Betaine Promotes Fat Accumulation and Reduces Injury in Landes Goose Hepatocytes by Regulating Multiple Lipid Metabolism Pathways
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics Statement
2.3. Culture of Goose Primary Hepatocytes
2.4. Construction of Cell Model of Landes Goose Fatty Liver In Vitro
2.5. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.6. TG Deposition Assay and Oil Red-O Staining
2.7. Betaine Effect on Landes Goose Fatty Liver Cells
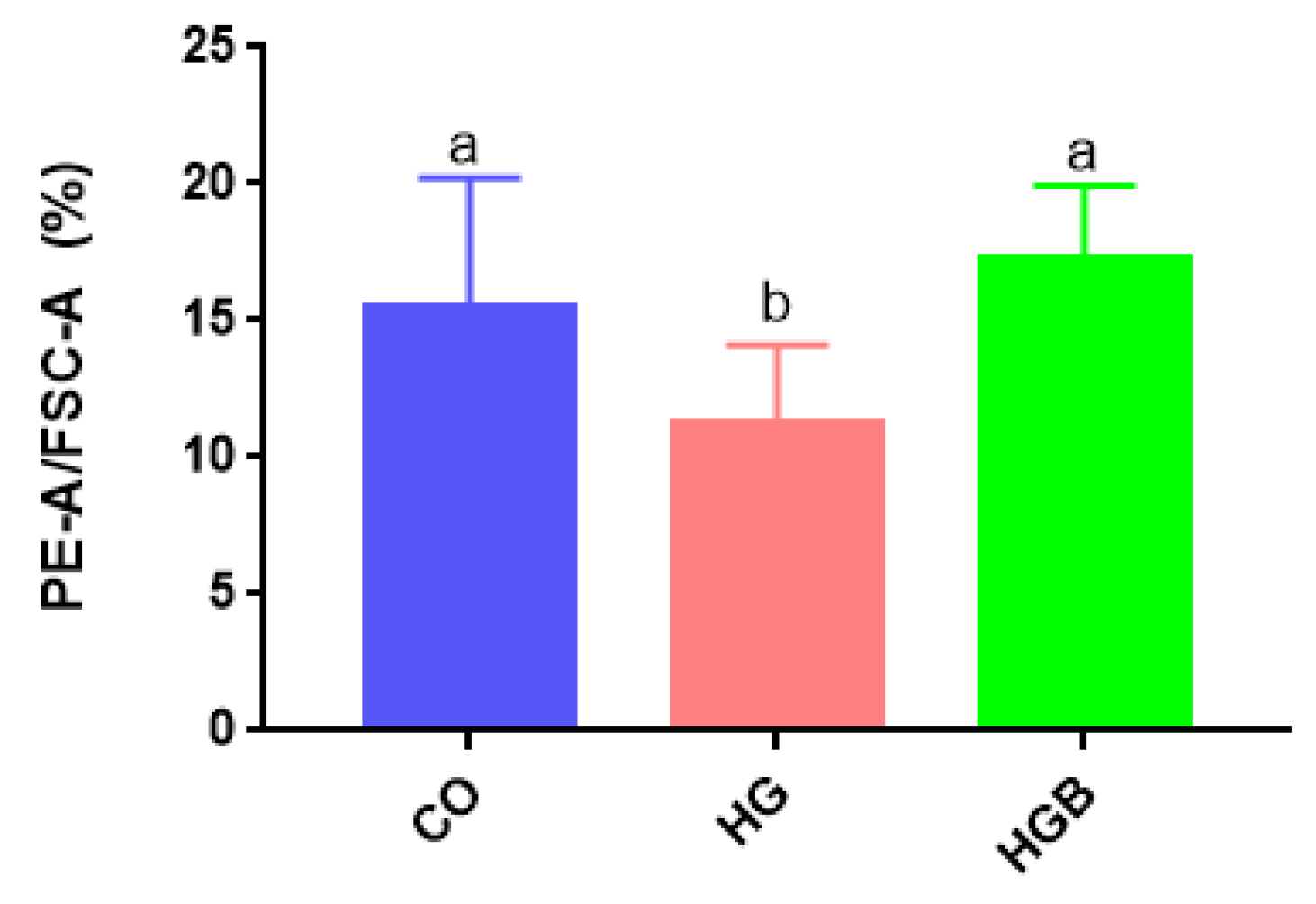
2.8. Determination of Mitochondrial Membrane Potential (MMP)
2.9. Analysis of Lipid Droplets’ Distributionin Hepatocytes
2.10. RNA Isolation and Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.11. Data Analysis
3. Results
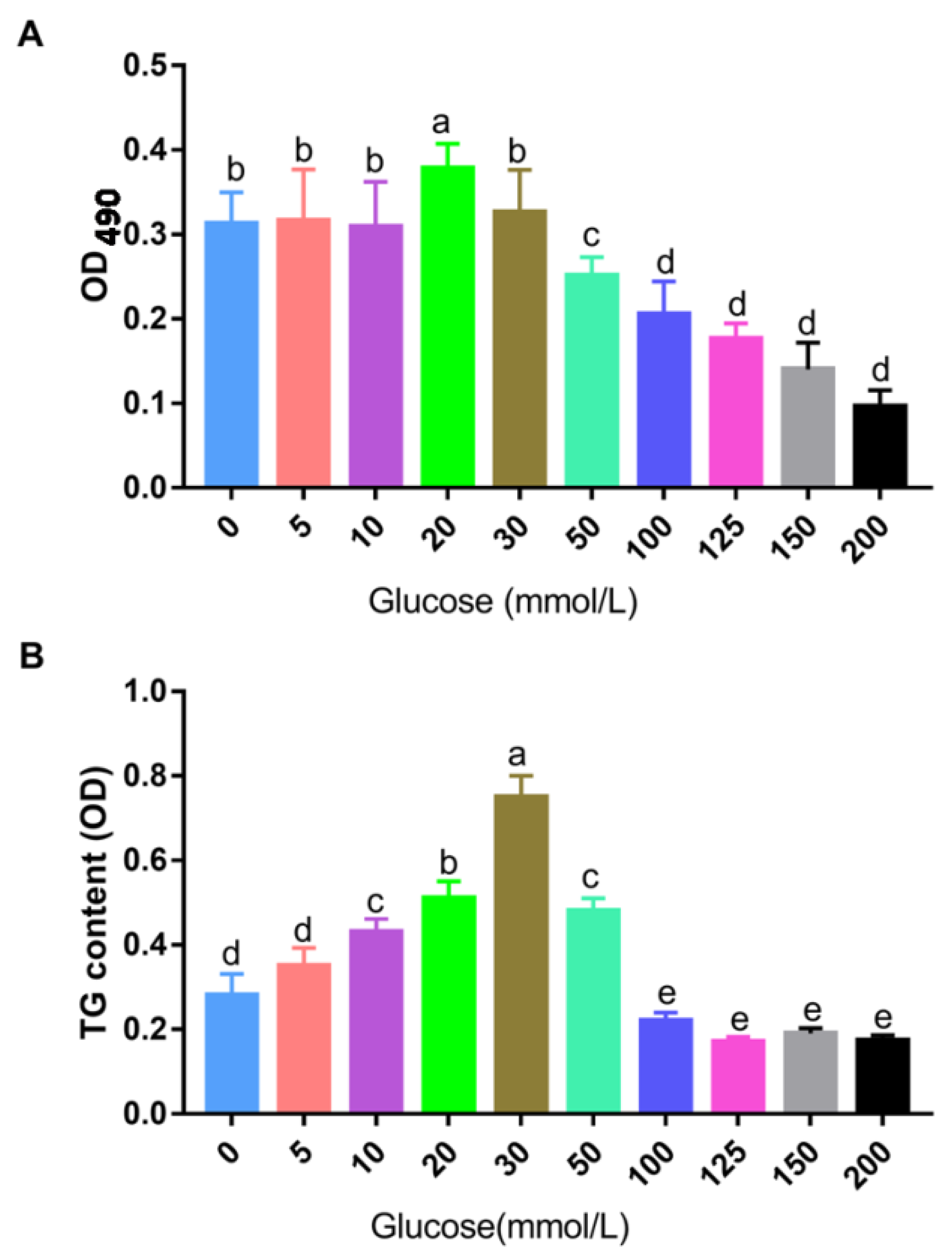
3.1. Construction of the Cell Model of Landes Goose Fatty Liver In Vitro
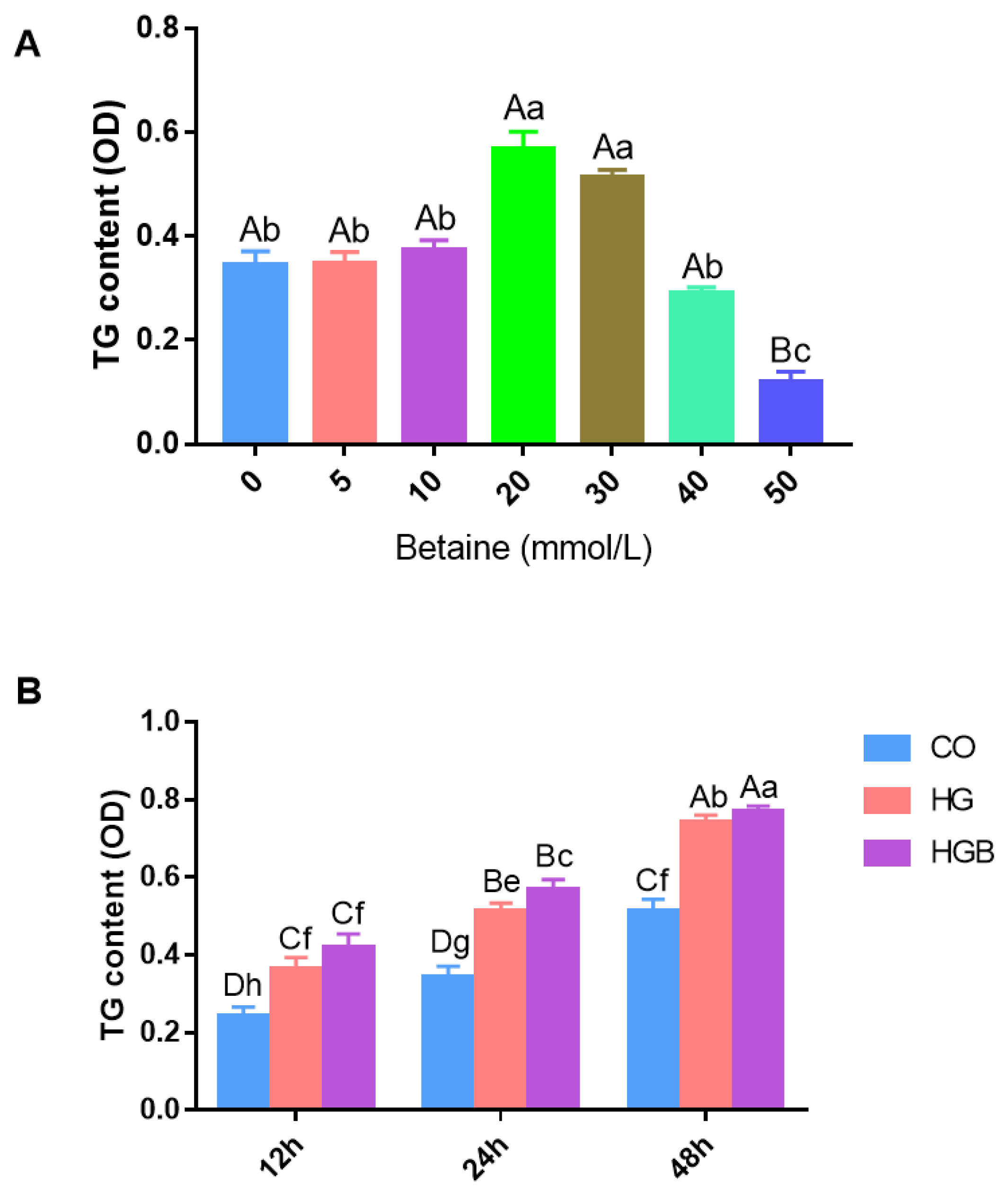
3.2. Effects of Betaine on TG Deposition in Landes Goose Fatty Liver Cells
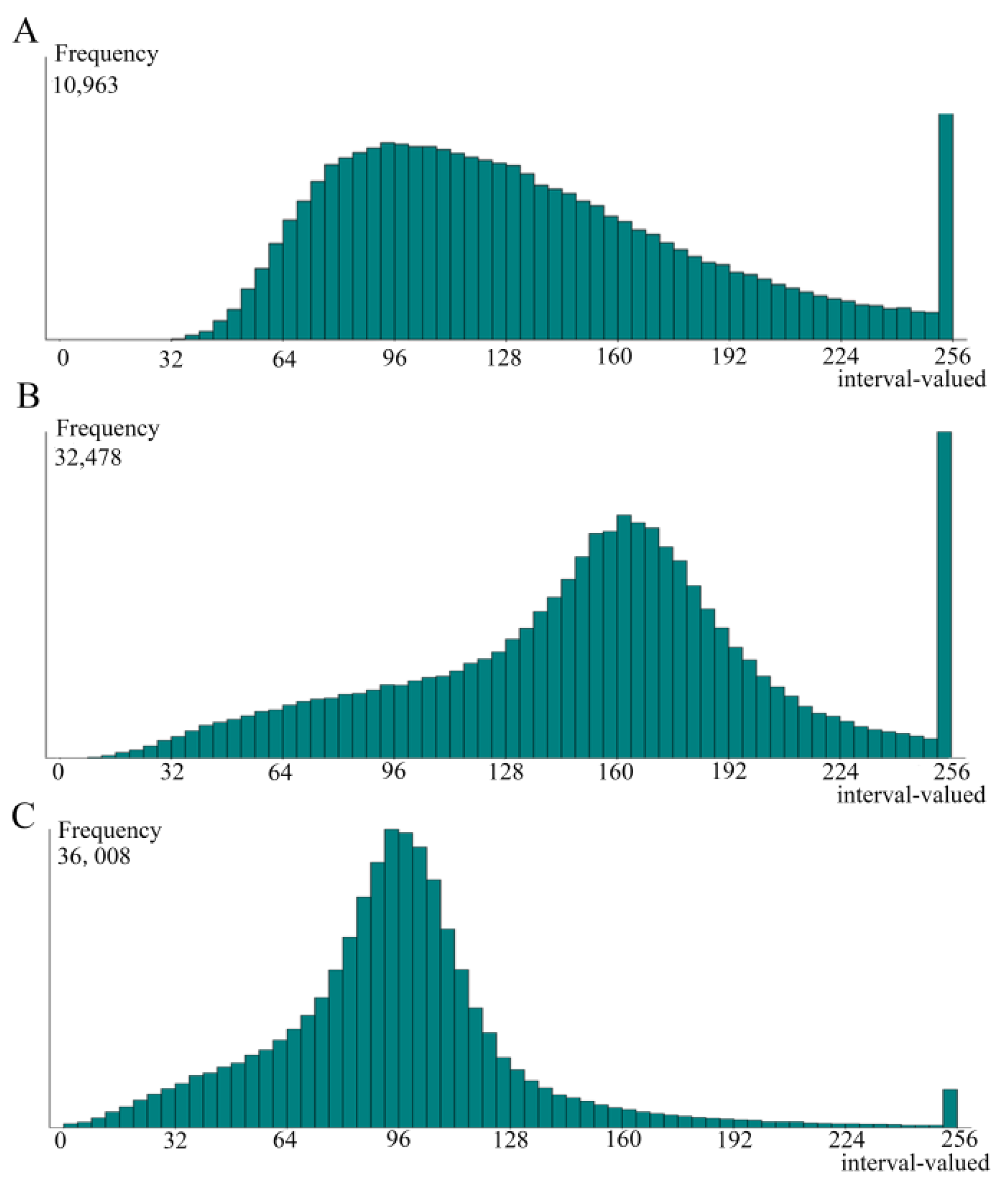
3.3. Betaine Modulated Lipid Droplets Distribution and Size in Landes Goose Fatty Liver Cells
3.4. Betaine Restored Fatty Acid β-Oxidation and Promoted Fat Oxidative Decomposition
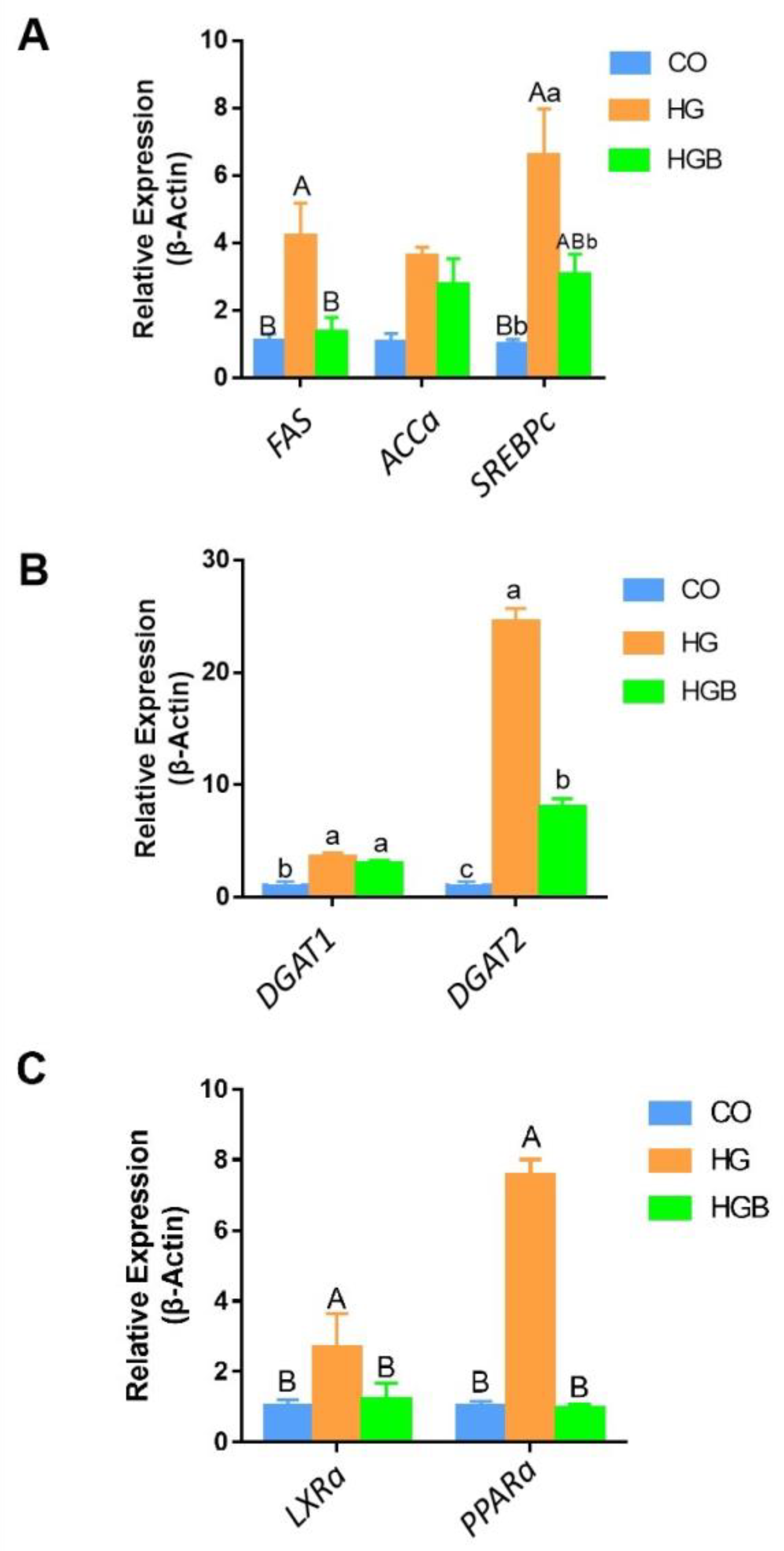
3.5. Betaine Inhibited the Expression of Key Genes in Fat Synthesis, Deposition, and Metabolism
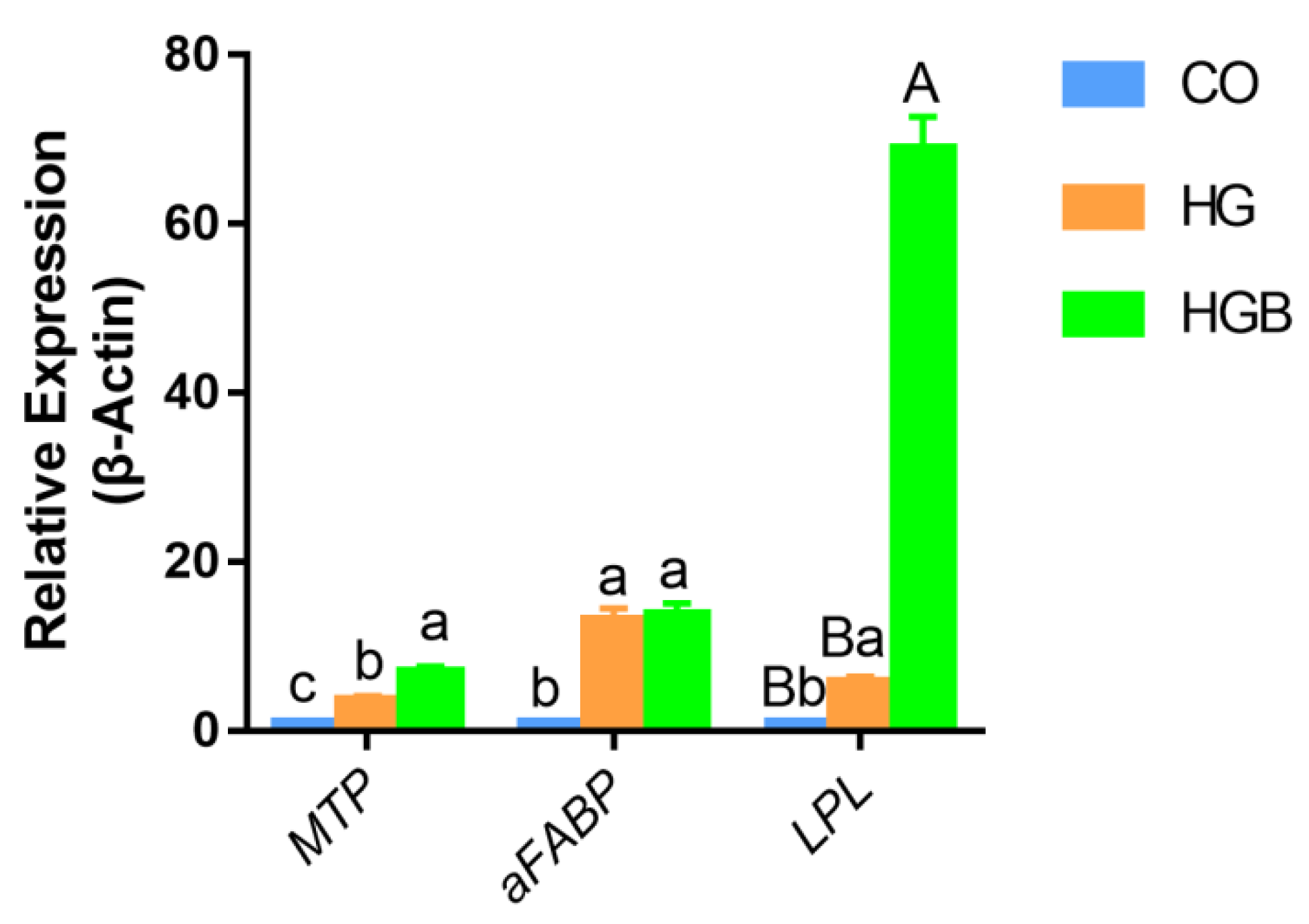
3.6. Betaine Promoted the Expression of Adipolytic and Transport-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Day, C.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. etBiophys. Acta (BBA) Gen. Subj. 2016, 1860, 1098–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.D.