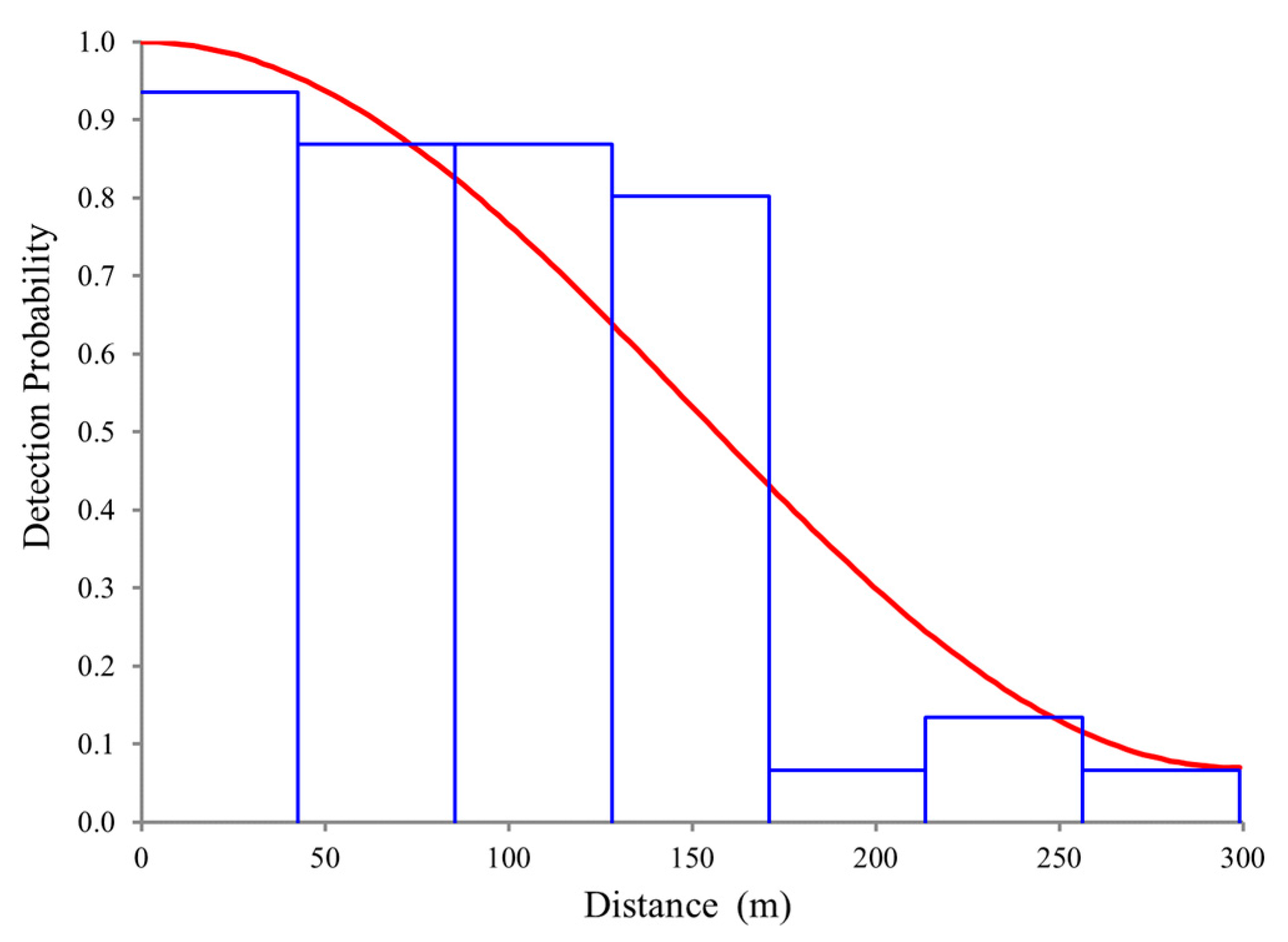
2.3. Abundance and Density Analysis
2.4. Ecological Niche Modelling (ENM)
2.4.1. Environmental Data
2.4.2. Modelling
3. Results
3.1. Abundance and Density Analysis
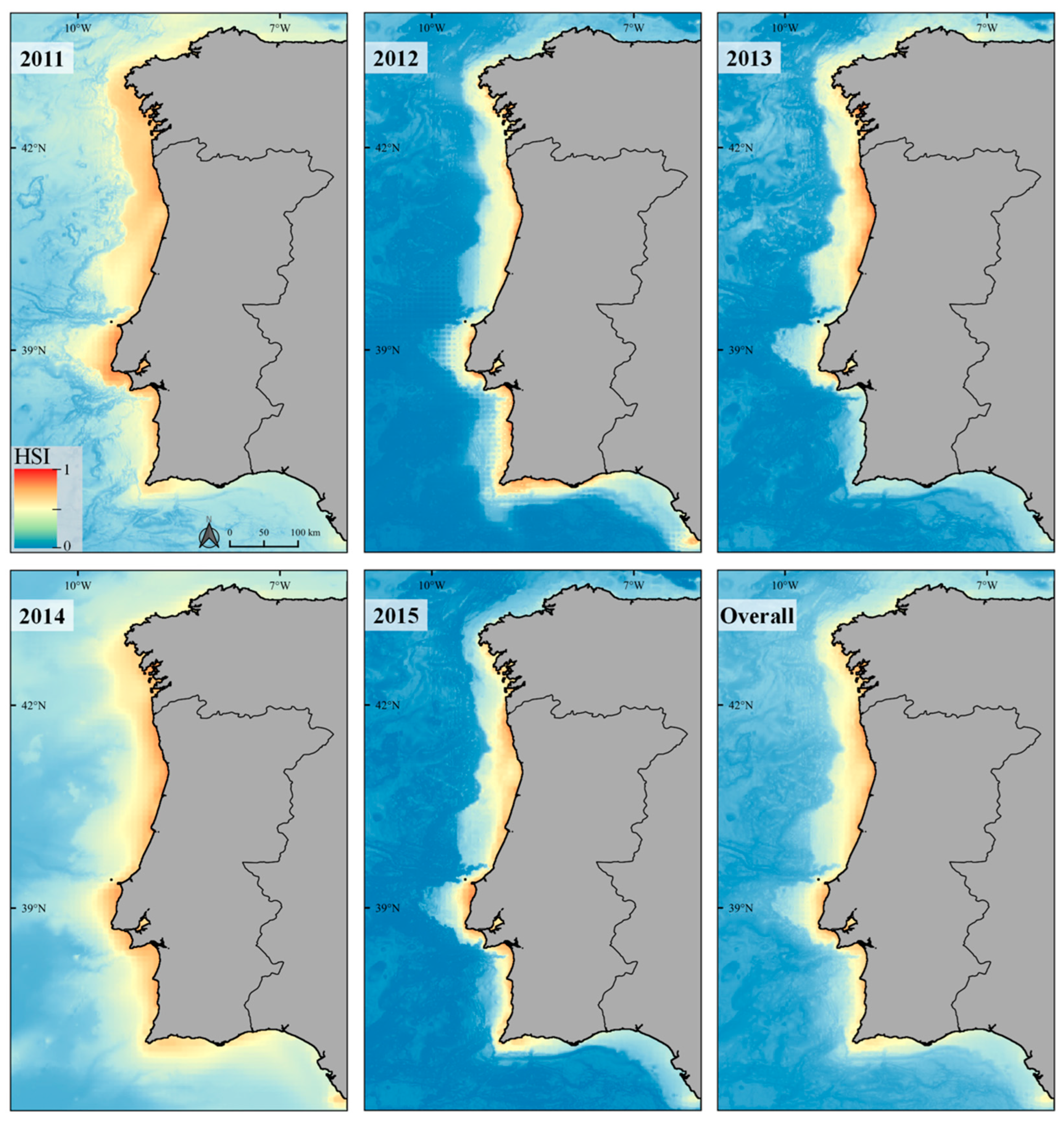
3.2. Ecological Niche Modelling
Response Curves
4. Discussion
4.1. Abundance and Density Analysis
4.2. Ecological Niche Model
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chehida, Y.B.; Stelwagen, T.; Hoekendijk, J.P.A.; Ferreira, M.; Eira, C.; Pereira, A.T.; Nicolau, L.; Marçalo, A.; Thumloup, J.; Fontaine, M.C. Harbor porpoise losing its edges: Genetic time series suggests a rapid population decline in Iberian waters over the last 30 years. BioRxiv 2021. [Google Scholar] [CrossRef]
- Fontaine, M.C.; Tolley, K.A.; Siebert, U.; Gobert, S.; Lepoint, G.; Bouquegneau, J.M.; Das, K. Long-term feeding ecology and habitat use in harbour porpoises Phocoena phocoena from Scandinavian waters inferred from trace elements and stable isotopes. BMC Ecol. 2007, 7, 1. [Google Scholar] [CrossRef][Green Version]
- Fontaine, M.; Roland, K.; Calves, I.; Austerlitz, F.; Palstra, F.P.; Tolley, K.A.; Ryan, S.; Ferreira, M.; Jauniaux, T.; Llavona, A.; et al. Postglacial climate changes and rise of three ecotypes of harbour porpoises, Phocoena phocoena, in western Palearctic waters. Mol. Ecol. 2014, 23, 3306–3321. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, M.C. Harbour Porpoises, Phocoena phocoena, in the Mediterranean Sea and Adjacent Regions: Biogeographic Relicts of the Last Glacial Period. Adv. Mar. Biol. 2016, 75, 333–358. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, M.C.; Thatcher, O.; Ray, N.; Piry, S.; Brownlow, A.; Davison, N.J.; Jepson, P.; Deaville, R.; Goodman, S.J. Mixing of porpoise ecotypes in southwestern UK waters revealed by genetic profiling. R. Soc. Open Sci. 2017, 4, 160992. [Google Scholar] [CrossRef] [PubMed]
- Vingada, J.; Ferreira, M.; Marçalo, A.; Santos, J.; Araújo, H.; Oliveira, I.; Monteiro, S.; Nicolau, L.; Gomes, P.; Tavares, C.; et al. SafeSea–Manual de Apoio para a Promoção de uma Pesca Mais Sustentável e de um mar seguro para cetáceos. In EEAGrants Programme—EEA Financial Mechanism 2004–2009 (Project 0039); Rui Carvalho Design: Braga, Portugal, 2011; p. 114. [Google Scholar]
- Vingada, J.; Eira, C. Conservation of Cetaceans and Seabirds in Continental Portugal. In The LIFE + MarPro Project; Rainho & Neves, Lda.: Aveiro, Portugal, 2018; p. 257. [Google Scholar]
- Pinn, E.H.; Macleod, K.; Tasker, M.L. Conservation of transnational species: The tensions between legal requirements and best scientific evidence. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 3291–3310. [Google Scholar] [CrossRef]
- INE—Instituto Nacional de Estatística–Estatísticas da Pesca. Technical Note 2019; INE: Lisboa, Portugal, 2020; p. 152. ISSN 0377-225-X. ISBN 978-989-25-0540-4. Available online: https://www.ine.pt/xurl/pub/435690295 (accessed on 1 April 2022).
