Locally Grown Crops and Immunocastration in Fattening Heavy Pigs: Effects on Performance and Welfare
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
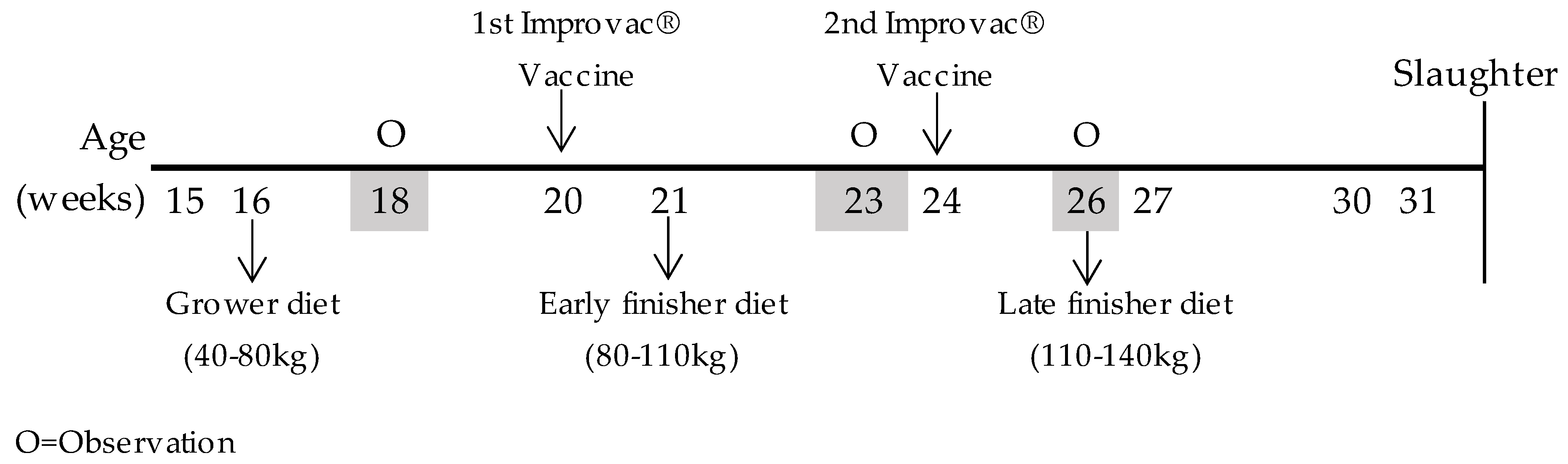
2.1. Animals and Experimental Design
2.2. Diets and Treatments
2.3. Performance and Feeding Behaviour Measurements
2.4. Tail and Ear Lesions
2.5. Abattoir Measurements
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Carcass Outcomes
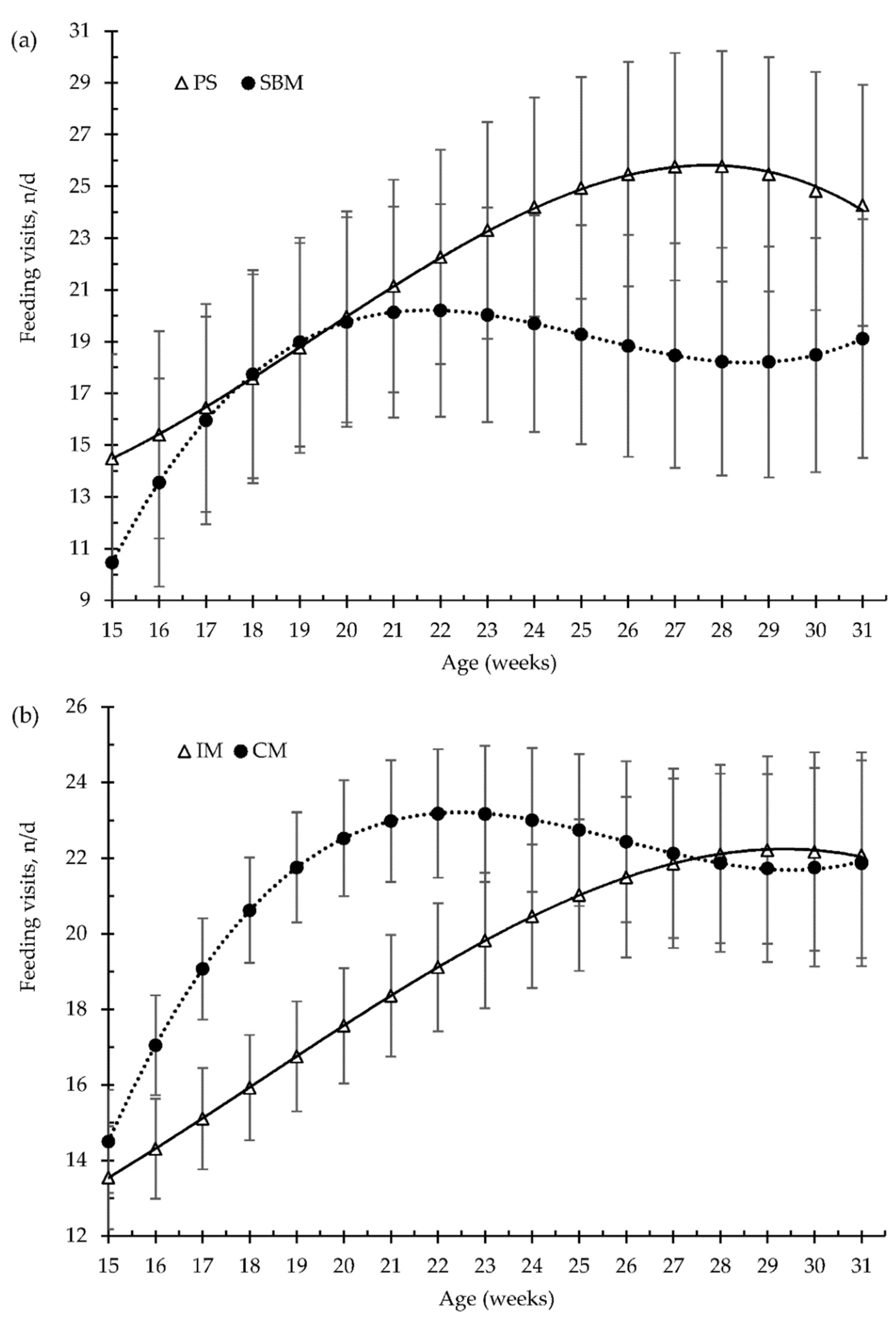
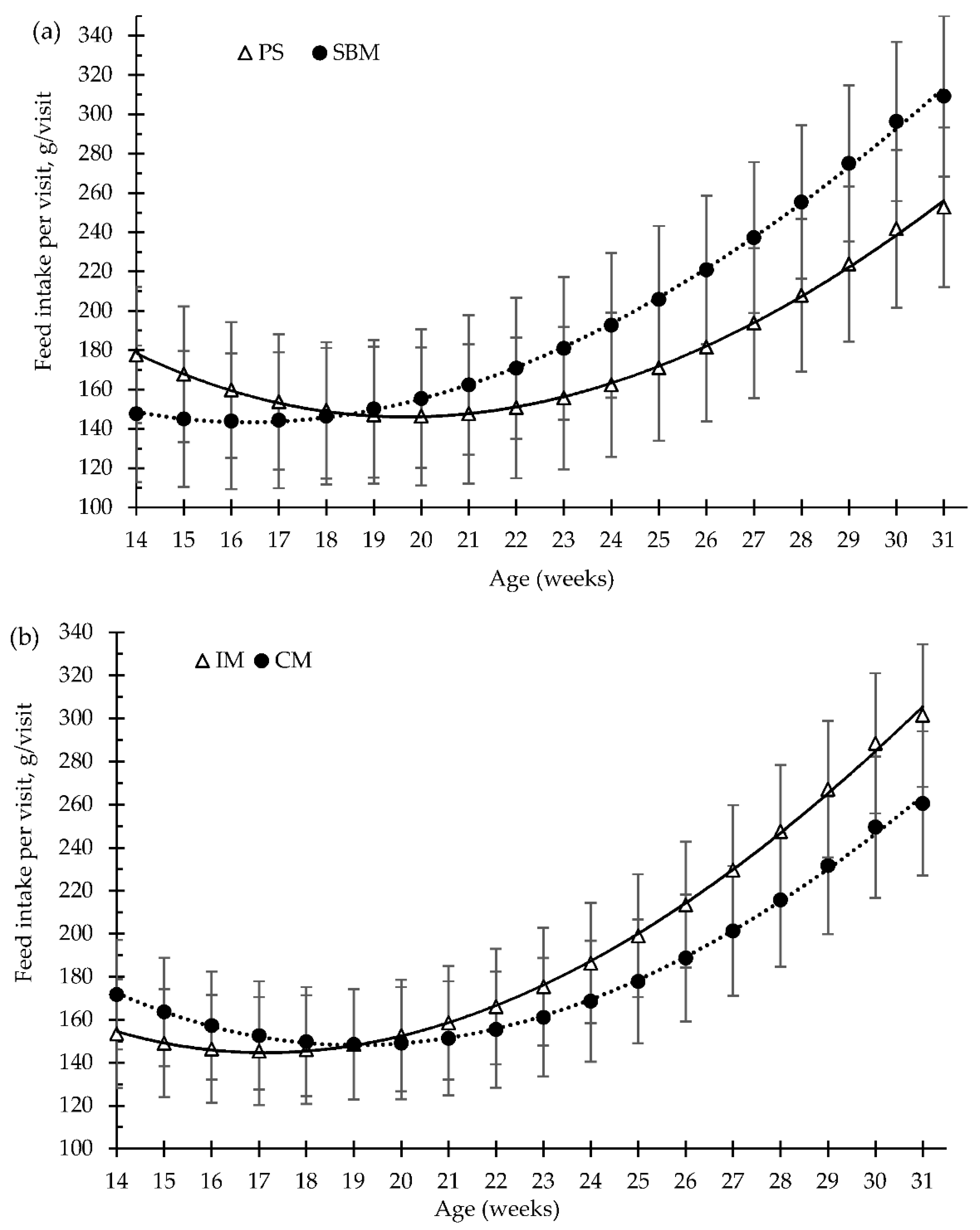
3.3. Patterns in Feeding Behaviour
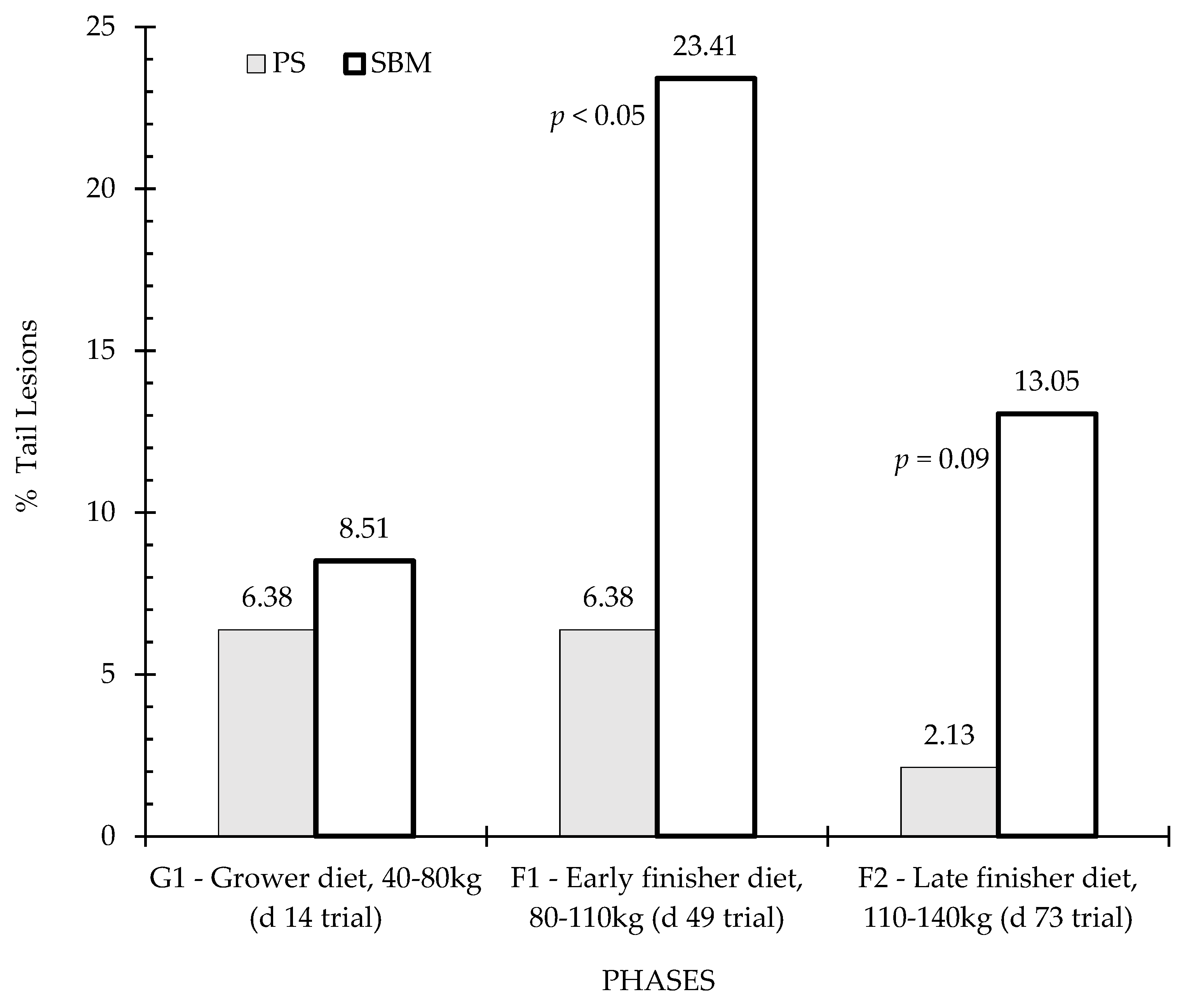
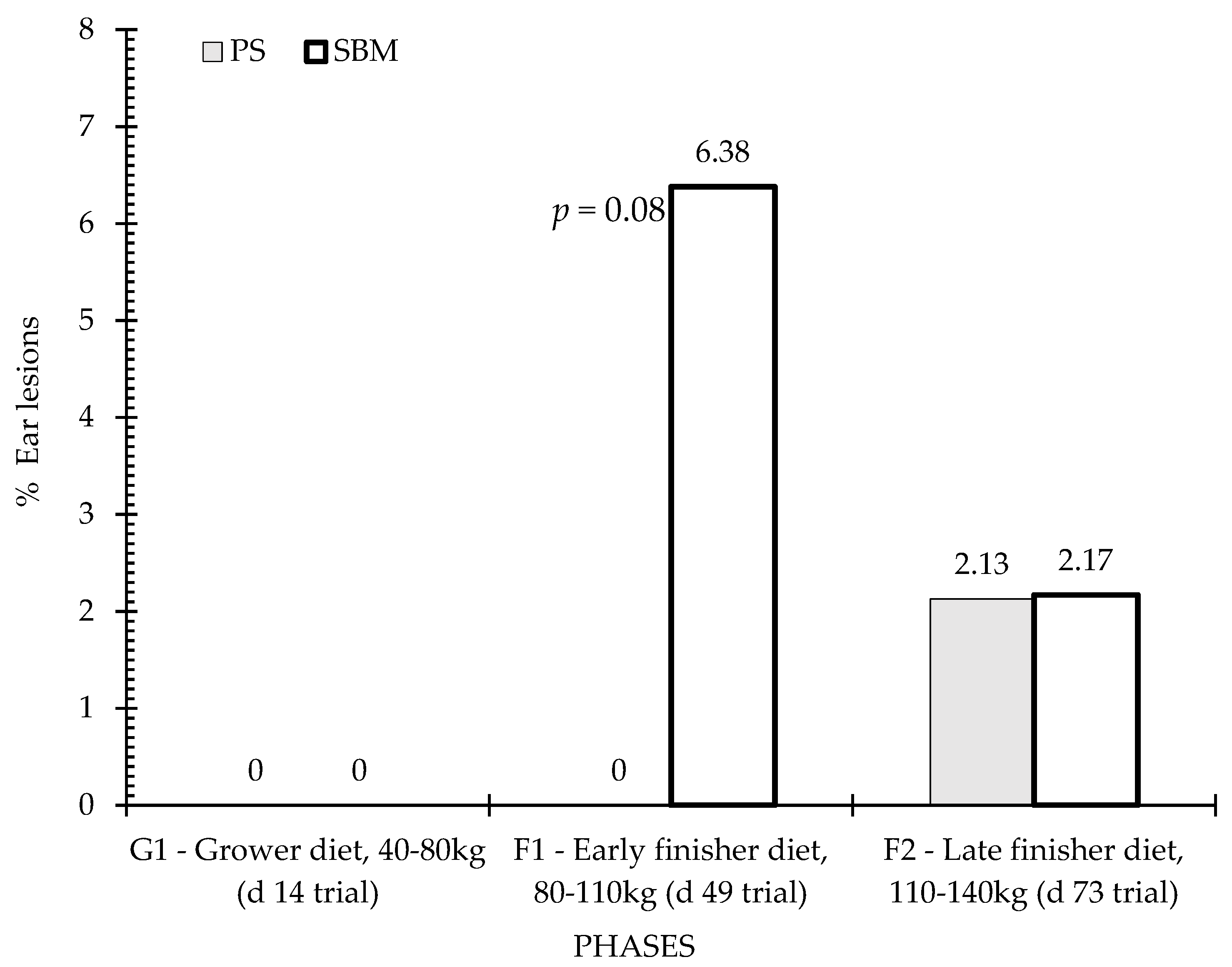