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J. Safety and efficacy of betaine anhydrous for food-producing animal species based on a dossier submitted by AB Vista. EFSA J. 2018, 16, e05335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, S.M.; Boyd, R.D.; Ferket, P.R.; Van Heugten, E. Effects of dietary supplementation of the osmolyte betaine on growing pig performance and serological and hematological indices during thermoneutral and heat-stressed conditions. J. Anim. Sci. 2017, 95, 5040–5053. [Google Scholar] [CrossRef] [PubMed]
- Park, S.O.; Kim, W.K. Effects of betaine on biological functions in meat-type ducks exposed to heat stress. Poult. Sci. 2017, 96, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Z.; Yang, H.; Zhao, F.; Kong, L. Response of growing goslings to dietary supplementation with methionine and betaine. Br. Poult. Sci. 2016, 57, 833–841. [Google Scholar] [CrossRef]
- Shah, A.M.; Ma, J.; Wang, Z.; Zou, H.; Hu, R.; Peng, Q. Betaine Supplementation Improves the Production Performance, Rumen Fermentation, and Antioxidant Profile of Dairy Cows in Heat Stress. Animals 2020, 10, 634. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.; Kang, L.; Jiang, Y. Effect of dietary betaine supplementation on lipogenesis gene expression and CpG methylation of lipoprotein lipase gene in broilers. Mol. Biol. Rep. 2010, 38, 1975–1981. [Google Scholar] [CrossRef]
- Omer, N.A.; Hu, Y.; Idriss, A.A.; Abobaker, H.; Hou, Z.; Yang, S.; Ma, W.; Zhao, R. Dietary betaine improves egg-laying rate in hens through hypomethylation and glucocorticoid receptor–mediated activation of hepatic lipogenesis-related genes. Poult. Sci. 2020, 99, 3121–3132. [Google Scholar] [CrossRef]
- Huang, T.; Yu, L.; Pan, H.; Ma, Z.; Wu, T.; Zhang, L.; Liu, K.; Qi, Q.; Miao, W.; Song, Z.; et al. Integrated Transcriptomic and Translatomic Inquiry of the Role of Betaine on Lipid Metabolic Dysregulation Induced by a High-Fat Diet. Front. Nutr. 2021, 8, 763. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Xu, M.; Jiang, L.; Zhou, M.; Liu, W.; Chen, Z.; Wang, Y.; Zou, Q.; Wang, L. Betaine prevented high-fat diet-induced NAFLD by regulating the FGF10/AMPK signaling pathway in ApoE−/− mice. Eur. J. Nutr. 2020, 60, 1655–1668. [Google Scholar] [CrossRef]
