Morphometry of Boar Spermatozoa in Semen Stored at 17 °C—The Influence of the Staining Technique
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Semen Collection
2.2. Analyses and Slide Preparation
2.3. Morphometric Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Dziekońska, A.; Fraser, L.; Strzeżek, J. Effect of different storage temperatures on the metabolic activity of spermatozoa following liquid storage of boar semen. J. Anim. Feed Sci. 2009, 18, 638–649. [Google Scholar] [CrossRef]
- Gączarzewicz, D.; Udała, J.; Piasecka, M.; Błaszczyk, B.; Stankiewicz, T. Storage temperature of boar semen and its relationship to changes in sperm plasma membrane integrity, mitochondrial membrane potential, and oxidoreductive capability. Turk. J. Biol. 2015, 39, 582–594. [Google Scholar] [CrossRef]
- Lopez Rodriguez, A.; Van Soom, A.; Arsenakis, I.; Maes, D. Boar management and semen handling factors affect the quality of boar extended semen. Porc. Health Manag. 2017, 3, 15. [Google Scholar] [CrossRef]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Bresciani, C.; Bianchera, A.; Berttini, R.; Buschini, A.; Marchi, L.; Cabassi, C.S.; Sabbioni, A.; Righi, F.; Mazzoni, C.; Parmigiani, E. Long-term liquid storage and reproductive evaluation of an innovative boar semen extender (Formula12) containing a non-reducing disaccharide and an enzymatic agent. Anim. Reprod. Sci. 2017, 180, 10–16. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Maxwell, W.M.C.; Johnson, L.A. Physiology of spermatozoa at high dilution rates: The influence of seminal plasma. Theriogenology 1999, 52, 1353–1362. [Google Scholar] [CrossRef]
- Boe-Hansen, G.B.; Annette, K.; Ersbøll, A.K.; Greve, T.; Christensen, P. Increasing storage time of extended boar semen reduces sperm DNA integrity. Theriogenology 2005, 63, 2006–2019. [Google Scholar] [CrossRef]
- Garcia-Vazquez, F.A.; Gadea, J.; Matas, C.; Holt, W.V. Importance of sperm morphology during sperm transport and fertilization in mammals. Asian J. Androl. 2016, 18, 844–850. [Google Scholar] [CrossRef]
- Yániz, J.L.; Soler, C.; Santolaria, P. Computer assisted sperm morphometry in mammals: A review. Anim. Reprod. Sci. 2015, 156, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kondracki, S.; Wysokińska, A.; Kania, M.; Górski, K. Application of two staining methods for sperm morphometric evaluation in domestic pigs. J. Vet. Res. 2017, 61, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Tsakmakidis, I.A.; Khalifa, T.A.A.; Boscos, C.M. Age-related changes in quality and fertility of porcine semen. Biol. Res. 2012, 45, 381–386. [Google Scholar] [CrossRef]
