Chitosan Oligosaccharide Inhibits the Synthesis of Milk Fat in Bovine Mammary Epithelial Cells through AMPK-Mediated Downstream Signaling Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Cell Culture and Treatments
2.3. CCK-8 and EdU (5-ethynyl-20-deoxyuridine) Assay
2.4. Detection of Cell Cycle Progression
2.5. Determination of Triglyceride (TG) and Cholesterol Content
2.6. Detection of Non-Esterified Fatty Acids (NEFA) Content
2.7. Real-Time Quantitative PCR (RT-qPCR) and Western Blot Analysis
2.8. Statistical Analysis
3. Results
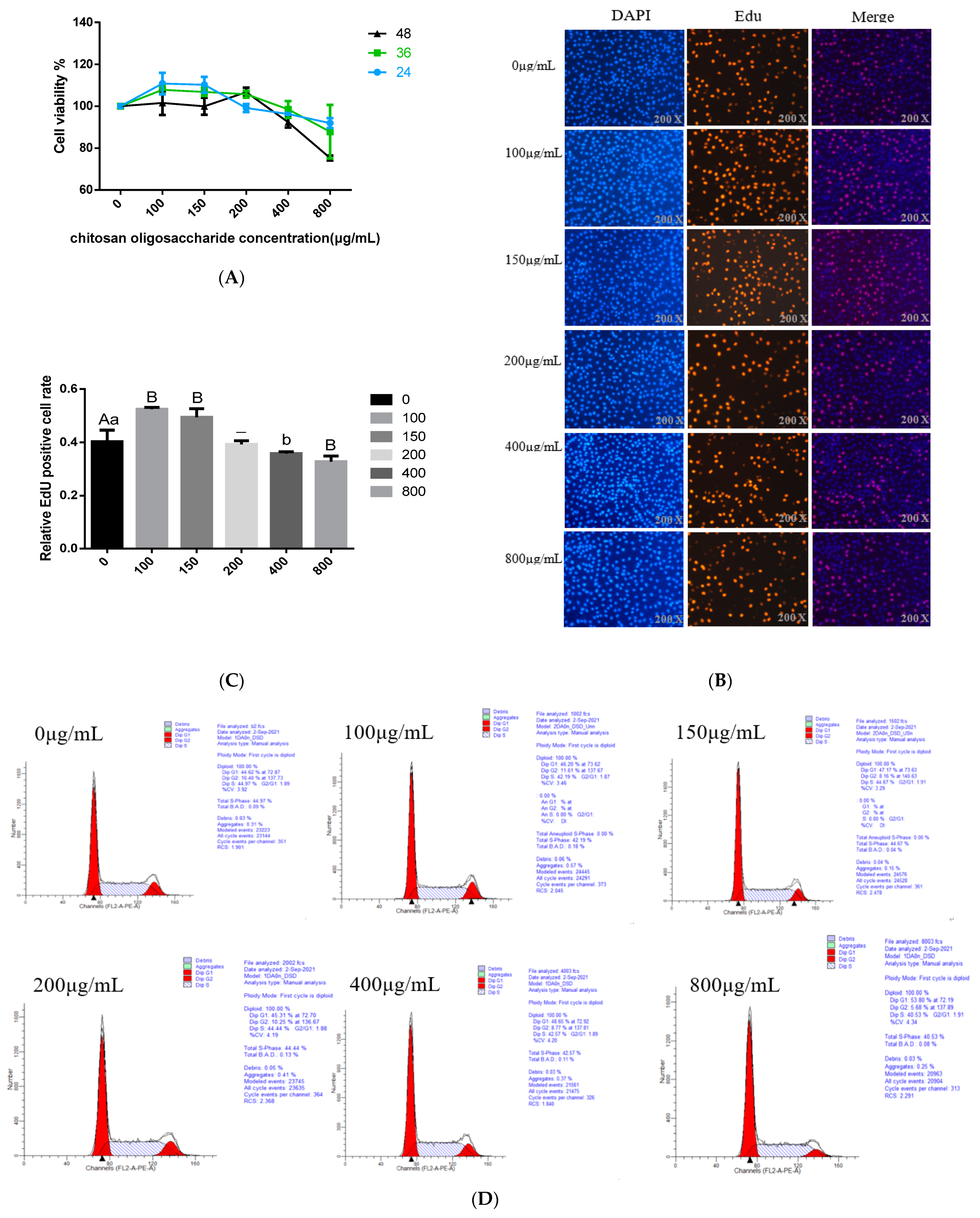
3.1. Effects of COS on BMECs Viability
3.2. Effects of COS on BMECs Proliferation
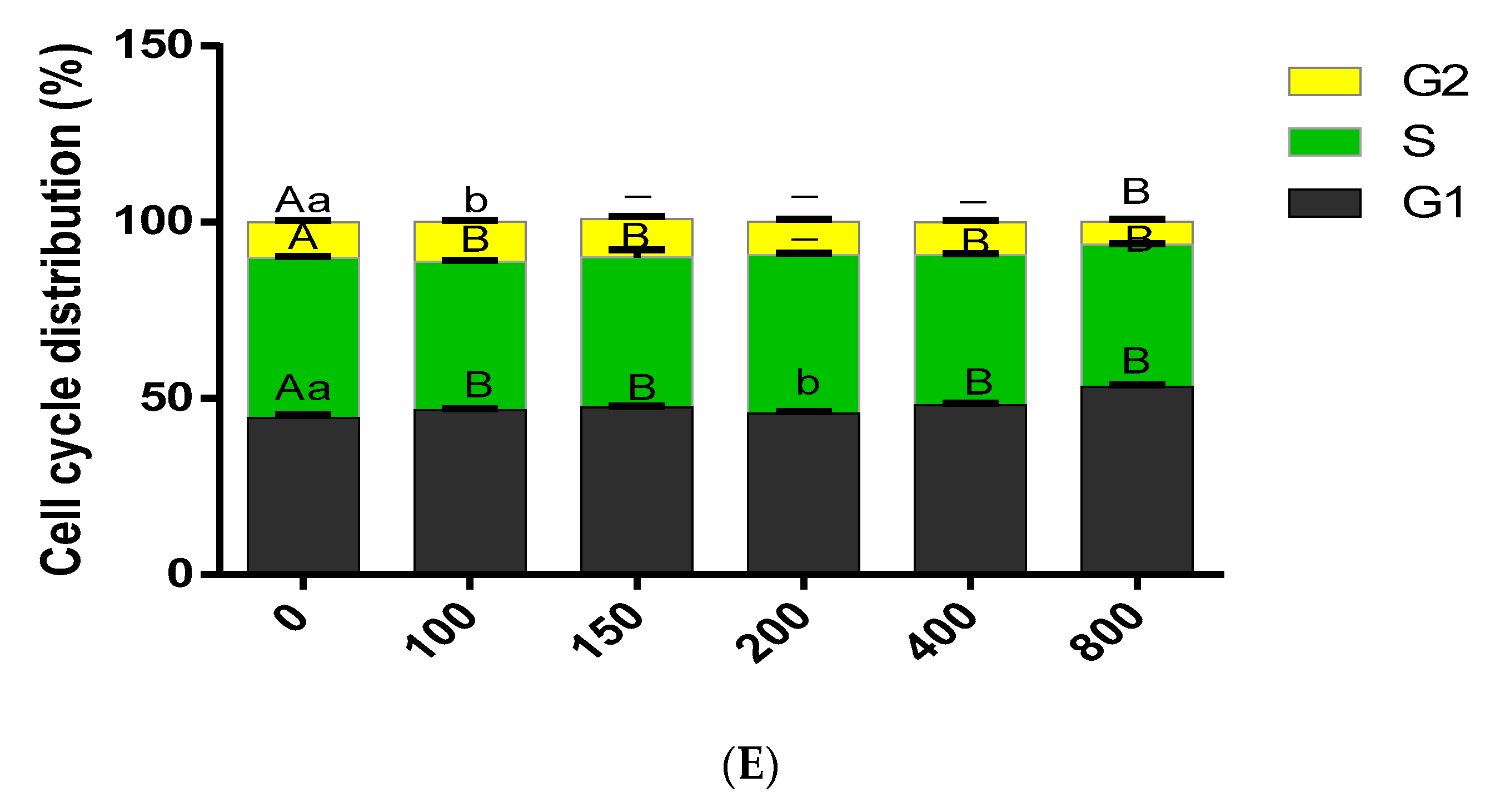
3.3. Effect of COS on Cell Cycle Progression
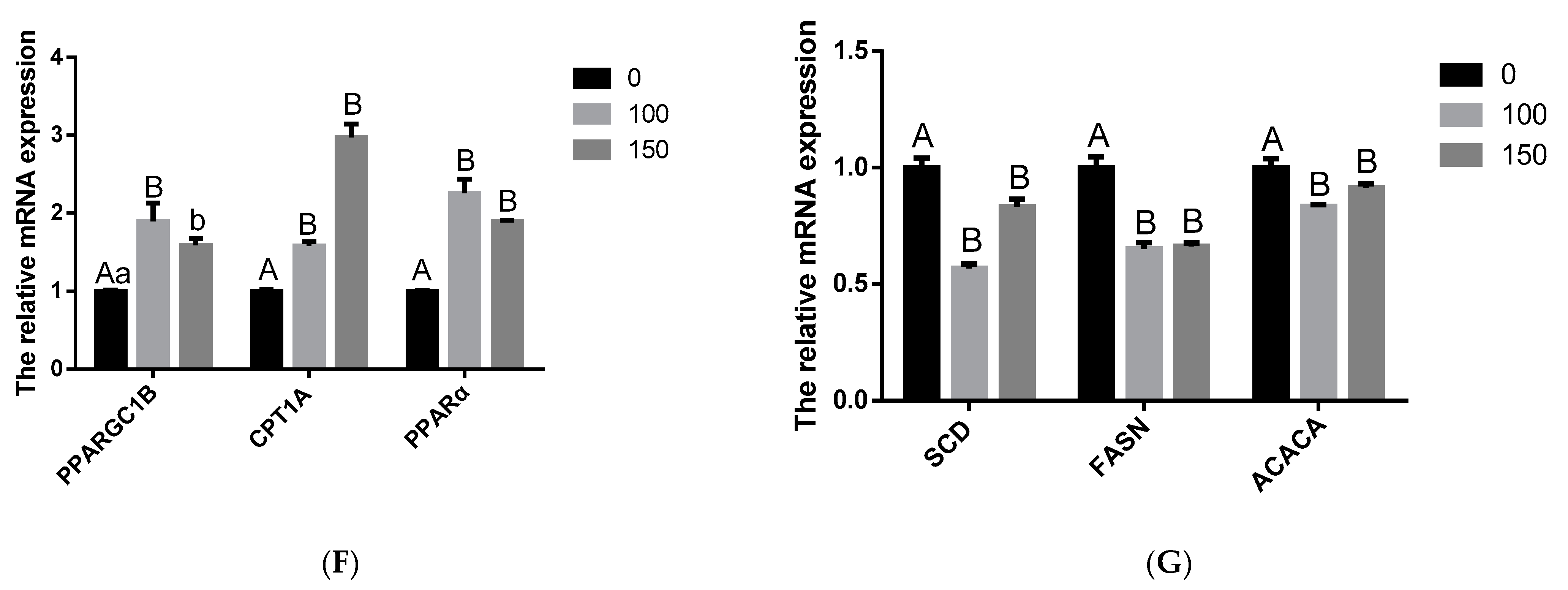
3.4. Effects of COS on BMECs Milk Fat Synthesis
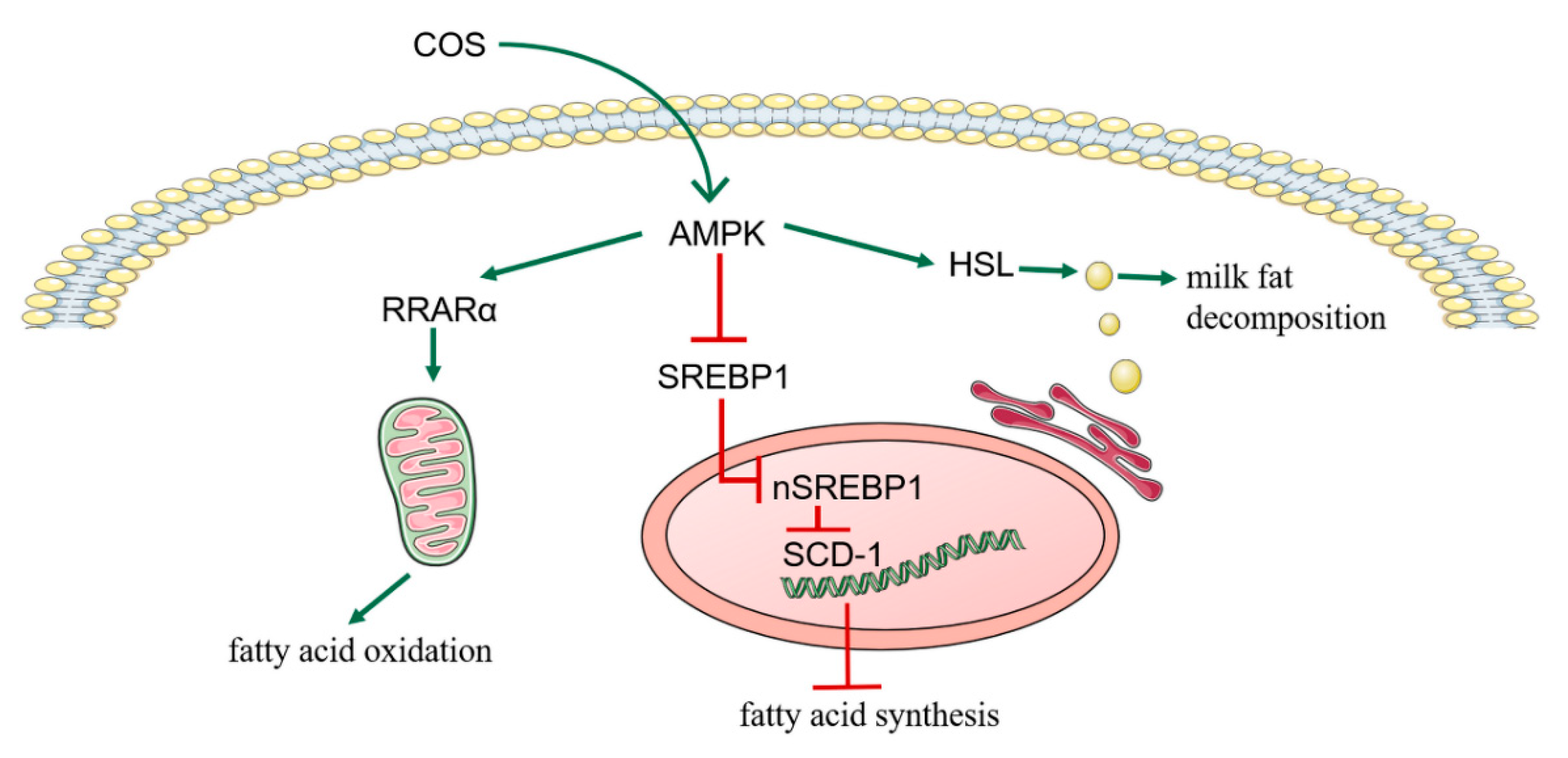
3.5. COS Inhibits Milk Fat Synthesis in BMECs through AMPK/SREBP1/SCD1, AMPK/HSL and AMPK/PPARα Signaling Pathways
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene | Sequence Number | Primer Sequence (5′ to 3′) | Product Size (bp) | Tm (°C) |
|---|---|---|---|---|
| DGAT1 | NM_174693.2 | F:CAACCACCTCATCTGGCTCA | 94 | 57.5 |
| R: GAACTCGCGGTCTCCAAACT | 57.8 | |||
| GPAM | NM_001012282.1 | F:GCTAGATGAGACGCCAGAT | 271 | 54.0 |
| R:CAGTTGTTCGCCATTGTAAT | 50.4 | |||
| HSL | NM_001080220.1 | F:GATGAGAGGGTAATTGCCG | 100 | 53.0 |
| R:GGATGGCAGGTGTGAACT | 55.1 | |||
| ATGL | NM_001046005.2 | F:TGACCACACTCTCCAACA | 155 | 52.9 |
| R:TGTCTGCTCCTTCATCCA | 52.8 | |||
| AMPKα1 | XM_005221557.3 | F:CCCGTATTATTTGCGTGTTCG | 160 | 54.2 |
| R:CTGTGGCGTAGCAGTCCCT | 60.5 | |||
| SCD1 | NM_173959.4 | F:ATTTATCCGACCTAAGAGCCG | 122 | 54.0 |