- Matthews, J.O.; Southern, L.L.; Higbie, A.D.; Persica, M.A.; Bidner, T.D. Effects of betaine on growth, carcass characteristics, pork quality, and plasma metabolites of finishing pigs. J. Anim. Sci. 2001, 79, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Bahr, S.M.; Shousha, S.; Khattab, W.; Shehab, A.; El-Garhy, O.; El-Garhy, H.; Mohamed, S.; Ahmed-Farid, O.; Hamad, A.; Sabike, I. Impact of Dietary Betaine and Metabolizable Energy Levels on Profiles of Proteins and Lipids, Bioenergetics, Peroxidation and Quality of Meat in Japanese Quail. Animals 2021, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.S.; El Nabtiti, A.A.; Hassan, M.A.; Mandour, M.A. Supplementary outcomes of betaine on economic and productive performance, some biochemical parameters, and lipoprotein lipase gene expression in finishing male broilers. Int. J. Veter- Sci. Med. 2018, 6, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Al-Sagan, A.A.; Al-Abdullatif, A.; Hussein, E.O.S.; Saadeldin, I.M.; Al-Mufarrej, S.I.; Qaid, M.; Albaadani, H.H.; Swelum, A.A.-A.; Alhotan, R. Effects of Betaine Supplementation on Live Performance, Selected Blood Parameters, and Expression of Water Channel and Stress-Related mRNA Transcripts of Delayed Placement Broiler Chicks. Front. Veter. Sci. 2021, 7, 632101. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Guo, X.-F.; Li, K.; Li, S.; Li, D. Effect of Betaine on Reducing Body Fat—A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 2480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Qiu, X.; Zhu, Y.; Tan, Z.; Li, Z.; Ouyang, D. Association between plasma betaine levels and dysglycemia in patients with coronary artery disease. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, C.; Lin, X.; Zhao, M.; Gong, D.; Liu, L.; Geng, T. Complement C3 participates in the development of goose fatty liver potentially by regulating the expression of FASN and ETNK1. Anim. Sci. J. 2021, 92, e13527. [Google Scholar] [CrossRef]