- van der Horst, G.; Maree, L. SpermBlue: A new universal stain for human and animal sperm which is also amenable to automated sperm morphology analysis. Biotech. Histochem. 2009, 84, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Niżański, W. Intravaginal insemination of bitches with fresh and frozen-thawed semen with addition of prostatic fluid: Use of an infusion pipette and the Osiris catheter. Theriogenology 2006, 66, 470–483. [Google Scholar] [CrossRef]
- Coetzee, K.; Kruger, T.F.; Lombard, C.J. Predictive value of normal sperm morphology: A structured literature review. Hum. Reprod. 1998, 4, 73–82. [Google Scholar] [CrossRef]
- Wysokińska, A.; Wójcik, E.; Chłopik, A. Evaluation of the morphometry of sperm from the epididymides of dogs using different staining methods. Animals 2021, 11, 227. [Google Scholar] [CrossRef]
- Kavak, A.; Lundeheim, N.; Aidnik, M.; Einarsson, S. Sperm morphology in Estonian and Tori Breed Stallions. Acta Vet. Scand. 2004, 45, 11–18. [Google Scholar] [CrossRef]
- Łącka, K.; Kondracki, S.; Iwanina, M.; Wysokińska, A. Assessment of stallion semen morphology using two different staining methods, microscopic techniques, and sample sizes. J. Vet. Res. 2016, 60, 99–104. [Google Scholar] [CrossRef]
- Wysokińska, A.; Szablicka, D. Integrity of Sperm Cell Membrane in the Semen of Crossbred and Purebred Boars during Storage at 17 °C: Heterosis Effects. Animals 2021, 11, 3373. [Google Scholar] [CrossRef]
- Gadella, B.M. Sperm membrane physiology and relevance for fertilization. Anim. Reprod. Sci. 2008, 107, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Rüdiger, K.; Waberski, D. Rotation of boar semen doses during storage sffects sperm quality. Reprod. Domest. Anim. 2015, 50, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Ammon, C.; Schaefer, J.; Luther, A.M.; Jung, M.; Waberski, D. Impact of different dilution techniques on boar sperm quality and sperm distribution of the extended ejaculate. Anim. Reprod. Sci. 2017, 182, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wysokińska, A.; Kondracki, S. Heterosis for morphometric characteristics of sperm cells from Duroc x Pietrain crossbred boars. Anim. Reprod. Sci. 2019, 211, 106217. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Rodriguez, I.; Dorado, J.; Sanz, J.; Soler, C. Effect of sample size and staining methods on stallion sperm morphometry by the Sperm Class Analyzer. Vet Med Czech 2005, 50, 24–32. [Google Scholar] [CrossRef]
