4.1. Growth Performance
Poultry by-product and black soldier fly meals have been extensively studied, mainly as sources of protein to replace fishmeal in the diet of different fish species [
9,
10,
62,
63,
64]. The novelty of the present study was a paradigm shift in that it evaluated the response of fish to graded levels of PBM and BSFM, either singly or in combination, to replace the protein from vegetable sources in fishmeal-free diets, including substantial levels of soybean meal (SBM). It is well known that in salmonids, feeding diets high in vegetable protein sources, particularly SBM, can result in impaired growth performance and gut disorders [
33,
65,
66,
67]. This has recently also been confirmed in this research, as previously reported by Randazzo et al. [
11]. In this study, the response of fish to varying dietary inclusion levels of PBM and BSFM was investigated in terms of the possible beneficial effects on nutrient retention, digestive function, and metabolic and welfare status. Relative to the CV treatment, including medium or high levels of both BSFM and PBM, either singly or in combination, led to improved nutrient and energy retention efficiency. These improvements were similar or even better than those observed in fish fed a fishmeal-based diet. A straight comparison of our results with other studies on salmonids is difficult as in most of the previous experiments the PBM and BSFM were used as fishmeal substitutes in diets including low proportions of vegetable protein sources [
34,
68]. In our study, PBM and BSFM were used to partially replace vegetable proteins in a basal vegetable diet. To what extent the improved growth, nutrient, and energy retention efficiency observed here reflect the improved overall nutritive value of the diets with PBM and BSFM inclusion or the concurrent shortage of vegetable protein (SBM) cannot be easily elucidated. However, as all the diets were designed to meet the rainbow trout nutrient requirements, the improvement observed in the aforementioned parameters seems conceivable, firstly, with a better overall digestible energy, amino acid supply/balance and/or improved gut health, or even improved gut microbiota composition when the plant proteins and SBM were gradually replaced by the test ingredients [
40]. On the other hand, Dumas et al. [
12] observed impaired growth performance, feed conversion, and a decline in the protein retention efficiency in rainbow trout of similar size in response to the increased replacement of fishmeal protein with PBM, defatted BSFM, and a blend of vegetable ingredients. These opposite outcomes could partly depend on different proportions of main alternate protein sources in the diet or in the composition of the vegetable protein blend as well as in the different protein to lipid (energy) dietary ratios between experiments. This also suggests that different ratios among major protein sources, in particular the ratio between PBM and BSFM in the diet, could be a crucial aspect that needs to be optimized in further trials to allow high growth performance to be attained even when the diets are deprived of fishmeal.
In this study, the whole-body composition of the fish was marginally affected by the dietary treatments. A few changes were observed, mostly in the water and lipid (energy) contents in the fish fed medium to high levels of BSFM, showing slightly increased body fat and, conversely, reduced water content. This is apparently in contrast with other studies where the inclusion of BSFM in the diet reduced the whole-body lipid content in rainbow trout [
69] and Atlantic salmon [
63]. In salmonids, lower lipid utilization and deposition in fish fed diets containing BSFM were associated with lowered lipid digestibility and with a putative adverse effect of chitin on lipid digestibility [
70]. In this study, we did not measure diet digestibility; thus, we could not explore the impact of chitin on the absorption and retention of lipids and other nutrients. However, based on growth response, whole-body composition, and nutrient and energy retention efficiency, it seems that the possible adverse effect of chitin contained in BSFM diets, which ranged from 0.4% up to 1.9%, was less pronounced than in other studies. This may be related to the increased relative abundance of chitin-degrading
Actinomyces and
Bacillus observed in the gut microbiota of rainbow trout fed the BSFM [
40].
4.2. Digestive Function
The host gene expression of digestive enzymes and nutrient transporters, as well as the activity of certain BBM enzymes, has been used as a complementary tool to evaluate the gut response to major dietary changes [
17,
20,
71,