3.4. Tail and Ear Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamnatou, C.; Ezcurra-Ciaurriz, X.; Chemisana, D.; Plà-Aragonés, L.M. Environmental assessment of a pork-production system in North-East of Spain focusing on life-cycle swine nutrition. J. Clean. Prod. 2016, 137, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Jezierny, D.; Mosenthin, R.; Bauer, E. The use of grain legumes as a protein source in pig nutrition: A review. Anim. Feed Sci. Technol. 2010, 157, 111–128. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Światkiewicz, M.; Grela, E.R. Effect of dietary inclusion of a herbal extract mixture and different oils on pig performance and meat quality. Meat Sci. 2015, 108, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Landero, J.L.; Wang, L.F.; Beltranena, E.; Zijlstra, R.T. Diet nutrient digestibility and growth performance of weaned pigs fed field pea. Anim. Feed Sci. Technol. 2014, 198, 295–303. [Google Scholar] [CrossRef]
- Prandini, A.; Sigolo, S.; Morlacchini, M.; Cerioli, C.; Masoero, F. Pea (Pisum sativum) and faba bean (Vicia faba L.) seeds as protein sources in growing-finishing heavy pig diets: Effect on growth performance, carcass characteristics and on fresh and seasoned Parma ham quality. Ital. J. Anim. Sci. 2011, 10, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.A.; Houdijk, J.G.M.; Homer, D.; Kyriazakis, I. Effects of dietary inclusion of pea and faba bean as a replacement for soybean meal on grower and finisher pig performance and carcass quality. J. Anim. Sci. 2013, 91, 3733–3741. [Google Scholar] [CrossRef] [Green Version]
- European Declaration on Alternatives to Surgical Castration of Pigs. 2010. Available online: https://ec.europa.eu/food/system/files/2016-10/aw_prac_farm_pigs_cast-alt_declaration_en.pdf (accessed on 1 June 2022).