- Czubaszek, M.; Andraszek, K.; Banaszewska, D.; Walczak-Jędrzejowska, R. The effect of the staining technique on morphological and morphometric parameters of boar sperm. PLoS ONE 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Banaszewska, D.; Andraszek, K.; Czubaszek, M.; Biesiada-Drzazga, B. The effect of selected staining techniques on bull sperm morphometry. Anim. Reprod. Sci. 2015, 159, 17–24. [Google Scholar] [CrossRef]
- Banaszewska, D.; Andraszek, K.; Zdrowowicz, E.; Danielewicz, A. The effect of selected staining techniques on stallion sperm morphometry. Livest. Sci. 2015, 175, 128–132. [Google Scholar] [CrossRef]
- Henkel, R.; Schreiber, G.; Sturmhoefel, A.; Hipler, U.C.; Zermann, D.H.; Menkveld, R. Comparison of three staining methods for the morphological evaluation of human spermatozoa. Fertil. Steril. 2008, 89, 449–455. [Google Scholar] [CrossRef]
- Chacon, J. Assessment of Sperm Morphology in Zebu Bulls, under Field Conditionsin the Tropics. Reprod. Dom. Anim. 2001, 36, 91–99. [Google Scholar] [CrossRef]
- Boersma, A.; Raûhofer, R.; Stolla, R. Influence of Sample Preparation, Staining Procedure and Analysis Conditions on Bull Sperm Head Morphometry using the Morphology Analyser Integrated Visual Optical System. Reprod. Dom. Anim. 2001, 36, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.F.; Greene, L.M.; Kelleman, A.; Knobbe, M.; Turner, R. Effect of method and clinician on stallion sperm morphology evaluation. Theriogenology 2011, 76, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz, E.; Jerysz, A.; Partyka, A.; Siudzinska, A. Efficacy of evaluation of rooster sperm morphology using different staining methods. Res. Vet. Sci. 2008, 85, 583–588. [Google Scholar] [CrossRef]
- Gacem, S.; Catalán, J.; Yánez-Ortiz, I.; Soler, C.; Miró, J. New Sperm Morphology Analysis in Equids: Trumorph® Vs Eosin-Nigrosin Stain. Vet. Sci. 2021, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Björndhal, L.; Soderlund, I.; Kvist, U. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 2003, 18, 813–816. [Google Scholar] [CrossRef]
- Saini, J.; Dhande, P.L.; Gaikwad, S.A.; Shankhapal, V.D.; Hmangaihzuali, E.V.L.; Walters, A. Comparative study on sperm morphology and morphometry of Holstein friesian and murrah buffalo bull. Buffalo Bull. 2018, 37, 559–567. [Google Scholar]
- Andraszek, K.; Banaszewska, D.; Biesiada-Drzazga, B. The use of two staining methods for identification of spermatozoon structure in roosters. Poult. Sci. 2018, 97, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Van Der Horst, G.; Medger, K.; Steckler, D.; Luther, I.; Bartels, P. Bottlenose dolphin (Tursiops truncatus) sperm revisited: Motility, morphology and ultrastructure of fresh sperm of consecutive ejaculates. Anim. Reprod. Sci. 2018, 195, 309–320. [Google Scholar] [CrossRef]