72]. The stomach is involved in important physico-chemical processes for the subsequent macronutrient transit and processing, and the mucous cells at the gastric surface function as an acidic-proteolytic organ in all vertebrates [
73]. It has been shown that the ability of fish to hydrolyze chitin fibers is governed by the natural feeding habits and depends on a specific set of chitinolytic enzymes, such as chitinases and chitobiase [
74,
75]. An important result of the present study is the finding that rainbow trout expressed the endogenous chitin hydrolysis-related gene (
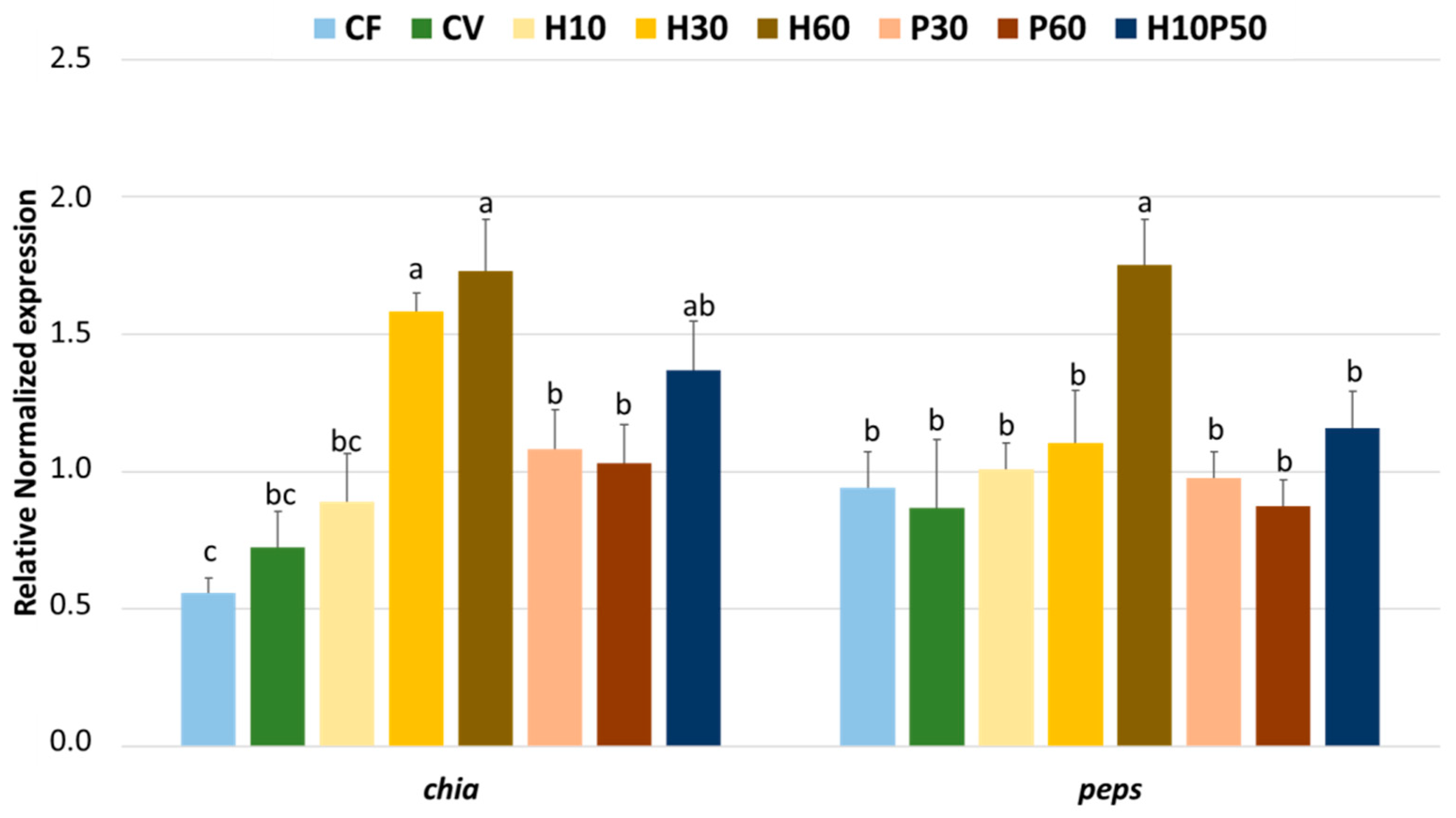
chia) in the stomach, which was upregulated in an apparent dose-dependent fashion by increasing the levels of BSFM in the diet. Moreover, the expression patterns of both
chia and
peps mRNAs agree with what was previously reported on other insectivorous or omnivorous animals [
76,
77], where the proteolytic enzyme accessibility was improved by gastric chitin-degrading enzymes [
78]. The results observed here in rainbow trout fed BSFM are also consistent with previous findings on zebrafish, where an upregulation of the
chia gene was noted in fish fed a diet where fishmeal was partially replaced by full-fat BSFM [
79,
80].
In the present experiment, replacing vegetable protein by moderate to high levels of PBM and/or BSFM increased the gene expression of peptide and amino acid intestinal transporters such as
PepT1 and
B(0)AT1. Our results first confirm that the
PepT1 is abundantly expressed in the proximal intestine of rainbow trout [
53,
81], as also reported on marine fish species [
17,
18,
82,
83]. Secondly, these results support the finding that such a biomarker can be modulated by the source of protein in the diet [
17,
18,
82]. The increased
PepT1 expression in the H10P50 diet, which is rich in Lys and Gly, is consistent with previous findings in trout fed diets supplemented with Lys–Gly dipeptide [
52], or in carp [
81] fed a diet supplemented with free lysine and glycine. In carp, this was found to be correlated with an improved feed conversion ratio and N retention. The expression and activity of
PepT1 probably depends on the availability, composition, and concentration of peptides, as well as the presence in the intestinal lumen of other components that may affect absorption dynamics [
84]. According to Gilbert et al. [
84], the uptake of free amino acids may be indirectly regulated by
PepT1 activity. Wenzel et al. [
85] observed that the uptake of dipeptides stimulates amino acid absorption, and this could partially explain the increased expression of both
PepT1 and
B(0)AT1 in rainbow trout fed the H10P50 diet in the present study.
Intestinal alkaline phosphatase (IAP) is the major homeostatic enzyme produced by enterocytes and is known to be involved in maintaining gut health in fish and other animals [
86]. Generally, the IAP activity responds to feed intake and diet composition; decreased expression is observed during starvation, and increased expression is observed after fat feeding. However, how dietary nutrients modulate both
iap gene expression and enzyme activity is still unclear [
87]. In the present study, the
iap gene expression was upregulated in the two intestinal regions examined, particularly in the fish fed the H60 diet. This could be related to the fatty acid composition of BSFM, known to be rich in medium chain fatty acids, particularly lauric acid [
88], which has been shown to increase
iap expression and/or activity in rats and enteroid models [
89,
90,
91]. In fact, the H60 diet was slightly higher in lauric acid than the others (
Table 3), and this seems consistent with this possible explanation. Another possible reason affecting the
iap expression evokes a role of the gut microbiota, as suggested by Bates et al. [
92] and Goldberg et al. [
93] and could be explained through an indirect effect of the bacterial components or metabolites on the intestinal epithelium physiology [
86,
87]. In our previous paper [
40], we reported that rainbow trout fed the H60 diet showed high relative abundances of the
Bacillus and
Actinomyces genus, which are known to act as chitin degraders, thus leading to chitosan production, which in turn was found to be a stimulator of IAP activity in the gut of trout fed this polymer in the form of nanoparticles [
94]. Although suggestive, this possible explanation must be considered with caution as in the present experiment there was no correspondence between the IAP gene expression and activity, probably due to the time shift between the transcription and the translation protein expression, inevitably generated by the experimental procedure.