| R:CGTTTCATCCCACAGATACCAT | 54.5 | |||
| SREBP1 | NM_001113302.1 | F:CTGTCCACAAAAGCAAATCGC | 191 | 55.2 |
| ACTTCCACCGCTGCTACTGC | 60.9 | |||
| PPARGC1B | XM_005209630.4 | F:TTCAACAGTGGACTCTACG | 133 | 51.1 |
| R:CAGCCAGACTCACAACAT | 52.0 | |||
| FASN | NM_001012669.1 | F:TCACCTACGAGGCCATTGTG | 99 | 57.4 |
| R:CTGAAGCCTCAGAGCCACTC | 58.4 | |||
| ACACA | NM_174224.2 | F:GAGACAAACAGGGACCATTAC | 166 | 53.0 |
| R:GACATAGATTCCCAGAGTTTG | 50.0 | |||
| CPT1A | NM_001304989.2 | F:GTCTTAGCTGAGCCTTGGAGA | 195 | 56.5 |
| R:GGAGAACTTGGAGGATATGTGG | 54.9 | |||
| PPARα | NM_001034036.1 | F:CAACCCGCCTTTCGTCAT | 106 | 55.9 |
| R:GACTTCCGCCTCCTTGT | 54.8 | |||
| ACTB | NM_173979.3 | F:AGAGCAAGAGAGGCATCC | 133 | 54.3 |
| R:TCGTTGTAGAAGGTGTGGT | 53.0 |
References
- Lucey, J.A.; Otter, D.; Horne, D.S. A 100-Year Review: Progress on the chemistry of milk and its components. J. Dairy Sci. 2017, 100, 9916–9932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Sanli, T.; Steinberg, G.R.; Singh, G.; Tsakiridis, T. AMP-activated protein kinase (AMPK) beyond metabolism A novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol. Ther. 2014, 15, 156–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quentin, T.; Kitz, J.; Steinmetz, M.; Poppe, A.; Baer, K.; Kraetzner, R. Different expression of the catalytic alpha subunits of the AMP activated protein kinase-an immunohistochemical study in human tissue. Histol. Histopathol. 2011, 26, 589–596. [Google Scholar]
- Gaidhu, M.P.; Fediuc, S.; Anthony, N.M.; So, M.; Mirpourian, M.; Perry, R.L.S.; Ceddia, R.B. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: Novel mechanisms integrating HSL and ATGL. J. Lipid Res. 2009, 50, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, G.S.; Yang, J.; Horton, J.D.; Hammer, R.E.; Goldstein, J.L.; Brown, M.S. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 2002, 277, 9520–9528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.J.; et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, J.; Rawson, R.B.; Espenshade, P.J.; Cheng, D.; Seegmiller, A.C.; Goldstein, J.L.; Brown, M.S. Molecular Identification of the Sterol-Regulated Luminal Protease that Cleaves SREBPs and Controls Lipid Composition of Animal Cells. Mol. Cell 1998, 2, 505–514. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPAR alpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Bronner, M.; Hertz, R.; Bar-Tana, J. Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor α by AMP-activated protein kinase. Biochem. J. 2004, 384, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-O.; Sakchaisri, K.; Asami, Y.; Ryoo, I.-J.; Choo, S.-J.; Yoo, I.-D.; Soung, N.-K.; Kim, Y.S.; Jang, J.-H.; Kim, B.Y.; et al. Illudins C-2 and C-3 Stimulate Lipolysis in 3T3-L1 Adipocytes and Suppress Adipogenesis in 3T3-L1 Preadipocytes. J. Nat. Prod. 2014, 77, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Yi, B.; Yao, B.Q.; Xia, T.; Yang, Y.F.; Zhang, Z.H.; Chen, C. Liraglutide attenuates renal tubular ectopic lipid deposition in rats with diabetic nephropathy by inhibiting lipid synthesis and promoting lipolysis. Pharmacol. Res. 2020, 156, 104778. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan Oligosaccharide Attenuates Nonalcoholic Fatty Liver Disease Induced by High Fat Diet through Reducing Lipid Accumulation, Inflammation and Oxidative Stress in C57BL/6 Mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Yuan, W.-P.; Liu, B.; Liu, C.-H.; Wang, X.-J.; Zhang, M.-S.; Meng, X.-M.; Xia, X.-K. Antioxidant activity of chito-oligosaccharides on pancreatic islet cells in streptozotocin-induced diabetes in rats. World J. Gastroenterol. 2009, 15, 1339–1345. [Google Scholar] [CrossRef]