- Van Der Horst, G.; Skosana, B.; Legendre, A.; Oyeyipo, P.; du Plessis, S.S. Cut-off values for normal sperm morphology and toxicology for automated analysis of rat sperm morphology and morphometry. Biotech. Histochem. 2018, 93, 49–58. [Google Scholar] [CrossRef]
- Maree, L.; Du Plessis, S.S.; Menkveld, R.; Van Der Horst, G. Morphometric dimensions of the human sperm head depend on the staining method used. Hum. Reprod. 2010, 25, 1369–1382. [Google Scholar] [CrossRef]
- Holt, W.V.; Van Look, K.J.W. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction 2004, 127, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Petrunkina, A.M.; Waberski, D.; Günzel-Apel, A.R.; Töpfer-Petersen, E. Determinants of sperm quality and fertility in domestic animals. Reproduction 2007, 134, 3–17. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Barth, A. In vitro assessment of sperm quality related to in vivo function and fertility. Soc. Reprod. Fertil. Suppl. 2007, 64, 39–54. [Google Scholar] [PubMed]
- Rodriguez-Martinez, H.; Saravia, F.; Wallgren, M.; Tienthai, P.; Johannisson, A.; Vázquez, J.M.; Martínez, E.; Roca, J.; Sanz, L.; Calvete, J.J. Boar spermatozoa in the oviduct. Theriogenology 2005, 63, 514–535. [Google Scholar] [CrossRef]
- Wysokińska, A. Effect of sperm concentration on boar spermatozoa mitochondrial membrane potential and motility in semen stored at 17 °C. Acta Vet. Brno 2021, 89, 333–340. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Volkera, G.; Weitze, K.; Beyerbach, M.; Töpfer-Petersen, E.; Waberski, D. Detection of cooling-induced membrane changes in the response of boar sperm to capacitating conditions. Theriogenology 2005, 63, 2278–2299. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.; Saravia, F.; Garcia-Herreros, M.; Núñezmartínez, I.; Tapia, J.A.; Wallgren, M.; Rodríguez-Martinez, H.; Johannisson, A. Identification of Sperm Morphometric Subpopulations in Two Different Portions of the Boar Ejaculate and Its Relation to Postthaw Quality. J. Androl. 2005, 26, 716–723. [Google Scholar] [CrossRef]
- Saravia, F.; Núñez-Martínez, I.; Morán, J.; Soler, C.; Muriel, A.; Rodríguez-Martínez, H.; Peña, F. Differences in boar sperm head shape and dimensions recorded by computer-assisted sperm morphometry are not related to chromatin integrity. Theriogenology 2007, 68, 196–203. [Google Scholar] [CrossRef]
- Hidalgo, M.; Rodríguez, I.; Dorado, J.; Soler, C. Morphometric classification of Spanish thoroughbred stallion sperm heads. Anim. Reprod. Sci. 2008, 103, 374–378. [Google Scholar] [CrossRef]