- Van den Broeke, A.; Aluwé, M.; Kress, K.; Stefanski, V.; Škrlep, M.; Batorek, N.; Ampe, B.; Millet, S. Effect of dietary energy level in finishing phase on performance, carcass and meat quality in immunocastrates and barrows in comparison with gilts and entire male pigs. Animal 2022, 16, 100437. [Google Scholar] [CrossRef]
- Moore, K.L.; Mullan, B.P.; Dunshea, F.R. Boar taint, meat quality and fail rate in entire male pigs and male pigs immunized against gonadotrophin releasing factor as related to body weight and feeding regime. Meat Sci. 2017, 125, 95–101. [Google Scholar] [CrossRef]
- Kress, K.; Millet, S.; Labussière, É.; Weiler, U.; Stefanski, V. Sustainability of Pork production with immunocastration in Europe. Sustainability 2019, 11, 3335. [Google Scholar] [CrossRef] [Green Version]
- Dunshea, F.R.; Allison, J.R.D.; Bertram, M.; Boler, D.D.; Brossard, L.; Campbell, R.; Crane, J.P.; Hennessy, D.P.; Huber, L.; De Lange, C.; et al. The effect of immunization against GnRF on nutrient requirements of male pigs: A review. Animal 2013, 7, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Aluwé, M.; Degezelle, I.; Depuydt, L.; Fremaut, D.; Van Den Broeke, A.; Millet, S. Immunocastrated male pigs: Effect of 4 v. 6 weeks time post second injection on performance, carcass quality and meat quality. Animal 2016, 10, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Puls, C.L.; Rojo, A.; Ellis, M.; Boler, D.D.; McKeith, F.K.; Killefer, J.; Gaines, A.M.; Matzat, P.D.; Schroeder, A.L. Growth performance of immunologically castrated (with Improvest) barrows (with or without ractopamine) compared to gilt, physically castrated barrow, and intact male pigs. J. Anim. Sci. 2014, 92, 2289–2295. [Google Scholar] [CrossRef]
- Veit, C.; Traulsen, I.; Hasler, M.; Tölle, K.-H.; Burfeind, O.; grosse Beilage, E.; Krieter, J. Influence of raw material on the occurrence of tail-biting in undocked pigs. Livest. Sci. 2016, 191, 125–131. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists, 17th ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Stein, H.H.; Benzoni, G.; Bohlke, R.A.; Peters, D.N. Assessment of the feeding value of South Dakota-grown field peas (Pisum sativum L.) for growing pigs. J. Anim. Sci. 2004, 82, 2568–2578. [Google Scholar] [CrossRef] [PubMed]
- Sońta, M.; Rekiel, A.; Więcek, J.; Batorska, M.; Puppel, K. Alternative protein sources vs. GM soybean meal as feedstuff for pigs—meat quality and health-promoting indicators. Animals 2021, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- White, G.A.; Smith, L.A.; Houdijk, J.G.M.; Homer, D.; Kyriazakis, I.; Wiseman, J. Replacement of soya bean meal with peas and faba beans in growing/finishing pig diets: Effect on performance, carcass composition and nutrient excretion. Anim. Feed Sci. Technol. 2015, 209, 202–210. [Google Scholar] [CrossRef] [Green Version]
- FEDNA (Fundación Española Para el Desarrollo de la Nutrición. Available online: http://www.fundacionfedna.org/ingredientes_para_piensos/guisantes-primavera (accessed on 1 June 2022).
- FEDNA (Fundación Española Para el Desarrollo de la Nutrición Animal) Necesidades Nutricionales Para Ganado Porcino. Available online: http://www.fundacionfedna.org/sites/default/files/NormasPORCINO_2013rev2_0.pdf (accessed on 1 June 2022).