- Choi, E.H.; Yang, H.P.; Chun, H.S. Chitooligosaccharide ameliorates diet-induced obesity in mice and affects adipose gene expression involved in adipogenesis and inflammation. Nutr. Res. 2012, 32, 218–228. [Google Scholar] [CrossRef]
- Kang, N.H.; Lee, W.K.; Yi, B.R.; Park, M.A.; Lee, H.R.; Park, S.K.; Hwang, K.A.; Park, H.K.; Choi, K.C. Modulation of lipid metabolism by mixtures of protamine and chitooligosaccharide through pancreatic lipase inhibitory activity in a rat model. Lab. Anim. Res. 2012, 28, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Xia, L.; Jin, Z.; Ali, S.; Wang, M.; Li, X.; Yang, R.; Fang, X.; Zhao, Z. New function of the CD44 gene: Lipid metabolism regulation in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 6661–6671. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, Z.; Li, X.; Wang, M.; Xia, L.; Cao, Y.; Yang, R.; Fang, X. RNA Interference Mediated Knockdown of ATP Binding Cassette Subfamily A Member 1 Decreases the Triglyceride Content of Bovine Mammary Epithelial Cells. Pak. J. Zool. 2020, 52, 239–245. [Google Scholar] [CrossRef]
- An, N.; Rausch-Fan, X.; Wieland, M.; Matejka, M.; Andrukhov, O. Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent. Mater. 2012, 28, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; He, H.; Cao, X.; Shen, X.; Zhu, Q. MiR-148a-3p Regulates Skeletal Muscle Satellite Cell Differentiation and Apoptosis via the PI3K/AKT Signaling Pathway by Targeting Meox2. Front. Genet. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jiang, P.; Iqbal, A.; Wang, M.; Li, X.; Fang, X.; Yu, H.; Zhao, Z. Transcriptomic Analysis of Short/Branched-Chain Acyl-Coenzyme a Dehydrogenase Knocked Out BMECs Revealed Its Reg-ulatory Effect on Lipid Metabolism. Front. Vet. Sci. 2021, 8, 744287. [Google Scholar] [CrossRef]
- Lillo, L.; Alarcon, J.; Cabello, G.; Cespedes, C.; Caro, C. Antibacterial Activity of Chitooligosaccharides. Z. Nat. C J. Biosci. 2008, 63, 644–648. [Google Scholar] [CrossRef]
- Shen, K.-T.; Chen, M.-H.; Chan, H.-Y.; Jeng, J.-H.; Wang, Y.-J. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem. Toxicol. 2009, 47, 1864–1871. [Google Scholar] [CrossRef]
- Kim, K. Production of chitin oligosaccharides with different molecular weights and their antioxidant effect in RAW 264.7 cells. J. Funct. Foods 2009, 1, 188–198. [Google Scholar]
- Li, X.; Zhou, C.; Chen, X.; Zhao, M. Subcellular localization of chitosan oligosaccharides in living cells. Chin. Sci. Bull. 2014, 59, 2449–2454. [Google Scholar] [CrossRef]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef]
- Cho, E.J.; Rahman, A.; Kim, S.W.; Baek, Y.M.; Hwang, H.J.; Oh, J.Y.; Hwang, H.S.; Lee, S.H.; Yun, J.W. Chitosan oligosaccharides inhibit adipogenesis in 3T3-L1 adipocytes. J. Microbiol. Biotechnol. 2008, 18, 80–87. [Google Scholar]
- Jiang, Y.; Fu, C.; Liu, G.; Guo, J.; Su, Z. Cholesterol-lowering effects and potential mechanisms of chitooligosaccharide capsules in hyperlipidemic rats. Food Nutr. Res. 2018, 62, 1446. [Google Scholar] [CrossRef] [Green Version]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Su, Z. Anti-Obese Effect of Glucosamine and Chitosan Oligosaccharide in High-Fat Diet-Induced Obese Rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef]