- Gravance, C.G.; Liu, I.K.M.; Davis, R.O.; Hughes, J.P.; Casey, P.J. Quantification of normal head morphometry of stallion spermatozoa. J. Reprod. Fertil. 1996, 108, 41–46. [Google Scholar] [CrossRef][Green Version]
- Severa, L.; Máchal, L.; Švábová, L.; Mamica, O. Evaluation of shape variability of stallion sperm heads by means of image analysis and Fourier descriptors. Anim. Reprod. Sci. 2010, 119, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Révay, T.; Nagy, S.; Kovács, A.; Edvi, M.E.; Hidas, A.; Rens, W.; Gustavsson, I. Head area measurements of dead, live, X- and Y-bearing bovine spermatozoa. Reprod. Fertil. Dev. 2004, 16, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Barquero, V.; Soler, C.; Sevilla, F.; Calderón-Calderón, J.; Valverde, A. A Bayesian analysis of boar spermatozoa kinematics and head morphometrics and their relationship with litter size fertility variables. Reprod. Dom. Anim. 2021, 56, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Balhorn, R. Sperm Chromatin: An Overview. In Sperm Chromatin. Biological and Clinical Aplications in Male Infertility and Assisted Reproduction; Zini, A., Agarwal, A., Eds.; Springer Science Business Media: New York, NY, USA, 2011; pp. 3–13. [Google Scholar]
- Sringam, S.; Kitiyanant, Y.; Lewin, L.M.; Saikhun, K. Semen quality and chromatin condensation in domestic cat sperm during passage through the epididymis. Agric. Nat. Resour. 2011, 45, 46–58. [Google Scholar]
- Núñez-Martinez, I.; Moran, J.M.; Peña, F. Identification of sperm morphometric subpopulations in the canine ejaculate: Do they reflect different subpopulations in sperm chromatin integrity? Zygote 2007, 15, 257–266. [Google Scholar] [CrossRef]
- Urbano, M.; Ortiz, I.; Dorado, J.; Hidalgo, M. Identification of sperm morphometric subpopulations in cooled-stored canine sperm and its relation with sperm DNA integrity. Reprod. Dom. Anim. 2017, 52, 468–476. [Google Scholar] [CrossRef]
- Malo, A.F.; Gomendio, M.; Garde, J.J.; Lang-Lenton, B.; Soler, A.J.; Roldan, E.R.S. Sperm design and sperm function. Biol. Lett. 2006, 2, 246–249. [Google Scholar] [CrossRef]
- Morrow, E.H.; Gage, M.J.G. Consistent significant variation between individual males in spermatozoa morphometry. J. Zool. Lond. 2001, 254, 147–153. [Google Scholar] [CrossRef]
- Cardullo, R.A.; Baltz, J.M. Metabolic regulation in mammalian sperm: Mitochondrial volume determines sperm length and flagellar beat frequency. Cell Motil. Cytoskelet. 1991, 19, 180–188. [Google Scholar] [CrossRef]
- Turner, R.M. Tales from the tail: What do we really know about sperm motility? J. Androl. 2003, 24, 790–803. [Google Scholar] [CrossRef]
