The Impact of Enhancing Diet Quality or Dietary Supplementation of Flavor and Multi-Enzymes on Primiparous Lactating Sows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Design
2.2. Experimental Diets and Husbandry
2.3. Sample Collection and Measurement
2.3.1. Performance of Sows
2.3.2. Performance of Piglets and Predicted Milk Yield
2.3.3. Blood Sample Collection and Measurement
2.3.4. Milk Sample Collection and Measurement
2.3.5. Apparent Total Tract Digestibility Sampling and Measurement
2.3.6. Lactation Energy Intake and Lactation Efficiency
2.4. Statistical Analysis
3. Results
3.1. Performance of Sows and Piglets and Predicted Milk Yield
3.2. Apparent Total Tract Digestibility, Sow Energy Intake and Lactation Efficiency
3.3. Serum Antioxidant Capacity and Biochemical Indicators
3.4. Milk Composition and Daily Output
3.5. Milk Supernatant Antioxidant Capacity
4. Discussion
4.1. Performance of Sows and Their Offspring
4.2. Apparent Total Tract Digestibility, Energy Intake and Lactation Efficiency
4.3. Serum Antioxidation Capacity and Biochemical Indicators
4.4. Milk Composition and Milk Supernatant Antioxidant Capacity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strathe, A.V.; Bruun, T.S.; Hansen, C.F. Sows with high milk production had both a high feed intake and high body mobilization. Animal 2017, 11, 1913–1921. [Google Scholar] [CrossRef]
- Cozannet, P.; Lawlor, P.G.; Leterme, P.; Devillard, E.; Geraert, P.A.; Rouffineau, F.; Preynat, A. Reducing BW loss during lactation in sows: A meta-analysis on the use of a nonstarch polysaccharide-hydrolyzing enzyme supplement. J. Anim. Sci. 2018, 96, 2777–2788. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, S.; Ringseis, R.; Hillen, S.; Becker, S.; Erhardt, G.; Reiner, G.; Eder, K. Genome-wide transcript profiling indicates induction of energy-generating pathways and an adaptive immune response in the liver of sows during lactation. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.; Solà-Oriol, D.; Guzmán-Pino, S.; Borda, E.; Pérez, J.F. Flavor preferences conditioned by postingestive effect of sucrose and porcine digestive peptides in postweaning pigs. J. Anim. Sci. 2012, 90, 381–383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; He, H.; Gong, L.; Lai, W.; Dong, B.; Zhang, L. Effects of sweetener sucralose on diet preference, growth performance and hematological and biochemical parameters of weaned piglets. Asian Aust. J. Anim. Sci. 2019, 33, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, M.; Xu, S.; Lin, Y.; Che, L.; Fang, Z.; Wu, D. Comparative effects of sodium butyrate and flavors on feed intake of lactating sows and growth performance of piglets. Anim. Sci. J. 2014, 85, 683–689. [Google Scholar] [CrossRef]
- He, L.; Zang, J.; Liu, P.; Fan, P.; Song, P.; Chen, J.; Ma, Y.; Ding, W.; Ma, X. Supplementation of milky flavors improves the reproductive performance and gut function using sow model. Protein Pept. Lett. 2017, 24, 449–455. [Google Scholar] [CrossRef]
- Silva, B.A.N.; Tolentino, R.L.S.; Eskinazi, S.; Jacob, D.V.; Raidan, F.S.S.; Albuquerque, T.V.; Oliverira, N.C.; Araujo, G.G.A.; Silva, K.F.; Alcici, P.F. Evaluation of feed flavor supplementation on the performance of lactating high-prolific sows in a tropical humid climate. Anim. Feed Sci. Technol. 2018, 236, 141–148. [Google Scholar] [CrossRef]
- Wang, R.; Cinar, M.; Macun, H.C.; Ozenc, E.; Salar, S. Flavor supplementation during late gestation and lactation periods increases the reproductive performance and alters fecal microbiota of the sows. Anim. Nutr. 2021, 7, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Jørgensen, H. Intestinal degradation of dietary carbohydrates–from birth to maturity. In Digestive Physiology of Pigs; Lindberg, J., Ogle, B., Eds.; CABI Publishing: Uppsala, Sweden, 2001; pp. 109–120. [Google Scholar]
- Kim, J.C.; Simmins, P.H.; Mullan, B.P.; Pluske, J.R. The digestible energy value of wheat for pigs, with special reference to the post-weaned animal. Anim. Feed Sci. Technol. 2005, 122, 257–287. [Google Scholar] [CrossRef]
- Le Gall, M.; Serena, A.; Jorgensen, H.; Theil, P.K.; Bach Knudsen, K.E. The role of whole-wheat grain and wheat and rye ingredients on the digestion and fermentation processes in the gut-a model experiment with pigs. Br. J. Nutr. 2009, 102, 1590–1600. [Google Scholar] [CrossRef] [Green Version]