- Gu, W.; Wen, K.; Yan, C.; Li, S.; Liu, T.; Xu, C.; Liu, L.; Zhao, M.; Zhang, J.; Geng, T.; et al. Maintaining intestinal structural integrity is a potential protective mechanism against inflammation in goose fatty liver. Poult. Sci. 2020, 99, 5297–5307. [Google Scholar] [CrossRef]
- Geng, T.; Xia, L.; Li, F.; Xia, J.; Zhang, Y.; Wang, Q.; Yang, B.; Montgomery, S.; Cui, H.; Gong, D. The role of endoplasmic reticulum stress and insulin resistance in the occurrence of goose fatty liver. Biochem. Biophys. Res. Commun. 2015, 465, 83–87. [Google Scholar] [CrossRef]
- Han, C.; Wei, S.; He, F.; Liu, D.; Wan, H.; Liu, H.; Li, L.; Xu, H.; Du, X.; Xu, F. The Regulation of Lipid Deposition by Insulin in Goose Liver Cells Is Mediated by the PI3K-AKT-mTOR Signaling Pathway. PLoS ONE 2015, 10, e0098759. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Dodson, M.; Li, X.; Li, Q.; Wang, H.; Xie, Z. The effects of dietary betaine supplementation on fatty liver performance, serum parameters, histological changes, methylation status and the mRNA expression level of Spot14α in Landes goose fatty liver. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2009, 154, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hermiera, D.; Saadoun, A.; Salichon, M.-R.; Sellier, N.; Rousselot-Paillet, D.; Chapman, M.J. Plasma lipoproteins and liver lipids in two breeds of geese with different susceptibility to hepatic steatosis: Changes induced by development and force-feeding. Lipids 1991, 26, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Han, C.; Deng, D.; Ye, F.; Gan, X.; Liu, H.; Li, L.; Xu, H.; Wei, S. Research progress into the physiological changes in metabolic