- Moore, J.E.; Barlow, J. Bayesian state-space model of fin whale abundance trends from a 1991–2008 time series of line-transect surveys in the California Current. J. Appl. Ecol. 2011, 48, 1195–1205. [Google Scholar] [CrossRef]
- Hammond, P.S.; Macleod, K.; Berggren, P.; Borchers, D.L.; Burt, L.; Cañadas, A.; Desportes, G.; Donovan, G.P.; Gilles, A.; Gillespie, D.; et al. Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biol. Conserv. 2013, 164, 107–122. [Google Scholar] [CrossRef]
- Rogan, E.; Read, A.J.; Berggren, P. Empty promises: The European Union is failing to protect dolphins and porpoises from fisheries by-catch. Fish Fish. 2021, 22, 865–869. [Google Scholar] [CrossRef]
- Panigada, S.; Lauriano, G.; Burt, L.; Pierantonio, N.; Donovan, G. Monitoring winter and summer abundance of cetaceans in the Pelagos Sanctuary (northwestern Mediterranean Sea) through aerial surveys. PLoS ONE 2011, 6, e22878. [Google Scholar] [CrossRef] [PubMed]
- Laran, S.; Doremus, G.; Mannocci, L.; Canneyt, O.V.; Watremez, P.; Cadinouche, A.; Dulau-Dronuot, V.; Mayer, F.M.; Monthy, D.; Andrianarivelo, N.; et al. Progress of the REMMOA aerial surveys conducted in the French EEZ and adjacent waters: Contrasted cetacean habitats in the southwest Indian Ocean (SC/64/E14). In Proceedings of the 64th Annual Meeting of the International Whaling Commission, Panama City, Panama, 8 June–6 July 2012. [Google Scholar]
- Hammond, P.S.; Lacey, C.; Gille, A.; Viquerat, S.; Börjesson, P.; Macleod, K.; Ridoux, V.; Santos, M.B.; Scheidat, M.; Teilmann, J.; et al. Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys. In Final Report; 2021; p. 42. Available online: https://synergy.st-andrews.ac.uk/scans3/category/researchoutput/ (accessed on 1 March 2022).
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Zanardo, N.; Parra, G.J.; Passadore, C.; Möller, L.M. Ensemble modelling of southern Australian bottlenose dolphin Tursiops sp. Distribution reveals important habitats and their potential ecological function. Mar. Ecol. Prog. Ser. 2017, 569, 253–266. [Google Scholar] [CrossRef]
- Araújo, H.; Bastos-Santos, J.; Rodrigues, P.C.; Ferreira, M.; Pereira, A.; Henriques, A.C.; Monteiro, S.; Eira, C.; Vingada, J. The importance of Portuguese Continental Shelf Waters to Balearic Shearwaters revealed by aerial ensos. Mar. Biol. 2017, 50, 71–80. [Google Scholar] [CrossRef]
- RCM 17/2019. Presidency of the Council of Ministers, Republic Diary No. 16/2019, Series I of 23 January 2019. pp. 474–475. Available online: https://data.dre.pt/eli/resolconsmin/17/2019/01/23/p/dre/pt/html (accessed on 1 March 2022).
- RCM 18/2019. Presidency of the Council of Ministers, Republic Diary No. 16/2019, Series I of 23 January 2019. pp. 475–476. Available online: https://data.dre.pt/eli/resolconsmin/18/2019/01/23/p/dre/pt/html (accessed on 1 March 2022).
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.B.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: London, UK, 2001. [Google Scholar]
- Scheidat, M.; Gilles, A.; Kock, K.H.; Siebert, U. Harbour porpoise Phocoena phocoena abundance in the southwestern Baltic Sea. Endanger. Species Res. 2008, 5, 215–223. [Google Scholar] [CrossRef]
- Geelhoed, S.C.; Scheidat, M. Abundance of harbour porpoises (Phocoena phocoena) on the Dutch Continental Shelf, aerial surveys 2012–2017. Lutra 2018, 61, 127–136. [Google Scholar]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011; p. 328. [Google Scholar]
- Breen, P.; Brown, S.; Reid, D.; Rogan, E. Modelling cetacean distribution and mapping overlap with fisheries in the northeast Atlantic. Ocean Coast. Manag. 2016, 134, 140–149. [Google Scholar] [CrossRef]
- Breen, P.; Brown, S.; Reid, D.; Rogan, E. Ocean & Coastal Management Where is the risk? Integrating a spatial distribution model and a risk assessment to identify areas of cetacean interaction with fisheries in the northeast Atlantic. Ocean Coastal Manag. 2017, 136, 148–155. [Google Scholar] [CrossRef]
- Edrén, S.M.C.; Wisz, M.S.; Teilmann, J.; Dietz, R.; Söderkvist, J. Modelling spatial patterns in harbour porpoise satellite telemetry data using maximum entropy. Ecography 2010, 33, 698–708. [Google Scholar] [CrossRef]
- Embling, C.B.; Gillibrand, P.A.; Gordon, J.; Shrimpton, J.; Stevick, P.T.; Hammond, P.S. Using habitat models to identify suitable sites for marine protected areas for harbour porpoises (Phocoena phocoena). Biol. Conserv. 2010, 143, 267–279. [Google Scholar] [CrossRef]
- Panigada, S.; Zanardelli, M.; MacKenzie, M.; Donovan, C.; Mélin, F.; Hammond, P.S. Modelling habitat preferences for fin whales and striped dolphins in the Pelagos Sanctuary (Western Mediterranean Sea) with physiographic and remote sensing variables. Remote Sens. Environ. 2008, 112, 3400–3412. [Google Scholar] [CrossRef]
- Natoli, A.; Moura, A.E.; Sillero, N. Citizen science data of cetaceans in the Arabian / Persian Gulf: Occurrence and habitat preferences of the three most reported species. Mar. Mamm. Sci. 2021, 38, 235–255. [Google Scholar] [CrossRef]
- Bouza, R.R. Modelling the Biogeochemical Dynamics of the Iberian Upwelling System. Doctoral Thesis, University of Porto Repository, Porto, Portugal, 12 December 2013. Available online: https://hdl.handle.net/10216/84701 (accessed on 15 March 2022).