In this study, some minor but significant changes in BBM gene expression and enzyme activity due to dietary treatments were observed only for the maltase in the anterior intestine, which appeared to be in response to the amount of dietary starch rather than the protein source. This seems consistent with the outcomes of previous studies on salmon and trout by Krogdahl et al. [
95], where disaccharidase activity was found in a positive correlation with the dietary starch level. Leucine aminopeptidase (LAP) enzyme activity was used as a rough marker of the ability of fish to process dietary proteins from ingredients of different origin. In general, replacing graded levels of vegetable proteins with BSFM did not affect LAP activity along the gut apart from a numerically negligible decline with the H60 diet in the distal intestine. This is in contrast with the results of a study on Atlantic salmon [
70], where a diet including 60% BSFM to replace 85% of the fishmeal protein, resulted in a marked decrease in LAP activity in the proximal and medium intestine. This adverse effect on the capability of the BBM enzyme to hydrolyze peptides into amino acids was ascribed to the chitin supplied by BSFM, which was assumed to interfere with intestinal homeostasis. Apart from the different fish species, the fish size range, the dietary levels, the composition of the plant protein, and the general culture conditions, the different outcomes of the two experiments could also depend on the higher level of dietary BSFM (chitin) in the experiment with salmon relative to the present study (i.e., 60 vs. 45%), which could have emphasized the adverse effect of chitin on intestinal mucosal homeostasis and function. In this study, the LAP activities were slightly reduced in the pyloric caeca and distal intestine when dietary plant proteins were substituted by PBM. In a previous experiment on gilthead seabream, increasing the levels of PBM in a substitution of dietary fishmeal did not affect intestinal LAP activity [
96], while Hekmatpour et al. [
97] found the activity of LAP to be stimulated in response to graded levels of fishmeal replacement by PBM in sobaity seabream (