pathways in waterfowl with hepatic steatosis. Br. Poult. Sci. 2020, 62, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lyu, W.; Fu, Z.; Fan, Q.; Xiao, Y.; Ren, Y.; Yang, H. Metabolic Profiling Analysis of Liver in Landes Geese During the Formation of Fatty Liver via GC-TOF/MS. Front. Physiol. 2022, 12, 783498. [Google Scholar] [CrossRef]
- Seglen, P.O. Chapter 4 Preparation of Isolated Rat Liver Cells. Methods Cell Biol. 1976, 13, 29–83. [Google Scholar] [CrossRef]
- Zhu, L.H.; Meng, H.; Duan, X.J.; Xu, G.Q.; Zhang, J.; Gong, D.Q. Gene expression profile in the liver tissue of geese after overfeeding. Poult. Sci. 2011, 90, 107–117. [Google Scholar] [CrossRef]
- Liu, X.; Li, P.; He, C.; Qu, X.; Guo, S. Comparison of overfed Xupu and Landes geese in performance, fatty acid composition, enzymes and gene expression related to lipid metabolism. Asian-Australasian J. Anim. Sci. 2020, 33, 1957–1964. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.L.; Su, S.Y.; Li, Q.F.; Zhang, X.; Xie, Z. Duplicated CCAAT/enhancer-binding protein (C/EBP) gene: Transcription and methylation changes in response to dietary betaine in Landes goose liver. Poult. Sci. 2013, 92, 1878–1887. [Google Scholar] [CrossRef]
- Fernández-Fígares, I.; Lachica, M.; Martínez-Pérez, M.; Ramsay, T.G. Conjugated linoleic acid and betaine affect lipolysis in pig adipose tissue explants. Animal 2019, 13, 2840–2846. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Qi, Y.; Aluo, Z.; Liu, S.; Zhang, Z.; Zhou, L. Betaine increases mitochondrial content and improves hepatic lipid metabolism. Food Funct. 2018, 10, 216–223. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, Y.; Ding, Z.; Lv, L.; Sui, Y.; Sun, Q.; Abobaker, H.; Cai, D.; Zhao, R. Maternal betaine supplementation decreases hepatic cholesterol deposition in chicken offspring with epigenetic modulation of SREBP2 and CYP7A1 genes. Poult. Sci. 2020, 99, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huang, L.; Gao, J.; Wen, S.; Tai, Y.; Chen, M.; Huang, Z.; Liu, R.; Tang, C.; Li, J. Betaine attenuates chronic alcohol-induced fatty liver by broadly regulating hepatic lipid metabolism. Mol. Med. Rep. 2017, 16, 5225–5234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, M.; Paal, M.; Donohue, T.; Ganesan, M.; Osna, N.; Kharbanda, K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.-Y.; Watt, M.J.; Rensen, S.S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajaz, S.; McPhail, M.J.; Gnudi, L.; Trovato, F.M.; Mujib, S.; Napoli, S.; Carey, I.; Agarwal, K. Mitochondrial dysfunction as a mechanistic biomarker in patients with non-alcoholic fatty liver disease (NAFLD). Mitochondrion 2021, 57, 119–130. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Q.; Li, X.; Wang, M.; Cai, D.; Li, X.; Zhao, R. In Ovo Injection of Betaine Affects Hepatic Cholesterol Metabolism through Epigenetic Gene Regulation in Newly Hatched Chicks. PLoS ONE 2015, 10, e0122643. [Google Scholar] [CrossRef] [Green Version]
- Teratani, T.; Tomita, K.; Furuhashi, H.; Sugihara, N.; Higashiyama, M.; Nishikawa, M.; Irie, R.; Takajo, T.; Wada, A.; Horiuchi, K.; et al. Lipoprotein Lipase Up-regulation in Hepatic Stellate Cells Exacerbates Liver Fibrosis in Nonalcoholic Steatohepatitis in Mice. Hepatol. Commun. 2019, 3, 1098–1112. [Google Scholar] [CrossRef] [Green Version]
- Pardina, E.; Baena-Fustegueras, J.A.; Llamas, R.; Catalán, R.; Galard, R.; Lecube, A.; Fort, J.M.; Llobera, M.; Allende, H.; Vargas, V.; et al. Lipoprotein Lipase Expression in Livers of Morbidly Obese Patients Could be Responsible for Liver Steatosis. Obes. Surg. 2009, 19, 608–616. [Google Scholar] [CrossRef]
- Davail, S.; Guy, G.; André, J.-M.; Hermier, D.; Hoo-Paris, R. Metabolism in two breeds of geese with moderate or large overfeeding induced liver-steatosis. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2000, 126, 91–99. [Google Scholar] [CrossRef]