- Austin, D.; Bowen, W.D.; McMillan, J.I.; Iverson, S.J. Linking movement, diving, and habitat to foraging success in a large marine predator. Ecology 2006, 87, 3095–3108. [Google Scholar] [CrossRef]
- Redfern, J.V.; Ferguson, M.C.; Becker, E.A.; Hyrenbach, K.D.; Good, C.; Barlow, J.; Kaschner, K.; Baumgartner, M.F.; Forney, K.A.; Balance, L.T.; et al. Techniques for cetacean–habitat modeling. Mar. Ecol. Prog. Ser. 2006, 310, 271–295. [Google Scholar] [CrossRef]
- Soldevilla, M.S.; Wiggins, S.M.; Hildebrand, J.A.; Oleson, E.M.; Ferguson, M.C. Risso’s and Pacific white-sided dolphin habitat modeling from passive acoustic monitoring. Mar. Ecol. Prog. Ser. 2011, 423, 247–260. [Google Scholar] [CrossRef]
- Sbrocco, E.J.; Barber, P.H. MARSPEC: Ocean climate layers for marine spatial ecology. Ecology 2013, 94, 979. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R package version 3.5-2. CRAN. 2021. Available online: https://CRAN.R-project.org/package=raster (accessed on 30 September 2021).
- Pebesma, E.J. Multivariable geostatistics in S: The gstat package. Comput. Geosci. 2004, 30, 683–691. [Google Scholar] [CrossRef]
- Gräler, B.; Pebesma, E.; Heuvelink, G. Spatio-Temporal Interpolation using gstat. R J. 2016, 8, 204–218. [Google Scholar] [CrossRef]
- Leroy, B.; Meynard, C.N.; Bellard, C.; Courchamp, F. Virtualspecies: An R package to generate virtual species distributions. Ecography 2016, 39, 599–607. [Google Scholar] [CrossRef]
- Franzblau, A.N. A Primer of Statistics for Non-Statisticians; Harcourt, Brace and World, Inc.: New York, NY, USA, 2008; pp. 154–196. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Peterson, A.T.; Loiselle, B.A.; NCEAS Predicting Species Distributions Working Group. The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 2008, 45, 239–247. [Google Scholar] [CrossRef]
- Braunisch, V.; Coppes, J.; Arlettaz, R.; Suchant, R.; Schmid, H.; Bollmann, K. Selecting from correlated climate variables: A major source of uncertainty for predicting species distributions under climate change. Ecography 2013, 36, 971–983. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 30 September 2021).
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, J.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M. BIOMOD—A platform for ensemble forecasting of species distribution. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K.A. Evaluating resource selection functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Scherrer, D.; Christe, P.; Guisan, A. Modelling bat distributions and diversity in a mountain landscape using focal predictors in ensemble of small models. Divers. Distrib. 2018, 25, 770–782. [Google Scholar] [CrossRef]
- Torres-Pereira, A.; Araújo, H.; Matos, F.L.; Bastos-Santos, J.; Sá, S.; Nicolau, L.; Marçalo, A.; Marques, C.; Ferreira, M.; Covelo, P.; et al. Assessment of harbour porpoise bycatch along the Portuguese and Galician coast: Insights from strandings over two decades (SC/68D/HIM/14). Working paper presented at the 68D Scientific Committee Meeting of the International Whaling Commission, Virtual, 25 April–13 May 2022. [Google Scholar]
- Martínez-Cedeira, J.; Vázquez, J.A.; López, A. Proyecto PHOCOEVAL: Evaluación y Determinación de la Categoría de Conservación de la Marsopa Común (Phocoena phocoena) en el NW de la Península Ibérica. In Technical Note; 2016; p. 136. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5782445&orden=0&info=link (accessed on 17 March 2022).