Sparidentex hasta). Beyond the different fish species, culture conditions, and major differences in the protein source being replaced (veg proteins vs. fishmeal), which could probably play a role in affecting intestinal LAP activities, there is no easy explanation to reconcile such contrasting results among the experiments. It should be stressed, however, that the magnitude of the diet-induced differences observed here in the enzyme activities was minor and were poorly reflected in terms of overall growth and protein or energy retention efficiency.
4.3. Fish Welfare
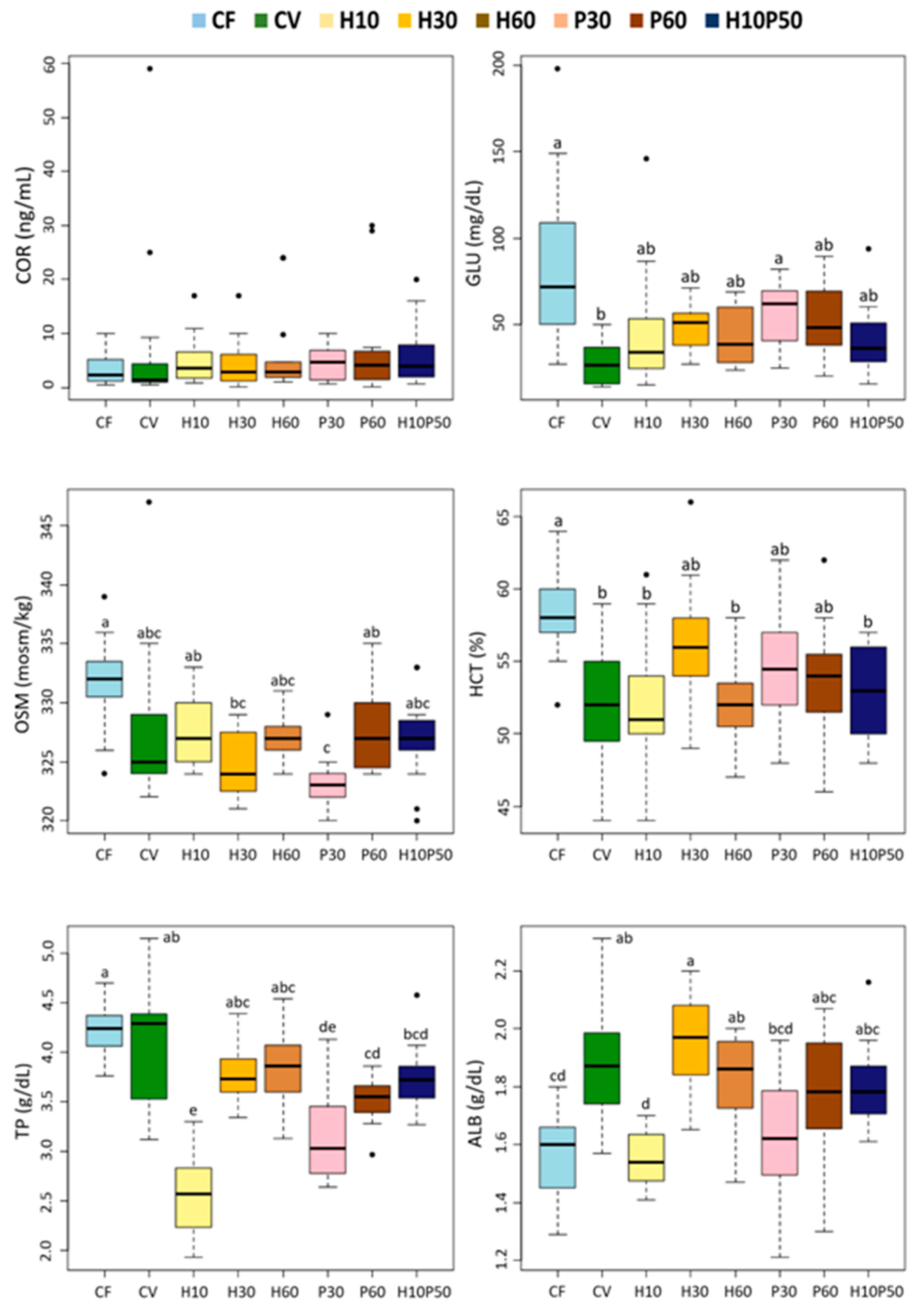
In this study, fish welfare was evaluated according to a function-based approach by assessing stress, metabolic, immune responses, liver health, and fish condition and by ensuring appropriate water quality and stocking density during the trial [
22]. Despite its physiological consequences on welfare, stress response has been little investigated in relation to the source of dietary protein in fish. Moreover, the knowledge on the involvement of dietary protein/amino acids in modulating the primary and secondary stress response is still quite unexplored [
27,
98,
99]. Previous studies have shown that plant-based diets induce chronic stress or reduced stress tolerance in rainbow trout [
33,
100,
101], but little or no information is available on the stress response to diets containing insect or poultry by-product meals. In this study, the serum cortisol level did not differ among dietary treatments and was close to the basal levels reported for rainbow trout, indicating no primary stress response [
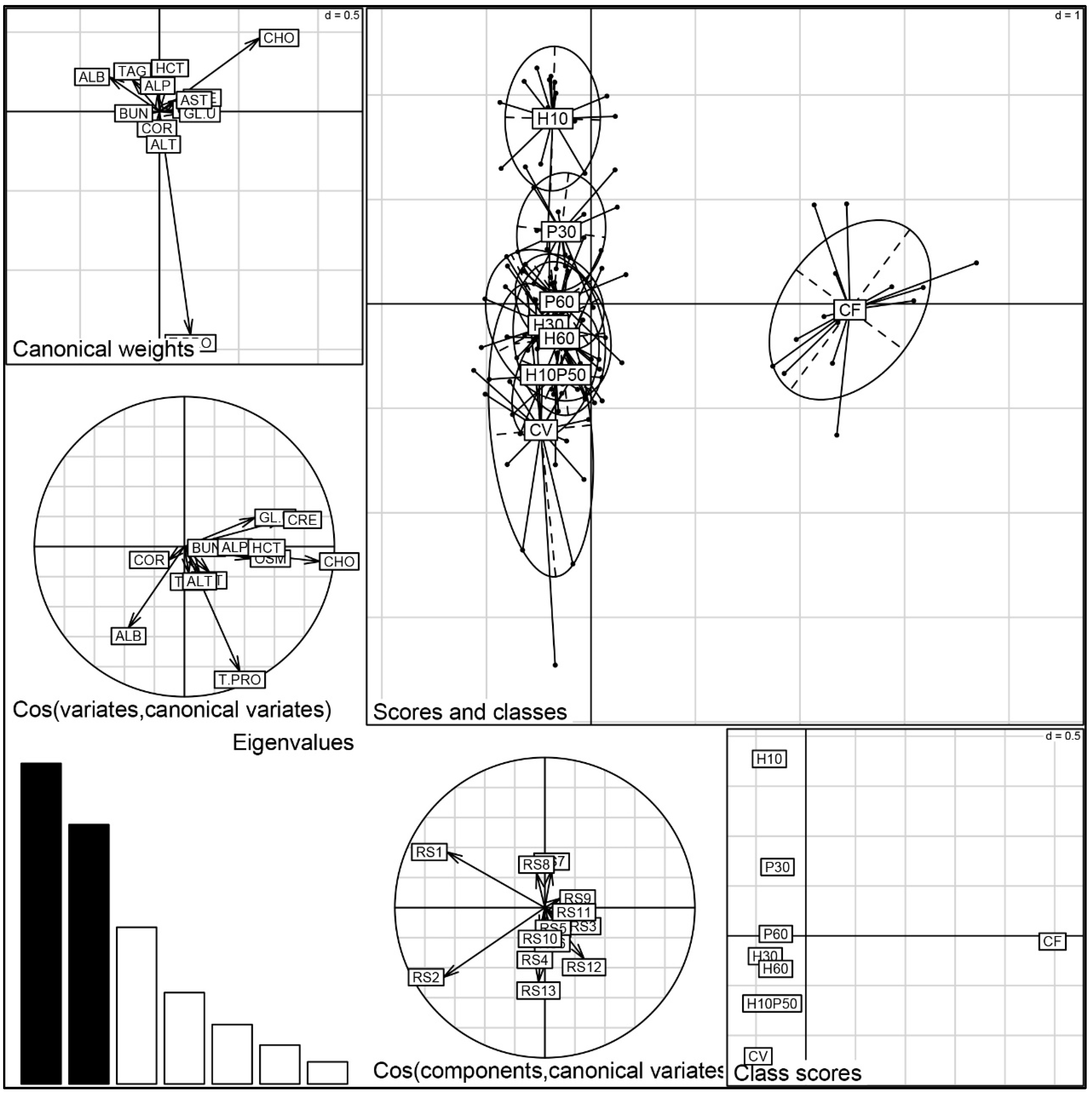
102]. The osmolality and hematocrit levels, generally regarded as secondary stress indicators, were similar in the fish fed the CV and CV-substituted diets. Their values were higher in the fish fed the CF relative to those given the CV diet, probably reflecting the fact that the CF contained a large proportion of fishmeal rich in cholesterol and minerals. This also seems to be suggested by the strong correlation between cholesterol, osmolality, and hemoglobin upon the discriminant analysis (
Figure 7). A close relationship between cholesterol and hematocrit has already been described in rainbow trout [
58]. Nonetheless, the levels of these latter parameters measured in all the dietary groups are consistent with the normal values in rainbow trout [
57,
58]. In accordance with our results, osmolality was not affected in freshwater Atlantic salmon fed insect-based diets [
70].