- André, J.; Guy, G.; Gontier-Latonnelle, K.; Bernadet, M.; Davail, B.; Hoo-Paris, R.; Davail, S. Influence of lipoprotein-lipase activity on plasma triacylglycerol concentration and lipid storage in three genotypes of ducks. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2007, 148, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, Y.; Wang, Z.; Li, X.; Chen, W.; Tao, Z.; Shen, J.; Tian, Y.; Wang, D.; Li, G.; et al. The goose genome sequence leads to insights into the evolution of waterfowl and susceptibility to fatty liver. Genome Biol. 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, J.D.; Suzuki, T.; Robinson, D.B.; Harris, C.M.; Johnson, J.E.; Mohler, P.J.; Jerome, W.G.; Swift, L.L. Microsomal Triglyceride Transfer Protein (MTP) Associates with Cytosolic Lipid Droplets in 3T3-L1 Adipocytes. PLoS ONE 2015, 10, e0135598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swift, L.L.; Kakkad, B.; Boone, C.; Jovanovska, A.; Jerome, W.G.; Mohler, P.J.; Ong, D.E. Microsomal triglyceride transfer protein expression in adipocytes: A new component in fat metabolism. FEBS Lett. 2005, 579, 3183–3189. [Google Scholar] [CrossRef] [Green Version]
- Swift, L.L.; Love, J.D.; Harris, C.M.; Chang, B.H.; Jerome, W.G. Microsomal triglyceride transfer protein contributes to lipid droplet maturation in adipocytes. PLoS ONE 2017, 12, e0181046. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| GenBank No. | Target Genes | Anneal. T (℃) | Primer Sequence | Product |
|---|---|---|---|---|
| M26111 | β-actin | 65 | F: 5′-ACCACCGGTATTGTTATGGACT-3′ R: 5′-TTGAAGGTGGTCTCGTGGAT-3′ | 398 bp |
| J03541 | ACCα | 59 | F: 5′-TGTGGCTGATGTGAGCTTTC-3′ R: 5′-ACTGTCGGGTCACCTTCAAC-3′ | 152 bp |
| NM205155 | FAS | 59 | F: 5′-AATCCATGGCTAAACGCATC-3′ R: 5′-GGCCATTTACTTCCTGTGGA-3′ | 216 bp |