- Arcos, J.M.; Bécares, J.; Gil-Velasco, M.; Bonales, J.A.V.; Cardona, L. EsMarES—Estrategias Marinas de España, protegendo el mar para todos. PARTE IV. In Evaluación Del Estado Del Medio Marino y Definición Del Buen Estado Ambiental em la Demarcación Marina Noratlántica; Ministerio para la Transición Ecológica (MITECO): Madrid, Spain, 2019; p. 157. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi60vTauuv3AhUowQIHHWp8BYwQFnoECAgQAQ&url=https%3A%2F%2Fwww.miteco.gob.es%2Fes%2Fcostas%2Ftemas%2Fproteccion-medio-marino%2Fparteivevaluacionestadoydefinicionbeadmnor_tcm30-498362.pdf&usg=AOvVaw2fFZiwnPmT49tP29mjp4tt (accessed on 17 March 2022).
- Martínez-Cedeira, J.; Izquierdo Ferreiro, I.; Fernández Acevedo, O. Informe Campaña Aérea Proyecto VIRADA; CEMMA: Galicia, Spain, 2021; p. 46. [Google Scholar]
- Castro, J.; Gonçalves, J.N.; Alexandre, P.; de Stephanis, R.; Verborgh, P.; Laborde, M. Distribution of harbour porpoise (Phocoena phocoena) in the South of Portugal. In Proceedings of the 25th Conference of the European Cetacean Society, Cadiz, Spain, 21–23 March 2011. [Google Scholar]
- ACCOBAMS. Estimates of Abundance and Distribution of Cetaceans in the Black Sea from 2019 Surveys; Paiu, R.M., Panigada, S., Cañadas, A., Gol’din, P., Popov, D., David, L., Amaha Ozturk, A., Glazov, D., Eds.; ACCOBAMS—ACCOBAMS Survey Initiative/CeNoBS Projects: Monaco City, Monaco, 2021; p. 54. [Google Scholar]
- Bjørge, A.; Tolley, K.A. Harbor porpoise: Phocoena phocoena. In Encyclopedia of Marine Mammals, 3rd ed.; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: Amsterdam, The Netherlands, 2018; pp. 448–451. [Google Scholar]
- Becker, E.A.; Forney, K.A.; Ferguson, M.C.; Foley, D.G.; Smith, R.C.; Barlow, J.; Redfern, J.V. Comparing California Current cetacean–habitat models developed using in situ and remotely sensed sea surface temperature data. Mar. Ecol. Prog. Ser. 2010, 413, 163–183. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.; Lusseau, D.; Li, S. The biogeography of group sizes in humpback dolphins (Sousa spp.). Integr. Zool. 2021, 16, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.A.; Franks, D.W.; Morrell, L.J.; Balcomb, K.C.; Parsons, K.M.; van Ginneken, A.; Croft, D.P. Social network correlates of food availability in an endangered population of killer whales, Orcinus orca. Anim. Behav. 2012, 83, 731–736. [Google Scholar] [CrossRef]
- Walker, D.; Cresswell, G. Whales and Dolphins of the European Atlantic: The Bay of Biscay, English Channel, Celtic Sea and Coastal SW Ireland, 2nd ed.; ABC Print: Plymouth, UK, 2008. [Google Scholar]
- Torres Ortiz, S.; Stedt, J.; Midtiby, H.S.; Egemose, H.D.; Wahlberg, M. Group hunting in harbour porpoises (Phocoena phocoena). Can. J. Zool. 2021, 99, 511–520. [Google Scholar] [CrossRef]
- Fontaine, M.C.; Tolley, K.A.; Michaux, J.R.; Birkun, A., Jr.; Ferreira, M.; Jauniaux, T.; Llavona Vallina, A.; Öztürk, B.; Öztürk, A.A.; Ridoux, V.; et al. Genetic and historic evidence for climate-driven population fragmentation in a top cetacean predator: The harbour porpoises in European water. Proc. R. Soc. B Biol. Sci. 2010, 277, 2829–2837. [Google Scholar] [CrossRef]
- Macleod, C.D.; Weir, C.R.; Pierpoint, C.; Harland, E.J. The habitat preferences of marine mammals west of Scotland (UK). J. Mar. Biol. Assoc. UK 2007, 87, 157–164. [Google Scholar] [CrossRef]
- Bjørge, A.; Tolley, K.A. Harbor porpoise: Phocoena phocoena. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: London, UK, 2009; pp. 530–533. [Google Scholar]
- Lambert, C.; Pettex, E.; Dorémus, G.; Laran, S.; Van Canneyt, O.; Ridoux, V. How does ocean seasonality drive habitat preferences of highly mobile top predators? Part II: The eastern North-Atlantic Reference. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 141, 133–154. [Google Scholar] [CrossRef]
- Fiúza, A.F. Upwelling patterns off Portugal. In Coastal Upwelling Its Sediment Record; Suess, E., Thiede, J., Eds.; NATO Conference Series Springer: Boston, MA, USA, 1983; Volume 10B, pp. 85–98. [Google Scholar] [CrossRef]
- Gilles, A.; Adler, S.; Kaschner, K.; Scheidat, M.; Siebert, U. Modelling harbour porpoise seasonal density as a function of the German Bight environment: Implications for management. Endanger. Species Res. 2011, 14, 157–169. [Google Scholar] [CrossRef]
- Wingfield, J.E.; O’Brien, M.; Lyubchich, V.; Roberts, J.J.; Halpin, P.N.; Rice, A.N.; Bailey, H. Year-round spatiotemporal distribution of harbour porpoises within and around the Maryland wind energy area. PLoS ONE 2017, 12, e0176653. [Google Scholar] [CrossRef] [PubMed]
- Sveegaard, S.; Balle, J.D.; Kyhn, L.; Larsen, J.; Mohn, C.; Teilmann, J.; Nabe Nielsen, J. Monthly Variation in Fine-Scale Distribution of Harbour Porpoises at St. Middelgrund Reef. Aarhus University, DCE-Danish Centre for Environment and Energy. In Technical Report from DCE-Danish Centre for Environment and Energy; 2017; p. 34. Available online: http://dce2.au.dk/pub/TR97.pdf (accessed on 1 March 2022).