- Srivastava, N.; Pande, M. Mitochondrion: Features, functions and comparative analysis of specific probes in detecting sperm cell damages. Asian Pac. J. Reprod. 2016, 5, 445–452. [Google Scholar] [CrossRef]
- Kahrl, A.F.; Snook, R.R.; Fitzpatrick, J.L. Fertilization mode drives sperm length evolution across the animal tree of life. Nat. Ecol. Evol. 2021, 5, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.L.; Bridge, C.D.; Snook, R.R. Repeated evidence that the accelerated evolution of sperm is associated with their fertilization function. Proc. R. Soc. B 2020, 287, 20201286. [Google Scholar] [CrossRef] [PubMed]
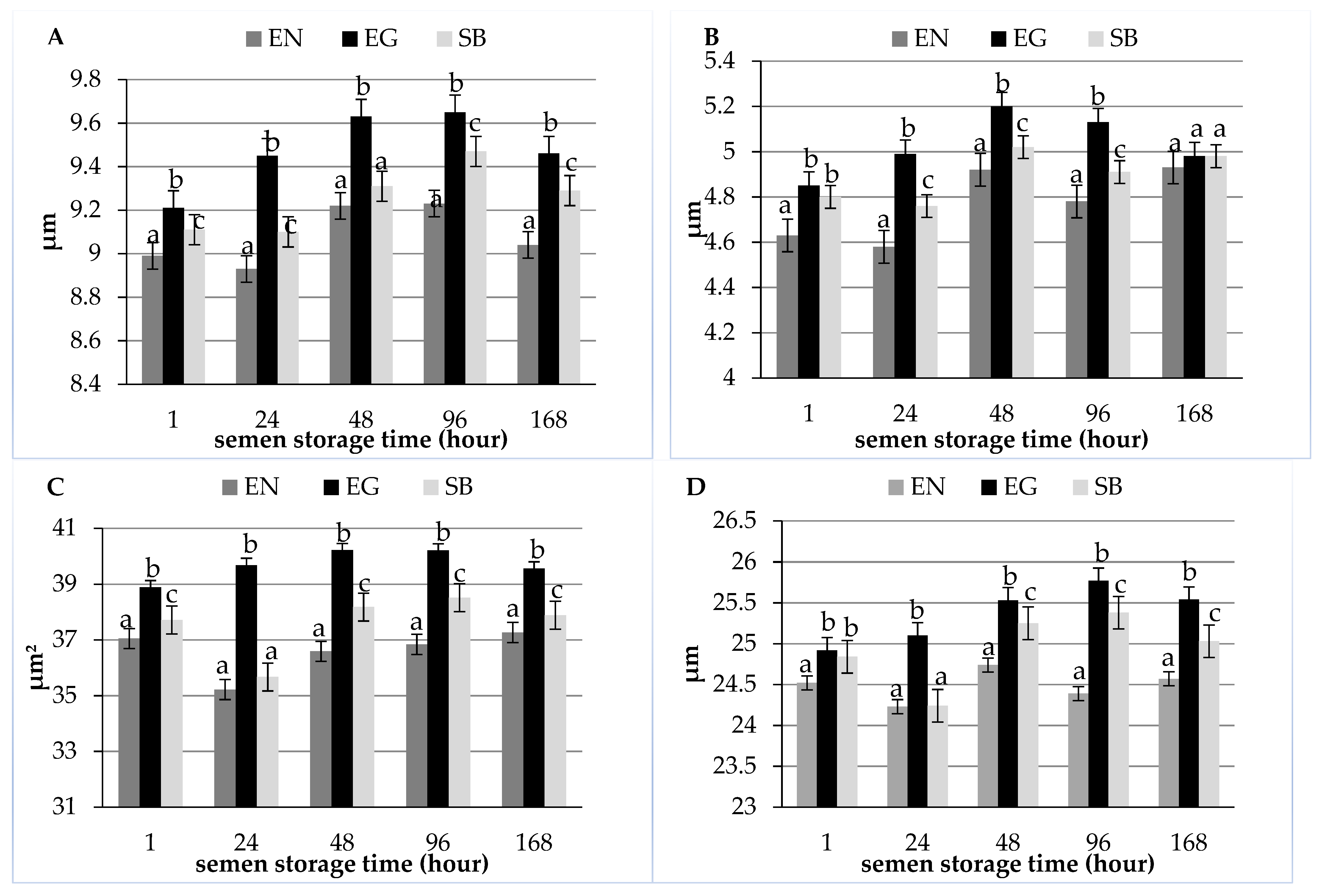
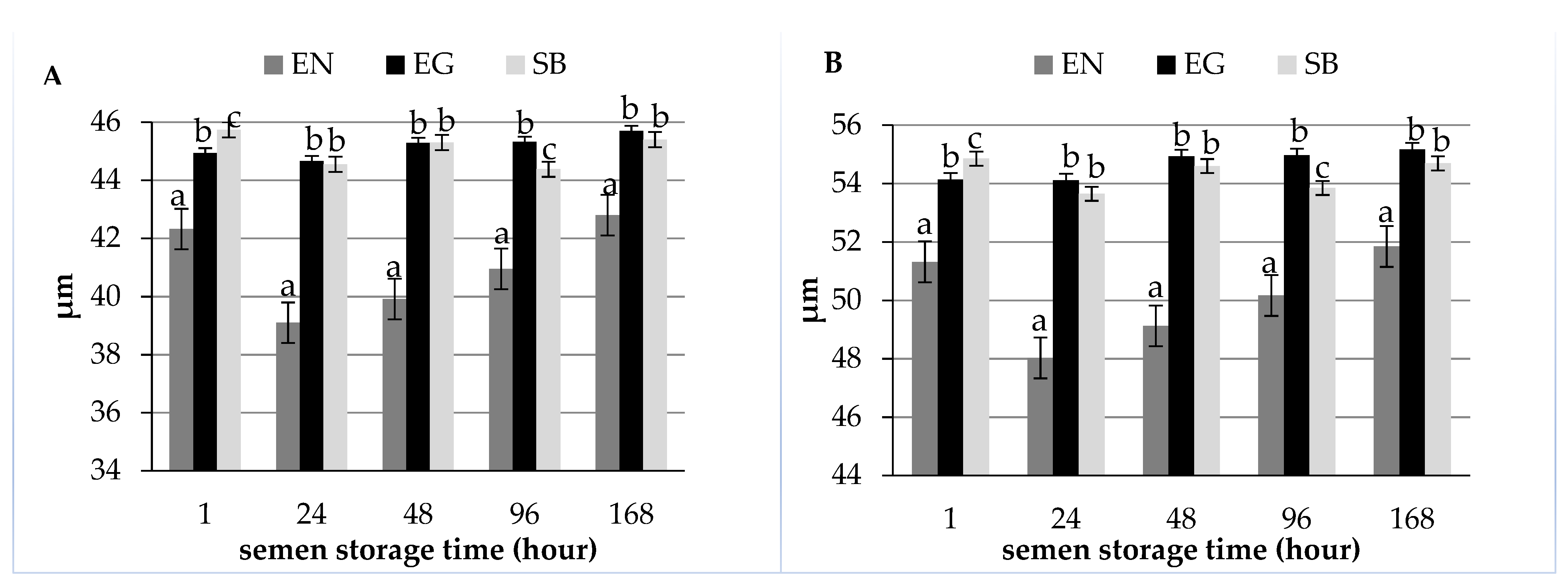
| Item | Semen Storage Time (Hour) | ||||
|---|---|---|---|---|---|
| 1 | 24 | 48 | 96 | 168 | |
| Number of analyzed cells | 1200 | 1200 | 1200 | 1200 | 1200 |
| Head | |||||
| Length (µm) | 8.99 ± 0.88 a | 8.93 ± 0.87 a | 9.22 ± 0.85 b | 9.23 ± 0.76 b | 9.04 ± 0.76 ab |
| Width (µm) | 4.63 ± 0.57 a | 4.58 ± 0.61 a | 4.92 ± 0.66 b | 4.78 ± 0.65 b | 4.93 ± 0.58 b |
| Area (µm2) | 37.05 ± 4.96 a | 35.22 ± 3.86 b | 36.59 ± 4.44 ab | 36.84 ± 4.23 ab | 37.27 ± 4.12 a |
| Perimeter (µm) | 24.52 ± 1.81 ab | 24.23 ± 1.61 a | 24.74 ± 1.76 b | 24.39 ± 1.71 ab | 24.57 ± 1.58 ab |
| Tail | |||||
| Length (µm) | 42.32 ± 5.45 a | 39.10 ± 6.42 b | 39.92 ± 6.29 bc | 40.95 ± 7.06 c | 42.80 ± 6.34 a |
| Sperm total length (µm) | 51.32 ± 5.67 ac | 48.03 ± 6.53 b | 49.13 ± 6.40 ab | 50.17 ± 7.10 ac | 51.85 ± 6.38 c |
| Shape indices | |||||
| Ellipticity | 1.97 ± 0.28 a | 1.99 ± 0.33 a | 1.90 ± 0.28 bc | 1.96 ± 0.31 a | 1.86 ± 0.27 c |
| Elongation | 0.32 ± 0.06 a | 0.32 ± 0.07 a | 0.30 ± 0.06 b | 0.32 ± 0.07 a | 0.29 ± 0.06 b |
| Rugosity | 0.77 ± 0.06 a | 0.76 ± 0.07 ab | 0.75 ± 0.07 b | 0.78 ± 0.07 a | 0.78 ± 0.06 a |
| Regularity | 0.89 ± 0.16 a | 0.92 ± 0.17 ac | 0.98 ± 0.17 b | 0.95 ± 0.15 bc | 0.94 ± 0.15 bc |
| Item | Semen Storage Time (Hour) | ||||
|---|---|---|---|---|---|
| 1 | 24 | 48 | 96 | 168 | |
| Number of analyzed cells | 1200 | 1200 | 1200 | 1200 | 1200 |
| Head | |||||
| Length (µm) | 9.21 ± 0.78 a | 9.45 ± 0.78 b | 9.63 ± 0.80 b | 9.65 ± 0.77 b | 9.46 ± 0.72 b |
| Width (µm) | 4.85 ± 0.61 a | 4.99 ± 0.72 a | 5.20 ± 0.65 bc | 5.13 ± 0.69 c | 4.98 ± 0.76 a |
| Area (µm2) | 38.89 ± 4.17 a | 39.68 ± 4.53 ab | 40.21 ± 4.64 b | 40.20 ± 4.75 b | 39.56 ± 4.79 ab |
| Perimeter (µm) | 24.92 ± 1.38 a | 25.10 ± 1.47 ab | 25.53 ± 1.80 bc | 25.77 ± 1.77 c | 25.54 ± 2.33 bc |
| Tail | |||||
| Length (µm) | 44.93 ± 2.89 a | 44.66 ± 4.28 a | 45.29 ± 4.87 a | 45.32 ± 4.27 a | 45.70 ± 4.38 a |
| Sperm total length (µm) | 54.14 ± 3.09 a | 54.11 ± 4.28 a | 54.93 ± 4.95 a | 54.97 ± 4.28 a | 55.17 ± 4.38 a |