- Pan, H.; Fu, C.; Huang, L.; Jiang, Y.; Deng, X.; Guo, J.; Su, Z. Anti-Obesity Effect of Chitosan Oligosaccharide Capsules (COSCs) in Obese Rats by Ameliorating Leptin Resistance and Adipogenesis. Mar. Drugs 2018, 16, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Ye, Z.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Chitosan oligosaccharide ameliorated obesity by reducing endoplasmic reticulum stress in diet-induced obese rats. Food Funct. 2020, 11, 6285–6296. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Han, J.; Yu, Y.; Li, X.; Wang, Y.; Tian, H.; Guo, S.; Jin, S.; Luo, T.; Qin, S. Chitosan oligosaccharide decreases very-low-density lipoprotein triglyceride and increases high-density lipoprotein cholesterol in high-fat-diet-fed rats. Exp. Biol. Med. 2011, 236, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zheng, J.; Yuan, X.; Jiao, S.; Cui, F. Chitosan Oligosaccharides Improve Glucolipid Metabolism Disorder in Liver by Suppression of Obesity-Related Inflammation and Restoration of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ). Mar. Drugs 2018, 16, 455. [Google Scholar] [CrossRef] [Green Version]
- Lubary, M.; Hofland, G.W.; ter Horst, J.H. The potential of milk fat for the synthesis of valuable derivatives. Eur. Food Res. Technol. 2011, 232, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhong, H.; Yin, Y.; Jiang, Z. Genistein has beneficial effects on hepatic steatosis in high fat-high sucrose diet-treated rats. Biomed Pharmacother 2017, 91, 964–969. [Google Scholar] [CrossRef]