All the dietary treatments including the test ingredients, resulted in reduced serum glucose levels relative to the CF diet, with a more evident drop in the fish fed the CV diet. In this study, glycemia reflected the total carbohydrate content or levels of starch-containing ingredients in the diets. Similar to our results, no changes in plasma glucose levels were reported in rainbow trout fed diets with BSFM as a substitute for fishmeal or soybean meal [
12,
34,
37].
The serum protein content, mainly consisting of albumin and globulins, is considered an indicator of nutritional status in fish [
24,
103]. In this study, the total protein and albumin concentrations were significantly lower in the fish fed the H10 and P30 diets, compared to the fish fed the CV diet, and remained in any case within the normal range of values for rainbow trout [
104,
105]. These results are consistent with the data reported for the sturgeon (
Acipenser schrenckii), showing a significant decrease in serum total protein and albumin without differences in fish growth with the increasing of the dietary soy protein isolate to over 50% of the substitution of fishmeal [
106]. In the sturgeon, the decline of serum proteins was ascribed to reduced protein synthesis or protein breakdown due to liver pathological conditions or intestinal mucosal inflammation/injury. This seems to be the case for rainbow trout in our experiment since, as reported by Randazzo et al. [
11], histological and molecular analyses showed signs of inflammation in the distal intestine in the fish fed the H10 diet, while a moderate liver steatosis and a significant reduction in liver protein content were observed in the fish fed the P30 diet, when compared to those fed the CV diet.
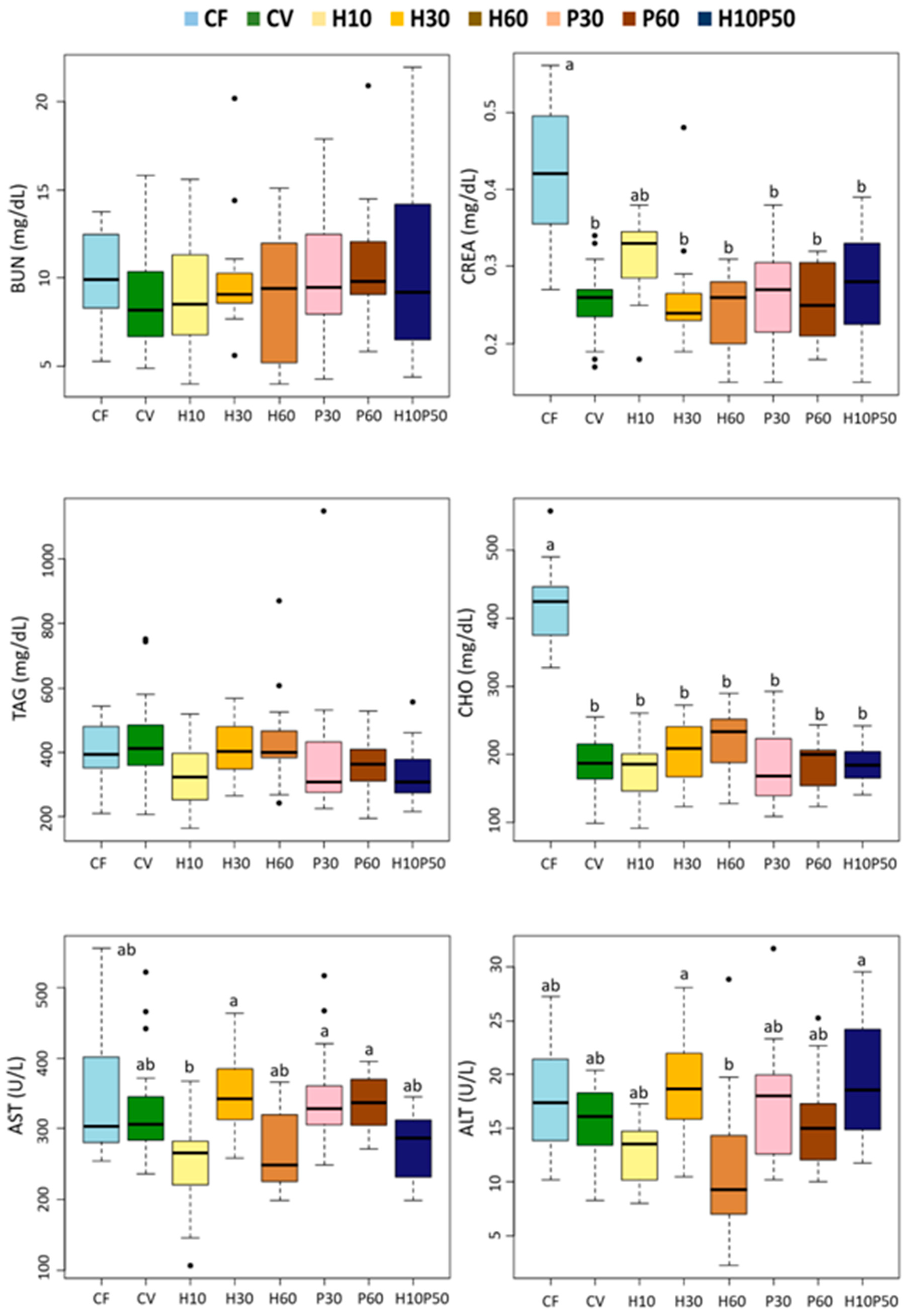
We found minor changes in the serum lipid components in response to the dietary treatments. The serum triglyceride concentrations were not affected by the diets and were within normal physiological range [
104]. As already noted, the fish fed the CF diet had higher serum cholesterol content, mirroring a higher cholesterol intake, while similar levels were measured in the fish fed the diets containing the test ingredients and those fed the CV diet. A concentration of around 200 mg/dL is consistent with the values reported in other studies with rainbow trout fed diets containing BSFM [
34]. The fish fed the CF diet also showed higher creatinine levels, thus reflecting the high creatine content in fishmeal compared to the other ingredients.