- Stalder, D.; van Beest, F.M.; Sveegaard, S.; Dietz, R.; Tilmann, J.; Nabe-Nielsen, J. Influence of environmental variability on harbour porpoise movement. Mar. Ecol. Prog. Ser. 2020, 648, 207–2019. [Google Scholar] [CrossRef]
- Solanki, H.U.; Mankodi, P.C.; Nayak, S.R.; Somvanshi, V.S. Evaluation of remote-sensing-based potential fishing zones (PFZs) forecast methodology. Cont. Shelf Res. 2005, 25, 2163–2173. [Google Scholar] [CrossRef]
- Mann, K.; Lazier, J. Dynamics of Marine Ecosystems. In Biological–Physical Interactions in the Oceans, 3rd ed.; Blackwell Publishing: Malden, MA, USA, 2006. [Google Scholar]
- Ballance, L.T.; Pitman, R.L.; Fiedler, P.C. Progress in Oceanography Oceanographic influences on seabirds and cetaceans of the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 360–390. [Google Scholar] [CrossRef]
- Mason, E.; Coombs, S.H.; Oliveira, P.B. An overview of the literature concerning the oceanography of the eastern North Atlantic region. Port. Inst. Sea Atmos. Sci. Tech. Rep. 2005, 33, 59. [Google Scholar]
- INE—Instituto Nacional de Estatística–Estatísticas da Pesca. Technical Note 2011; INE: Lisboa, Portugal, 2012; p. 132. ISBN 978-989-25-0156-7. ISSN 0377-225-x. Available online: https://www.ine.pt/xurl/pub/140610972. (accessed on 1 April 2022).
- INE—Instituto Nacional de Estatística–Estatísticas da Pesca. Technical Note 2012; INE: Lisboa, Portugal, 2013; p. 135. ISSN 0377-225-x. ISBN 978-989-25-0199-4. Available online: https://www.ine.pt/xurl/pub/153378507 (accessed on 1 April 2022).
- INE—Instituto Nacional de Estatística–Estatísticas da Pesca. Technical Note 2013; INE: Lisboa, Portugal, 2014; p. 135. ISSN 0377-225-x. ISBN 978-989-25-0263-2. Available online: https://www.ine.pt/xurl/pub/210756920 (accessed on 1 April 2022).
- INE—Instituto Nacional de Estatística–Estatísticas da Pesca. Technical Note 2014; INE: Lisboa, Portugal, 2015; ISSN 0377-225-x. ISBN 978-989-25-0303-5. Available online: https://www.ine.pt/xurl/pub/139431 (accessed on 1 April 2022).
- INE—Instituto Nacional de Estatística–Estatísticas da Pesca. Technical Note 2015; INE: Lisboa, Portugal, 2016; ISSN 0377-225-x. ISBN 978-989-25-0354-7. Available online: https://www.ine.pt/xurl/pub/261842006 (accessed on 1 April 2022).
- Martínez-Cedeira, J.; López, A. Actualización del Estado de Conservación de la Marsopa en las Demarcaciones Marinas Noratlántica y Sudatlántica y Elaboración del Borrador del Plan de Conservación; Ministerio de Agricultura y Pesca; Alimentación y Medio Ambiente: Madrid, Spain, 2018; 217p. [Google Scholar]
- BOE-A-2020-15296. Boletín Oficial del Estado, Ministerio Para la TRANSICIÓN Ecológica y el Reto Demográfico, Spain. 2020. No. 314. pp. 108167–108171. Available online: https://www.boe.es (accessed on 15 March 2022).
- Vingada, J.; Martínez-Cedeira, J.; López, A.; Eira, C. Do we need a transboundary Nature 2000 site? In Proceedings of the Workshop LIFE+MarPro: Marine Protected Areas and Management Plans for Cetaceans and Seabirds, Ílhavo, Portugal, 4–5 December 2017. [Google Scholar]
- Scheidat, M.; Verdaat, H.; Aarts, G. Using aerial surveys to estimate density and distribution of harbour porpoises in Dutch waters. J. Sea Res. 2012, 69, 1–7. [Google Scholar] [CrossRef]
- Gilles, A.; Viquerat, S.; Becker, E.A.; Forney, K.A.; Geelhoed, S.C.V.; Haelters, J.; Nabe-Nielsen, J.; Scheidat, M.; Siebert, U.; Sveegaard, S.; et al. Seasonal habitat-based density models for a marine top predator, the harbor porpoise, in a dynamic environment. Ecosphere 2016, 7, e01367. [Google Scholar] [CrossRef]
- Waggitt, J.J.; Evans, P.G.; Andrade, J.; Banks, A.N.; Boisseau, O.; Bolton, M.; Bardbury, G.; Brereton, T.; Campguysen, C.J.; Durnck, J.; et al. Distribution maps of cetacean and seabird populations in the North-East Atlantic. J. Appl. Ecol. 2019, 57, 253–269. [Google Scholar] [CrossRef]
- Ferreira, M.C.T. Monitorização de uma População de bôtos (Phocoena phocoena L.) na Região do Cabo Mondego; Internship Report; Nature and Forests Conservation Institute: Lisboa, Portugal, 2000; p. 44. [Google Scholar]
- Pereira, A.F.T. Monitorização do Uso Costeiro por Pequenos Cetáceos e Avaliação do Uso de Pingers Para Mitigação de Capturas Acidentais na Região Norte de Portugal. Master Thesis, University of Porto Open Repository, Porto, Portugal, 17 November 2015. Available online: https://repositorio-aberto.up.pt/handle/10216/82345 (accessed on 15 March 2022).