- Reed, J.D. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar] [CrossRef]
- Jezierny, D.; Mosenthin, R.; Sauer, N.; Roth, S.; Piepho, H.P.; Rademacher, M.; Eklund, M. Chemical composition and standardised ileal digestibilities of crude protein and amino acids in grain legumes for growing pigs. Livest. Sci. 2011, 138, 229–243. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Beltranena, E.; Zijlstra, R.T. Effect of anti-nutritional factors of oilseed co-products on feed intake of pigs and poultry. Anim. Feed Sci. Technol. 2017, 233, 76–86. [Google Scholar] [CrossRef]
- Gómez-Izquierdo, E.; De Mercado, E.; Gómez-Fernández, J.; Tomás, C.; Guillamón, E.; Varela, A.; Muzquiz, M.; Pedrosa, M.M.; López-Nuez, P.; Latorre, M.A. Sustitución de soja por guisante de invierno en dietas de cerdos pesados: Impacto productivo del nivel de inhibidores de proteasas. ITEA Inf. Tec. Econ. Agrar. 2017, 113, 138–157. [Google Scholar] [CrossRef]
- Gabert, V.M.; Sauer, W.C.; Shaoyan, L.; Fan, M.Z. Exocrine pancreatic secretions in young pigs fed diets containing faba beans (Vicia faba) and peas (Pisum sativum): Concentrations and flows of total, protein-bound and free amino acids. J. Sci. Food Agric. 1996, 70, 256–262. [Google Scholar] [CrossRef]
- Auzins, A.; Krievina, A.; Leimane, I. Modelling of locally grown plant protein costs for pig feeding. Eng. Rural Dev. 2021, 20, 1304–1311. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Communication. Circular Economy Action Plan: The European Green Deal. Publications Office 2020. Available online: https://data.europa.eu/doi/10.2775/458852 (accessed on 1 June 2022).
- De Quelen, F.; Brossard, L.; Wilfart, A.; Dourmad, J.Y.; Garcia-Launay, F. Eco-Friendly Feed Formulation and On-Farm Feed Production as Ways to Reduce the Environmental Impacts of Pig Production Without Consequences on Animal Performance. Front. Vet. Sci. 2021, 8, 703. [Google Scholar] [CrossRef] [PubMed]
- Carcò, G.; Dalla Bona, M.; Carraro, L.; Latorre, M.A.; Fondevila, M.; Gallo, L.; Schiavon, S. Influence of mild feed restriction and mild reduction in dietary amino acid content on feeding behaviour of group-housed growing pigs. Appl. Anim. Behav. Sci. 2018, 198, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Kobek-Kjeldager, C.; Schönherz, A.A.; Canibe, N.; Pedersen, L.J. Diet and microbiota-gut-brain axis in relation to tail biting in pigs: A review. Appl. Anim. Behav. Sci. 2022, 246, 105514. [Google Scholar] [CrossRef]
- Morales, J.I.; Serrano, M.P.; Cámara, L.; Berrocoso, J.D.; López, J.P.; Mateos, G.G. Growth performance and carcass quality of immunocastrated and surgically castrated pigs from crossbreds from Duroc and Pietrain sires1. J. Anim. Sci. 2013, 91, 3955–3964. [Google Scholar] [CrossRef]
- Prunier, A.; Brillouët, A.; Merlot, E.; Meunier-Salaün, M.C.; Tallet, C. Influence of housing and season on pubertal development, boar taint compounds and skin lesions of male pigs. Animal 2013, 7, 2035–2043. [Google Scholar] [CrossRef] [Green Version]
- Dalla-Costa, O.A.; Tavernari, F.D.C.; Lopes, L.D.S.; Costa, F.A.D.; Feddern, V.; de Lima, G.J. Performance, carcass and meat quality of pigs submitted to immunocastration and different feeding programs. Res. Vet. Sci. 2020, 131, 137–145. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; García-Gudiño, J.; Izquierdo, M.; Brun, A.; Gispert, M.; Blanco-Penedo, I.; Hernández-García, F.I. Non-destructive evaluation of carcass and ham traits and meat quality assessment applied to early and late immunocastrated Iberian pigs. Animal 2021, 15, 100189. [Google Scholar] [CrossRef]