We measured the circulating blood hepatic transaminases, AST and ALT, as biomarkers of liver tissue damage and general health condition [
107]. Similar to what was previously observed in Atlantic salmon fed insect-based diets [
71], in our study a high inclusion of BSFM in the H60 diet led to a lower serum ALT concentration compared to the other diets, although this difference did not reach statistical significance due to high data variability.
The results of the multivariate analysis provided a comprehensive evaluation of the physiological status of trout in response to the dietary treatments. A substantial similarity between the fish fed the CV and CV-derived diets emerged. Conversely, the H10 and P30 diets appeared to cluster separately from the other diets, and they were discriminated by lower total protein levels, denoting a physiological disturbance.
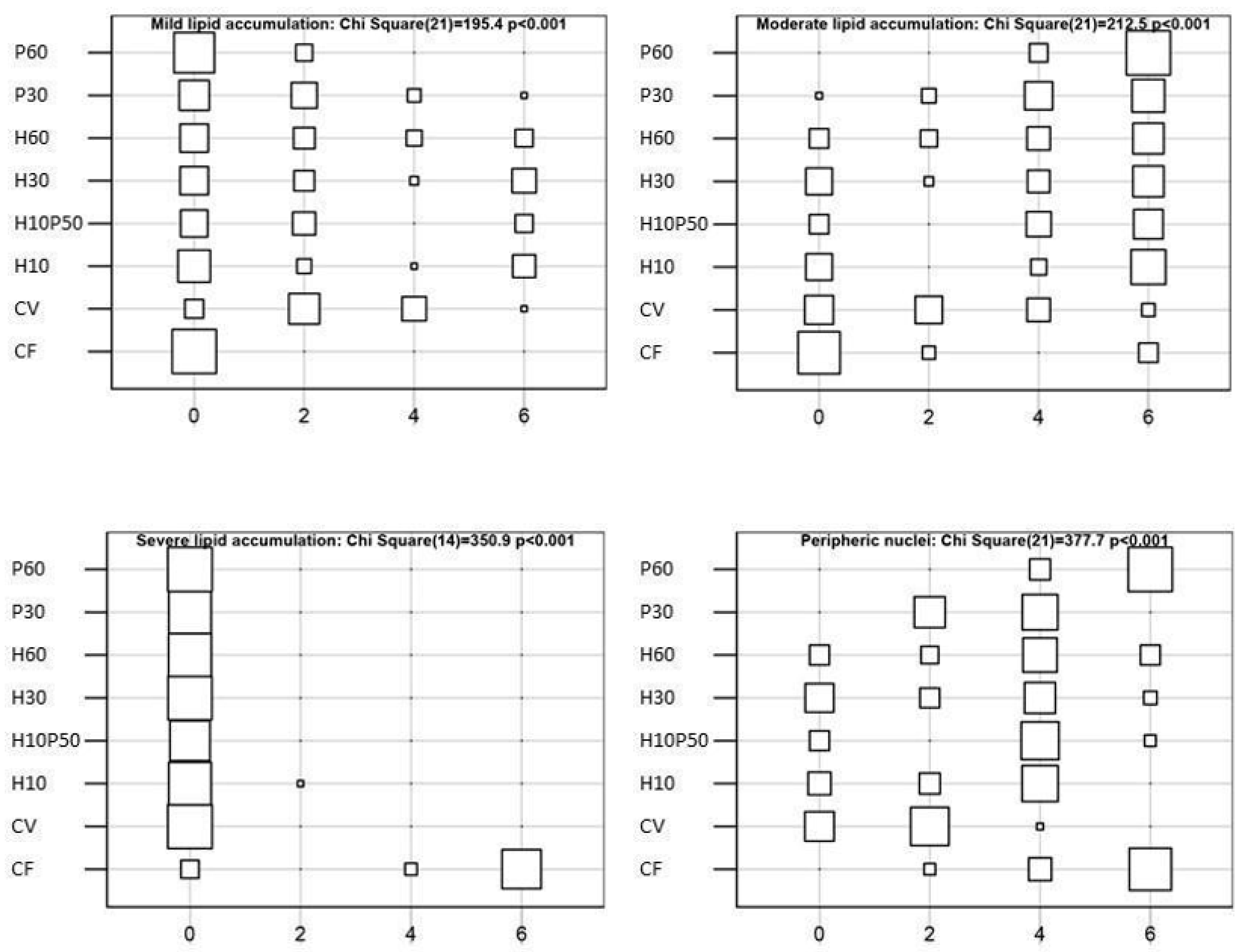
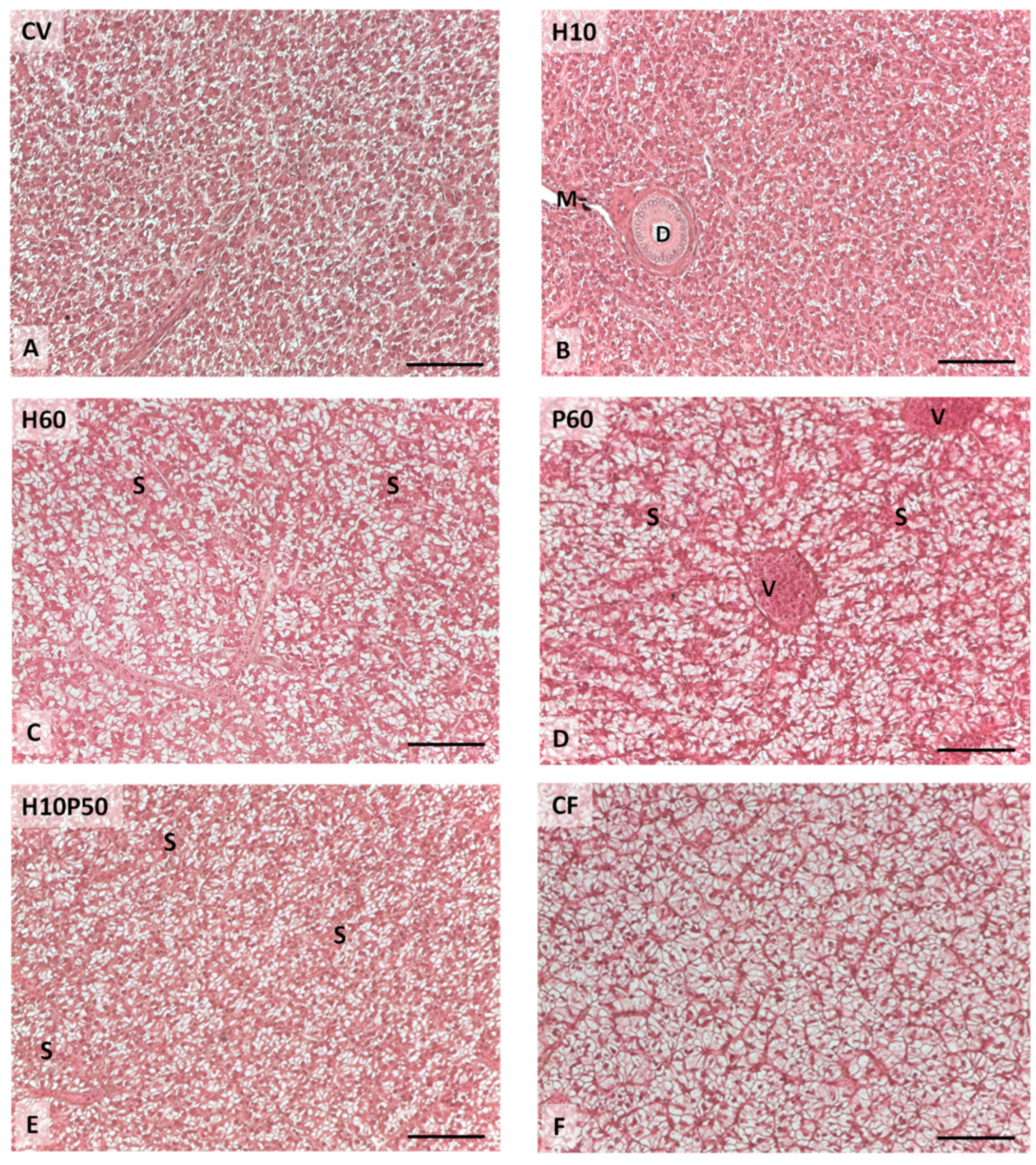
Liver health condition provides important information on fish nutritional status and welfare [
108]. The inclusion of alternative protein sources in the aquafeeds was shown to affect liver histology and fatty acid composition in several fish species, depending on ingredient type and inclusion level [
109,
110,
111,
112,
113]. In our analysis, several histopathological markers were used to evaluate liver health status, including lipid accumulation, circulatory disturbances, and inflammatory response [
61]. The inclusion of BSFM and/or PBM in the diet did not cause severe alterations in the hepatic tissue. The fish fed the CV and H10 diets showed lower liver lipid accumulation compared to the other dietary groups, due to the well-known lipid-lowering effect of soybean meal [
114]. A moderate hepatocyte lipid accumulation was observed in the fish fed the diets containing PBM and BSFM. The livers of the fish fed the P60 diet resulted in moderate lipid accumulation, which appeared slightly reduced with the diets containing BSFM alone or in combination with PBM, as already reported [
11]. These findings are consistent with the HSI values and circulating transaminase levels measured in the present study (
Table 7). Similar to what we observed here in the H10P50 fed trout, in
Lates calcarifer BSFM was also associated with reduced liver lipid deposition, when included in a diet together with PBM [
110]. This attenuating effect of BSFM on the liver lipid accumulation has been interpreted with regard to the higher amount of medium-chain fatty acids, in particular lauric acid, which are prone to a rapid oxidation rather than liver deposition [
70,
110]. Severe lipid accumulation was observed in the livers of the fish fed the CF diet (
Figure 8F), which also displayed the highest HSI and serum cholesterol level compared to the other dietary groups, thus confirming our previous observations [
11,
41].