- Wisniewska, D.M.; Johnson, M.; Teilmann, J.; Rojano-Doñate, L.; Shearer, J.; Sveegaard, S.; Miller, L.A.; Siebert, U.; Madsen, P.T. Ultra-high foraging rates of harbor porpoises make them vulnerable to anthropogenic disturbance. Curr. Biol. 2016, 26, 1441–1446. [Google Scholar] [CrossRef]
- Rojano-Doñate, L.; McDonald, B.I.; Wisniewska, D.M.; Johnson, M.; Teilmann, J.; Wahlberg, M.; Højer-Kristensen, J.; Madsen, P.T. High field metabolic rates of wild harbour porpoises. J. Exp. Biol. 2018, 221, jeb185827. [Google Scholar] [CrossRef]
- Aguiar, Z.V.P. Ecologia Alimentar do bôto (Phocoena phocoena) ao Longo da Costa Continental Portuguesa. Master’s Thesis, University of Porto Open Repository, Porto, Portugal, 11 December 2013. Available online: https://repositorio-aberto.up.pt/handle/10216/70768 (accessed on 15 March 2022).
- Pinheiro, G.A.J. Contribuição Para o Estudo da Dieta de Pequenos Cetáceos em Portugal Continental. Master Thesis, University of Aveiro Repository, Aveiro, Portugal, 18 December 2017. Available online: http://hdl.handle.net/10773/21949 (accessed on 15 March 2022).
- Heide-Jørgensen, M.P.; Iversen, M.; Nielsen, N.H.; Lockyer, C.; Stern, H.; Ribergaard, M.H. Harbour porpoises respond to climate change. Ecol. Evol. 2011, 1, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laakem, J.L. Distance Sampling: Estimating Abundance of Biological Populations; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Hiby, L.; Lovell, P. Using aircraft in tandem formation to estimate abundance of harbour porpoise. Biometrics 1998, 54, 1280–1289. [Google Scholar] [CrossRef]
- Birkun, A.A., Jr.; Frantzis, A. Phocoena phocoena ssp. Relicta. The IUCN Red List of Threatened Species. 2008. e.T17030A6737111. Available online: https://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T17030A6737111.en (accessed on 15 April 2022).
- Hammond, P.S.; Bearzi, G.; Bjørge, A.; Forney, K.A.; Karczmarski, L.; Kasuya, T.; Perrin, W.; Scott, M.D.; Wang, J.Y.; Wells, R.S.; et al. Phocoena phocoena (Baltic Sea Subpopulation) (Errata Version Published in 2016). The IUCN Red List of Threatened Species. 2008. e.T17031A98831650. Available online: https://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T17031A6739565.en (accessed on 11 July 2022).

| EGVs | Units | Original Resolution | Source | |
|---|---|---|---|---|
| Static variables | Bathymetry | Meters | 30 arc-sec (~1 km) | [36] http://marspec.org/, accessed on 18 June 2021 Distance to coast and slope: Derived from bathymetry |
| Distance to coast | ||||
| Slope | Degrees | 30 arc-sec (~1 km) | ||
| Dynamic variables | Dissolved oxygen (O2) | mmol m−3 | 0.083° (~8 km) (Monthly average, lag-1) | Copernicus Atlantic-Iberian Biscay Irish-Ocean Physics Reanalysis (CMEMS) https://resources.marine.copernicus.eu/product-detail/IBI_MULTIYEAR_BGC_005_003/INFORMATION, accessed on 18 June 2021 |
| Nitrate (NO3) | ||||
| Ammonium (NH4+) | ||||
| Phosphate (PO4) | ||||
| Silicate (Si) | ||||
| Iron (Fe) | ||||
| Chlorophyll concentration (Chl-a) | mg m−3 | |||
| pH | - | |||
| Salinity | Psu | Copernicus Atlantic-Iberian Biscay Irish-Ocean Physics Reanalysis (CMEMS) https://resources.marine.copernicus.eu/product-detail/IBI_MULTIYEAR_PHY_005_002/INFORMATION, accessed on 18 June 2021 | ||
| Sea Surface Temperature (SST) | °C |
| Survey Year | Transects | Distance (km) | Sightings | Individuals | Expected Group Size (CV) | Density (ind/km2) (95% CI) | Abundance (95% CI) | CV |
|---|---|---|---|---|---|---|---|---|
| g(0) = 0.364 | ||||||||
| 2011 | 39 | 1445.3 | 6 | 8 | 1.32 (13.72) | 0.048 (0.018–0.125) | 1196 (456–3135) | 50.70 |
| 2012 | 40 | 1482.4 | 16 | 23 | 1.28 (10.61) | 0.120 (0.061–0.236) | 2995 (1516–5917) | 34.95 |