| Parameter | |
|---|---|
| Dry matter | 11.5 |
| Crude protein, % | 18.6 |
| Ether-extract, % | 2.1 |
| Crude ash, % | 5.8 |
| Starch, % | 42.9 |
| Acid-detergent fibre, % | 7.5 |
| Neutral-detergent fibre, % | 22.0 |
| Crude fibre, % | 6.2 |
| Trypsin Protease Inhibitors (AOCS) | |
| Trypsin inhibitor activity (TIA, mg/g) | 3.9 |
| Amino Acids | |
| Lysine, % | 1.4 |
| Methionine, % | 0.2 |
| Threonine, % | 0.7 |
| Tryptophan, % | 0.2 |
| Isoleucine, % | 0.8 |
| Valine, % | 0.9 |
| Histidine, % | 0.4 |
| Arginine, % | 1.6 |
| Aspartic acid, % | 2.1 |
| Glutamic acid, % | 3.7 |
| Serine, % | 0.9 |
| Tyrosine, % | 0.7 |
| Phenylalanine, % | 1.0 |
| Leucine, % | 1.5 |
| Hydroxyproline, % | <0.03 |
| Proline, % | 1.1 |
| Item | Grower (40–80 kg) | Early Finisher (80–110 kg) | Late Finisher (110–140 kg) | |||
|---|---|---|---|---|---|---|
| 0% (SBM) | 25% (PS-L) | 0% (SBM) | 30% (PS-L) | 0% (SBM) | 40% (PS-L) | |
| Barley | 35 | 24.8 | 30 | 22.2 | 40 | 25.6 |
| Maize | 43 | 35 | 31.4 | 25 | 39 | 25 |
| Soybean meal, 44% | 18.9 | 12 | 12.5 | 4.7 | 10.8 | - |
| Soybean oil | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Wheat | - | - | 14.9 | 7.0 | - | - |
| Wheat bran | - | - | 8 | 8 | - | - |
| Dehydrated alfalfa pellets | - | - | - | - | 7.7 | 6.6 |
| Field peas | - | 25 | - | 30 | - | 40 |
| Calcium carbonate | 1.1 | 1.2 | 1.406 | 1.418 | 0.9 | 1.4 |
| Sodium chloride | 0.44 | 0.443 | 0.45 | 0.45 | 0.5 | 0.5 |
| Mineral and vitamin premix | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Bicalcic phosphate, 78.5% | 0.485 | 0.405 | 0.188 | 0.079 | 0.1 | - |
| L-Lysine HCl (Lys) | 0.11 | 0.02 | 0.171 | 0.05 | 0.147 | - |
| DL-Methionine, 99% (Met) | 0.018 | 0.028 | 0.027 | 0.05 | 0.01 | 0.04 |
| L-Tryptophan, 98% (Trp) | 0.005 | 0.017 | 0.005 | 0.022 | - | 0.01 |
| L-Threonine, 98.5% (Thr) | 0.018 | 0.028 | 0.04 | 0.048 | - | 0.01 |
| Parameter | Grower (40–80 kg) | Early Finisher (80–110 kg) | Late Finisher (110–140 kg) | |||
|---|---|---|---|---|---|---|
| 0% (SBM) | 25% (PS-L) | 0% (SBM) | 30% (PS-L) | 0% (SBM) | 40% (PS-L) | |
| NE, kcal/kg (calculated) | 2388 | 2388 | 2350 | 2350 | 2325 | 2325 |
| Dry matter | 88.0 | 88.1 | 89 | 89.3 | 89.0 | 89.3 |
| Crude protein, % | 14.9 | 14.7 | 13.2 | 13.1 | 13.6 | 13.3 |
| Ether-extract, % | 3.6 | 3.4 | 3.5 | 3.6 | 3.3 | 3.6 |
| Ash, % | 4.9 | 6.0 | 4.8 | 6.3 | 5.0 | 6.1 |
| Starch, % | 48.8 | 48.0 | 50.5 | 49.4 | 47.8 | 48.4 |
| Crude fibre, % | 4.4 | 4.4 | 4.1 | 5.2 | 5.2 | 5.7 |
| NDF, % | 12.1 | 12.1 | 12.2 | 13.8 | 13.8 | 15.4 |
| ADF, % | 6.2 | 6.5 | 5.0 | 5.1 | 6.0 | 7.4 |
| Amino acids | ||||||
| Lysine, % | 0.94 | 0.91 | 0.71 | 0.70 | 0.75 | 0.68 |
| Methionine, % | 0.3 | 0.33 | 0.24 | 0.26 | 0.22 | 0.22 |
| Threonine, % | 0.64 | 0.62 | 0.52 | 0.53 | 0.49 | 0.48 |
| Tryptophan, % | 0.17 | 0.19 | 0.17 | 0.15 | 0.16 | 0.13 |
| Isoleucine, % | 0.66 | 0.63 | 0.55 | 0.55 | 0.55 | 0.51 |
| Valine, % | 0.78 | 0.77 | 0.57 | 0.58 | 0.62 | 0.60 |
| Histidine, % | 0.46 | 0.44 | 0.35 | 0.34 | 0.36 | 0.32 |
| Arginine, % | 1.06 | 1.1 | 0.81 | 0.84 | 0.79 | 0.81 |
| Aspartic acid, % | 1.51 | 1.5 | 1.07 | 1.14 | 1.19 | 1.11 |