- Tang, T.; Song, J.; Li, J.; Wang, H.; Zhang, Y.; Suo, H. A synbiotic consisting of Lactobacillus plantarum S58 and hull-less barley β-glucan ameliorates lipid accumulation in mice fed with a high-fat diet by activating AMPK signaling and modulating the gut microbiota. Carbohydr. Polym. 2020, 243, 116398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, Q.; Li, X.; Jiang, M.; Cuis, B.W.; Xia, K.L.; Wu, Y.L.; Lian, L.H.; Nan, J.X. Amelioration of Alcoholic Liver Steatosis by Dihydroquercetin through the Modulation of AMPK-Dependent Lipogenesis Mediated by P2 × 7R-NLRP3-Inflammasome Activation. J. Agric. Food Chem. 2018, 66, 4862–4871. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Chen, J.; Wu, H.; Lu, X.; Fang, X.; Yin, F.; Zhao, Z.; Jiang, P.; Yu, H. Chitosan Oligosaccharide Inhibits the Synthesis of Milk Fat in Bovine Mammary Epithelial Cells through AMPK-Mediated Downstream Signaling Pathway. Animals 2022, 12, 1692. https://doi.org/10.3390/ani12131692
Fan J, Chen J, Wu H, Lu X, Fang X, Yin F, Zhao Z, Jiang P, Yu H. Chitosan Oligosaccharide Inhibits the Synthesis of Milk Fat in Bovine Mammary Epithelial Cells through AMPK-Mediated Downstream Signaling Pathway. Animals. 2022; 12(13):1692. https://doi.org/10.3390/ani12131692
Chicago/Turabian StyleFan, Jing, Jiayi Chen, Haochen Wu, Xin Lu, Xibi Fang, Fuquan Yin, Zhihui Zhao, Ping Jiang, and Haibin Yu. 2022. "Chitosan Oligosaccharide Inhibits the Synthesis of Milk Fat in Bovine Mammary Epithelial Cells through AMPK-Mediated Downstream Signaling Pathway" Animals 12, no. 13: 1692. https://doi.org/10.3390/ani12131692
APA StyleFan, J., Chen, J., Wu, H., Lu, X., Fang, X., Yin, F., Zhao, Z., Jiang, P., & Yu, H. (2022). Chitosan Oligosaccharide Inhibits the Synthesis of Milk Fat in Bovine Mammary Epithelial Cells through AMPK-Mediated Downstream Signaling Pathway. Animals, 12(13), 1692. https://doi.org/10.3390/ani12131692