Despite differences in the HSI values in the function of the dietary treatment, the organosomatic indices and condition factors [
30] were in line with data reported for healthy farmed rainbow trout [
115].
Leukocytes are involved in fish immune response and can be modulated by abiotic and biotic stressors. Their count and formula represent useful indicators of fish health [
28,
116]. No effect on leukocyte formula was found in Nile tilapia (
Oreochromis niloticus) and European sea bass (
Dicentrarchus labrax) fed diets including BSFM at different rates of fishmeal replacement [
117,
118], whereas no data are available on rainbow trout fed insect or poultry by-product meal diets. In our study, the dietary treatment significantly affected the differential count of the white blood cells (
Table 7). The lymphocytes resulted in being higher in the fish fed the H10 and H30 diets, whereas the monocytes and neutrophils tended to increase in the H60, P30, P60, and H10P50 diets. These results mirror the trend of increasing lysozyme activity observed in the H60 and P60 groups (
Figure 6), which is probably linked to increased blood phagocytes, which are the main source of serum lysozyme [
119]. The etiology of these variations remains uncertain, although it might be linked to nutrition, inflammation, or stress.
It is well recognized that the source of dietary protein affects the immune defense mechanisms in salmonids, with possible adverse effects when large proportions of fish or animal proteins are replaced in the diet by their vegetable counterparts. This is especially the case when certain soy protein derivatives such as SBM are used. In rainbow trout, several serologic and non-specific immune defense mechanisms were increased in the fish fed diets high in SBM compared to fishmeal-based diets, indicating an inflammatory or hypersensitivity reaction resulting in distal intestine pathological alterations [
66,
120,
121,
122]. Our CV diet contained a proportion of SBM at or below the level known to result in adverse effects on trout growth, gut health, and immune defense mechanisms [
121,
122]. When the outcomes of feeding the CV diet were compared to CF, the serum lysozyme was unaffected, but the peroxidase activity was clearly higher in the former. Peroxidases act as preventive antioxidants to detoxify peroxides that are potentially dangerous for lipids. Moreover, they function as starters to trigger oxidative reactions (stimulation of respiratory burst) activated as part of the immune defense against pathogens. Elevated peroxidase activity is likely to increase infection resistance [
123]. Head kidney leukocyte respiratory burst activity was only marginally increased with the CV diet compared to the CF. This outcome does not seem to present clear evidence of an abnormal response of innate immunity in response to a diet containing soybean meal.
Among novel dietary protein sources, insects have been claimed to stimulate certain immune mechanisms in fish. However, the studies on this topic are still limited [
124,
125,
126], and none of them evaluated the role of insect meals when included in diets high in vegetable proteins. The immune-boosting action of insects has mostly been attributed to their chitin content [
125,
127,
128,
129]. Chitin could act as stimulator of the innate immune system by binding to specific receptors, such as the macrophage mannose receptor, toll-like receptor 2 (TLR-2), interferon-γ (IFN-γ) receptor, and the Dectin-1 receptor [
130]. Experiments in which insect meals obtained from
Tenebrio molitor or
Musca domestica were used to replace dietary fishmeal have shown increased serum lysozyme activity in rainbow trout [
131] and increased phagocytic activity of peritoneal macrophages in red seabream [
132]. In the present study, the fish fed a diet high in BSFM when compared to those given the CV diet resulted in lower serum peroxidase and HK leukocyte respiratory burst activities but in similar serum lysozyme values. The fish fed the BSFM diets also showed reduced HK respiratory burst activity when compared to the CF. Hence, BSFM exhibited some immunomodulatory effects but only partially confirmed previous observations, and this warrants further investigation.
Few studies are available concerning the effects of diets including PBM on the innate immune response of carnivorous fish species. In the present study, the inclusion of this ingredient in a plant-protein-rich diet (P60) resulted in elevated serum lysozyme activity compared to the values observed in trout fed CV, CF, and H60 diets. An increased serum lysozyme activity is intended as a positive indicator of non-specific humoral defense. Lysozyme is one of the main lytic factors acting against Gram-positive bacteria [
133]. Rawles et al. [
134] found no differences in lysozyme activity in sunshine bass fed a fishmeal-based diet or a diet including nearly 30% SBM, 30% FM, and 30% PBM. The partial replacement of fishmeal with PBM associated with BSFM (45% PBM and 10% BSFM) in the diet of juvenile barramundi (
Lates calcifer) induced a marked increase in lysozyme activity when the fish were challenged with
Vibrio harveyi [
135]. On the other hand, a total replacement of fishmeal by PBM adversely affected the immune response, serum lysozyme activity, and stress-related genes (HSP70 and HSP90) of juvenile barramundi, whereas none of the diets had significant effects on bactericidal activity [
136]. Hence, PBM inclusion in the diet, used either singly or in combination with BSFM, resulted in conflicting effects on fish immunity when the results of the present study are compared with the outcomes of other experiments. Conversely to BSFM, where chitin acts as a bioactive compound, PBM does not contain known immune-modulating compounds, making it difficult to discern its role in affecting immune response from that of other dietary components.