| Shape indices | |||||
| Ellipticity | 1.93 ± 0.29 a | 1.93 ± 0.32 a | 1.88 ± 0.26 a | 1.93 ± 0.30 a | 1.94 ± 0.31 a |
| Elongation | 0.31 ± 0.06 a | 0.31 ± 0.07 a | 0.30 ± 0.06 a | 0.31 ± 0.07 a | 0.31 ± 0.08 a |
| Rugosity | 0.81 ± 0.05 a | 0.78 ± 0.06 b | 0.78 ± 0.08 b | 0.75 ± 0.08 c | 0.77 ± 0.08 b |
| Regularity | 0.88 ± 0.13 a | 0.96 ± 0.17 b | 0.99 ± 0.16 b | 0.99 ± 0.15 b | 0.94 ± 0.14 ab |
| Item | Semen Storage Time (Hour) | ||||
|---|---|---|---|---|---|
| 1 | 24 | 48 | 96 | 168 | |
| Number of analyzed cells | 1200 | 1200 | 1200 | 1200 | 1200 |
| Head | |||||
| Length (µm) | 9.11 ± 0.10 a | 9.10 ± 0.84 a | 9.31 ± 0.78 ab | 9.47 ± 0.90 b | 9.29 ± 0.78 ab |
| Width (µm) | 4.80 ± 0.72 ac | 4.76 ± 0.73 a | 5.02 ± 0.72 b | 4.91 ± 0.65 ab | 4.98 ± 0.59 bc |
| Area (µm2) | 37.72 ± 4.28 a | 35.67 ± 4.42 b | 38.18 ± 4.88 a | 38.52 ± 4.43 a | 37.89 ± 4.75 a |
| Perimeter (µm) | 24.84 ± 1.85 a | 24.24 ± 1.74 b | 25.25 ± 2.02 ac | 25.38 ± 1.75 c | 25.03 ± 1.65 ac |
| Tail | |||||
| Length (µm) | 45.74 ± 4.37 a | 44.55 ± 4.80 a | 45.30 ± 4.98 a | 44.38 ± 6.17 a | 45.40 ± 4.97 a |
| Sperm total length (µm) | 54.85 ± 4.65 a | 53.65 ± 4.93 a | 54.60 ± 5.14 a | 53.85 ± 6.43 a | 54.69 ± 5.18 a |
| Shape indices | |||||
| Ellipticity | 1.93 ± 0.30 a | 1.96 ± 0.35 a | 1.88 ± 0.27 a | 1.96 ± 0.30 a | 1.89 ± 0.28 a |
| Elongation | 0.31 ± 0.07 a | 0.31 ± 0.08 a | 0.30 ± 0.06 a | 0.32 ± 0.07 a | 0.30 ± 0.07 a |
| Rugosity | 0.77 ± 0.07 a | 0.76 ± 0.08 a | 0.76 ± 0.09 a | 0.75 ± 0.08 a | 0.76 ± 0.07 a |
| Regularity | 0.92 ± 0.20 a | 0.96 ± 0.17 a | 0.97 ± 0.17 a | 0.95 ± 0.15 a | 0.97 ± 0.15 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szablicka, D.; Wysokińska, A.; Pawlak, A.; Roman, K. Morphometry of Boar Spermatozoa in Semen Stored at 17 °C—The Influence of the Staining Technique. Animals 2022, 12, 1888. https://doi.org/10.3390/ani12151888
Szablicka D, Wysokińska A, Pawlak A, Roman K. Morphometry of Boar Spermatozoa in Semen Stored at 17 °C—The Influence of the Staining Technique. Animals. 2022; 12(15):1888. https://doi.org/10.3390/ani12151888
Chicago/Turabian StyleSzablicka, Dorota, Anna Wysokińska, Angelika Pawlak, and Klaudia Roman. 2022. "Morphometry of Boar Spermatozoa in Semen Stored at 17 °C—The Influence of the Staining Technique" Animals 12, no. 15: 1888. https://doi.org/10.3390/ani12151888
APA StyleSzablicka, D., Wysokińska, A., Pawlak, A., & Roman, K. (2022). Morphometry of Boar Spermatozoa in Semen Stored at 17 °C—The Influence of the Staining Technique. Animals, 12(15), 1888. https://doi.org/10.3390/ani12151888