| Glutamic acid, % | 3.05 | 2.85 | 2.55 | 2.55 | 2.47 | 2.37 |
| Serine, % | 0.74 | 0.7 | 0.56 | 0.57 | 0.60 | 0.52 |
| Tyrosine, % | 0.57 | 0.54 | 0.44 | 0.44 | 0.48 | 0.42 |
| Phenylalanine, % | 0.83 | 0.79 | 0.66 | 0.67 | 0.71 | 0.63 |
| Leucine, % | 1.24 | 1.16 | 0.93 | 0.93 | 1.04 | 0.95 |
| Hydroxyproline, % | 0.034 | <0.030 | <0.030 | <0.030 | 0.05 | 0.04 |
| Proline, % | 0.97 | 0.86 | 0.91 | 0.9 | 0.98 | 0.82 |
| Parameter | Feeding Strategy (F) | Method of Castration (C) | SE | Level of Significance 1 | |||
|---|---|---|---|---|---|---|---|
| SBM | PS-L | IM | CM | F | C | ||
| Body-Weight (BW), kg | |||||||
| Initial, day (d) 0 | 38.7 | 39.8 | 38.4 | 40 | 2.2 | 0.66 | 0.40 |
| Grower diet (40–80kg), d 49 | 81.1 | 83 | 80.3 | 83.8 | 2.4 | 0.50 | 0.12 |
| Early finisher diet (80–110kg), d 80 | 108.8 | 109.5 | 108.6 | 109.8 | 2.8 | 0.81 | 0.63 |
| Late finisher diet (110–140 kg), d 116 | 141.7 | 139.5 | 142.9 | 138.4 | 3.3 | 0.53 | 0.15 |
| Average Daily Gain (ADG), g/day | |||||||
| Grower diet (40–80 kg) | 867 | 882 | 856 | 893 | 21.8 | 0.49 | 0.10 |
| Early finisher diet (80–110 kg) | 893 | 856 | 910 | 839 | 21.3 | 0.09 | <0.01 |
| Late finisher diet (110–140 kg) | 914 | 834 | 954 | 793 | 21.7 | <0.001 | <0.0001 |
| Average Daily Feed Intake (ADFI), g/day | |||||||
| Initial, day (d) 0 | 1504 | 1631 | 1389 | 1745 | 117 | 0.51 | <0.01 |
| Grower diet (40–80 kg), d 49 | 2675 | 3007 | 2695 | 2987 | 125 | 0.12 | 0.02 |
| Early finisher diet (80–110 kg), d 80 | 3273 | 3628 | 3455 | 3447 | 145 | 0.12 | 0.96 |
| Late finisher diet (110–140 kg), d 116 | 3829 | 4108 | 4272 | 3665 | 178 | 0.30 | <0.01 |
| Feed Conversion Ratio (FCR) | |||||||
| Grower diet, 40–80 kg | 3.35 | 3.78 | 3.37 | 3.76 | 0.23 | 0.18 | 0.01 |
| Early finisher diet, 80–110 kg | 3.91 | 4.62 | 4.07 | 4.47 | 0.28 | 0.06 | 0.07 |
| Late finisher diet, 110–140 kg | 4.48 | 5.46 | 4.76 | 5.18 | 0.35 | 0.02 | 0.16 |
| Feeding Strategy (F) | Method of Castration (C) | SE | Level of Significance 1 | ||||
|---|---|---|---|---|---|---|---|
| SBM | PS-L | IM | CM | F | C | ||
| Final body mass, kg | 145.2 | 145.1 | 147.4 | 142.9 | 1.92 | 0.94 | 0.11 |
| Carcass weight, kg | 105.5 | 104.8 | 105.3 | 105.0 | 1.53 | 0.76 | 0.87 |
| Carcass yield, % | 72.7 | 72.3 | 71.5 | 73.4 | 0.46 | 0.54 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argemí-Armengol, I.; Villalba, D.; Vall, L.; Coma, R.; Roma, J.; Álvarez-Rodríguez, J. Locally Grown Crops and Immunocastration in Fattening Heavy Pigs: Effects on Performance and Welfare. Animals 2022, 12, 1629. https://doi.org/10.3390/ani12131629
Argemí-Armengol I, Villalba D, Vall L, Coma R, Roma J, Álvarez-Rodríguez J. Locally Grown Crops and Immunocastration in Fattening Heavy Pigs: Effects on Performance and Welfare. Animals. 2022; 12(13):1629. https://doi.org/10.3390/ani12131629
Chicago/Turabian StyleArgemí-Armengol, Immaculada, Daniel Villalba, Laura Vall, Ramon Coma, Josep Roma, and Javier Álvarez-Rodríguez. 2022. "Locally Grown Crops and Immunocastration in Fattening Heavy Pigs: Effects on Performance and Welfare" Animals 12, no. 13: 1629. https://doi.org/10.3390/ani12131629
APA StyleArgemí-Armengol, I., Villalba, D., Vall, L., Coma, R., Roma, J., & Álvarez-Rodríguez, J. (2022). Locally Grown Crops and Immunocastration in Fattening Heavy Pigs: Effects on Performance and Welfare. Animals, 12(13), 1629. https://doi.org/10.3390/ani12131629