| 2013 | 40 | 1482.4 | 16 | 25 | 1.42 (21.11) | 0.128 (0.061–0.268) | 3207 (1531–6718) | 38.14 |
| 2014 | 37 | 1408.3 | 8 | 11 | 1.32 (16.55) | 0.066 (0.029–0.152) | 1653 (717–3809) | 43.27 |
| 2015 | 39 | 1482.4 | 10 | 15 | 1.44 (15.79) | 0.086 (0.037–0.199) | 2147 (923–4997) | 43.86 |
| Total | 195 | 8635.0 | 56 | 83 | 1.39 (7.80) | 0.090 (0.051–0.158) | 2254 (1287–3949) | 21.99 |
| Survey Sector | Transects | Distance (km) | Sightings | Individuals | Expected Group Size (CV) | Density (ind/km2) (95% CI) | Abundance (95% CI) | CV |
|---|---|---|---|---|---|---|---|---|
| g(0) = 0.364 | ||||||||
| North | 58 | 6710.99 | 27 | 34 | 1.26 (7.44) | 0.140 (0.061–0.318) | 935 (409–2139) | 43.42 |
| Centre | 56 | 9145.15 | 17 | 26 | 1.52 (8.16) | 0.110 (0.051–0.237) | 1010 (470–2168) | 39.75 |
| Alentejo | 32 | 3424.86 | 4 | 5 | 1.14 (23.10) | 0.033 (0.012–0.092) | 113 (40–315) | 53.90 |
| Algarve | 49 | 5771.52 | 8 | 17 | 1.90 (24.79) | 0.071 (0.026–0.197) | 412 (150–1136) | 55.99 |
| EGVs | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| Bathymetry | 20.599 | 20.691 | 20.562 | 19.093 | 20.188 |
| Chl-a | 20.619 | 20.663 | 21.313 | 21.786 | 20.452 |
| Ammonium | 19.835 | 19.385 | 18.970 | 19.556 | 18.959 |
| SST | 19.716 | 20.570 | 19.129 | 20.046 | 21.335 |
| Slope | 19.231 | 18.691 | 20.026 | 19.519 | 19.066 |
| Survey Block | Location | Platform | Survey Years | Area (km2) | Abundance (n) | Density (n/km2) | CV (%) | |
|---|---|---|---|---|---|---|---|---|
| SCANS II | W | SW France and Atlantic Iberian Peninsula (Portugal and Spain) | ship | 2005 | 138,639.00 | 2357 | 0.017 | 92 |
| Present study | Portuguese Coast | airplane | 2011–2015 | 25,052.52 | 2254 | 0.090 | 21.99 | |
| SCANS III | AB | Western Iberian coast (Portugal and Galicia, Spain) | airplane | 2016 | 26,668.00 | 2715 | 0.102 | 30.8 |
| SCANS III | AA + AB + AC | Atlantic Iberian Peninsula 1 (Portugal and Spain) | airplane | 2016 | 72,863.00 | 2898 | 0.04 | 32.00 |
| Survey Year | Density (95% CI) | Abundance (95% CI) | CV |
|---|---|---|---|
| g(0) = 0.45 | |||
| 2011 | 0.039 (0.016–0.100) | 991 (391–2515) | 48.90 |
| 2012 | 0.096 (0.053–0.174) | 2416 (1338–4363) | 30.22 |
| 2013 | 0.120 (0.059–0.246) | 3020 (1482–6157) | 36.78 |
| 2014 | 0.057 (0.026–0.125) | 1437 (658–3135) | 40.36 |
| 2015 | 0.074 (0.034–0.162) | 1859 (853–4051) | 40.32 |
| Total | 0.061 (0.029–0.126) | 1523 (733–3163) | 31.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Pereira, A.; Araújo, H.; Matos, F.L.; Bastos-Santos, J.; Sá, S.; Ferreira, M.; Martínez-Cedeira, J.; López, A.; Sequeira, M.; Vingada, J.; et al. Harbour Porpoise Abundance in Portugal over a 5-Year Period and Estimates of Potential Distribution. Animals 2022, 12, 1935. https://doi.org/10.3390/ani12151935
Torres-Pereira A, Araújo H, Matos FL, Bastos-Santos J, Sá S, Ferreira M, Martínez-Cedeira J, López A, Sequeira M, Vingada J, et al. Harbour Porpoise Abundance in Portugal over a 5-Year Period and Estimates of Potential Distribution. Animals. 2022; 12(15):1935. https://doi.org/10.3390/ani12151935
Chicago/Turabian StyleTorres-Pereira, Andreia, Hélder Araújo, Fábio L. Matos, Jorge Bastos-Santos, Sara Sá, Marisa Ferreira, José Martínez-Cedeira, Alfredo López, Marina Sequeira, José Vingada, and et al. 2022. "Harbour Porpoise Abundance in Portugal over a 5-Year Period and Estimates of Potential Distribution" Animals 12, no. 15: 1935. https://doi.org/10.3390/ani12151935
APA StyleTorres-Pereira, A., Araújo, H., Matos, F. L., Bastos-Santos, J., Sá, S., Ferreira, M., Martínez-Cedeira, J., López, A., Sequeira, M., Vingada, J., & Eira, C. (2022). Harbour Porpoise Abundance in Portugal over a 5-Year Period and Estimates of Potential Distribution. Animals, 12(15), 1935. https://doi.org/10.3390/ani12151935