| AF481797 | PPARα | 50 | F: 5′-AGACCCTTGTGGCAAAACTG-3′ R: 5′-TAGGCTACCAGCATCCCATC -3′ | 247 bp |
| AF432506 | aFABP | 60 | F: 5′-CAGCATCAATGGTGATGTGA-3′ R: 5′-TCTCTTTGCCATCCCACTT-3′ | 179 bp |
| AF492498 | LXRα | 57 | F: AGCAGGTCTGCAGTTCGAGT R: GGCTTCCACATAGGTGTGCT | 181 bp |
| AJ310768 | SREBPc | 66 | F: 5′-GCGCTACCGCTCATCCATCA-3′ R: 5′-GGTCGGCATCTCCATCACCT-3′ | 283 bp |
| GW342946 | DGAT1 | 60 | F: 5′-CCTGAGGAACTTGGACACG-3′ R: 5′-CAGGGACTGGTGGAACTCG-3′ | 265 bp |
| GW342947 | DGAT2 | 60 | F: 5′-CGCCATCATCATCGTGGT-3′ R: 5′-CGTGCCGTAGAGCCAGTTT-3′ | 113 bp |
| XM_013199906 | MTP | 60 | F: 5′-CCCGATGAAGGAGAGGAA-3′ R: 5′-AAAATGTAACTGGCCTGAGT-3′ | 85 bp |
| XM_013188252 | LPL | 55 | F: 5′-TGCCCTCACACGCCTCTC-3′ R: 5′-TCTGAATGCCGATGCTGC-3′ | 95 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Song, R.; Su, S.; Qi, N.; Li, Q.; Xie, Z.; Yu, S. Betaine Promotes Fat Accumulation and Reduces Injury in Landes Goose Hepatocytes by Regulating Multiple Lipid Metabolism Pathways. Animals 2022, 12, 1530. https://doi.org/10.3390/ani12121530
Liu J, Song R, Su S, Qi N, Li Q, Xie Z, Yu S. Betaine Promotes Fat Accumulation and Reduces Injury in Landes Goose Hepatocytes by Regulating Multiple Lipid Metabolism Pathways. Animals. 2022; 12(12):1530. https://doi.org/10.3390/ani12121530
Chicago/Turabian StyleLiu, Jiying, Ruilong Song, Shengyan Su, Nannan Qi, Qifa Li, Zhuang Xie, and Shali Yu. 2022. "Betaine Promotes Fat Accumulation and Reduces Injury in Landes Goose Hepatocytes by Regulating Multiple Lipid Metabolism Pathways" Animals 12, no. 12: 1530. https://doi.org/10.3390/ani12121530
APA StyleLiu, J., Song, R., Su, S., Qi, N., Li, Q., Xie, Z., & Yu, S. (2022). Betaine Promotes Fat Accumulation and Reduces Injury in Landes Goose Hepatocytes by Regulating Multiple Lipid Metabolism Pathways. Animals, 12(12), 1530. https://doi.org/10.3390/ani12121530