- Clarke, L.C.; Sweeney, T.; Curley, E.; Gath, V.; Duffy, S.K.; Vigors, S.; Rajauria, G.; O’Doherty, J.V. Effect of β-glucanase and β-xylanase enzymes supplemented barley diets on nutrient digestibility, growth performance and expression of intestinal nutrient transporter genes in finisher pigs. Anim. Feed Sci. Technol. 2018, 238, 98–110. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Tian, Y.; Song, Z.; Ai, L. Interaction between barley β-glucan and corn starch and its effects on the in vitro digestion of starch. Int. J. Biol. Macromol. 2019, 141, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Geraert, P.A.; Maillard, R.; Kluess, J.; Lawlor, P.G. The effect of a non-starch polysaccharide-hydrolysing enzyme (Rovabio® Excel) on feed intake and body condition of sows during lactation and on progeny growth performance. Animal 2012, 6, 1627–1633. [Google Scholar] [CrossRef] [Green Version]
- Cozannet, P.; Kidd, M.T.; Yacoubi, N.; Geraert, P.-A.; Preynat, A. Dietary energy and amino acid enhancement from a multi-enzyme preparation. J. Appl. Poult. Res. 2019, 28, 136–144. [Google Scholar] [CrossRef]
- Zeng, Z.K.; Li, Q.Y.; Tian, Q.Y.; Xu, Y.T.; Piao, X.S. The combination of carbohydrases and phytase to improve nutritional value and non-starch polysaccharides degradation for growing pigs fed diets with or without wheat bran. Anim. Feed Sci. Technol. 2018, 235, 138–146. [Google Scholar] [CrossRef]
- Petry, A.L.; Patience, J.F. Xylanase supplementation in corn-based swine diets: A review with emphasis on potential mechanisms of action. J. Anim. Sci. 2020, 98, skaa318. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Dourmad, J.Y.; Étienne, M.; Valancogne, A.; Dubois, S.; van Milgen, J.; Noblet, J. InraPorc: A model and decision support tool for the nutrition of sows. Anim. Feed Sci. Technol. 2008, 143, 372–386. [Google Scholar] [CrossRef]
- Hansen, A.V.; Strathe, A.B.; Kebreab, E.; France, J.; Theil, P.K. Predicting milk yield and composition in lactating sows: A Bayesian approach. J. Anim. Sci. 2012, 90, 2285–2298. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC Int.: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Brestenský, M.; Nitrayová, S.; Heger, J.; Patráš, P. Chromic oxide and acid-insoluble ash as markers in digestibility studies with growing pigs and sows. J. Anim. Physiol. Anim. Nutr. 2017, 101, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooney, H.B.; O'Driscoll, K.; O'Doherty, J.V.; Lawlor, P.G. Effect of increasing dietary energy density during late gestation and lactation on sow performance, piglet vitality, and lifetime growth of offspring. J. Anim. Sci. 2020, 98, skz379. [Google Scholar] [CrossRef]
- Aldinger, S.M.; Speer, V.C.; Hays, V.W.; Catron, D.V. Effect of saccharin on consumption of starter rations by baby pigs. J. Anim. Sci. 1959, 18, 1350–1355. [Google Scholar] [CrossRef]
- Park, M.S.; Yang, Y.X.; Shinde, P.L.; Choi, J.Y.; Jo, J.K.; Kim, J.S.; Lohakare, J.D.; Yang, B.K.; Lee, J.K.; Kwon, I.K.; et al. Effects of dietary glucose inclusion on reproductive performance, milk compositions and blood profiles in lactating sows. J. Anim. Physiol. Anim. Nutr. 2010, 94, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Sang, I.L.; Lee, I.S.; Jin, H.C.; Kim, I.H. Effects of saccharin (sweetener) supplementation on growth performance, fecal moisture and litter performance of lactating sows. Korean J. Agric. Sci. 2017, 44, 228–234. [Google Scholar]
- Eissen, J.J.; Apeldoorn, E.J.; Kanis, E.; Verstegen, M.W.; de Greef, K.H. The importance of a high feed intake during lactation of primiparous sows nursing large litters. J. Anim. Sci. 2003, 81, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Beyer, M.; Jentsch, W.; Kuhla, S.; Wittenburg, H.; Kreienbring, F.; Scholze, H.; Rudolph, P.E.; Metges, C.C. Effects of dietary energy intake during gestation and lactation on milk yield and composition of first, second and fourth parity sows. Arch. Anim. Nutr. 2007, 61, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Review: Nutrient requirements of the modern high-producing lactating sow, with an emphasis on amino acid requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojgaard, C.K.; Bruun, T.S.; Theil, P.K. Impact of milk and nutrient intake of piglets and sow milk composition on piglet growth and body composition at weaning. J. Anim. Sci. 2020, 98, 1–12. [Google Scholar] [CrossRef]
- Theil, P.K.; Jørgensen, H.; Jakobsen, K. Energy and protein metabolism in lactating sows fed two levels of dietary fat. Livest. Prod. Sci. 2004, 89, 265–276. [Google Scholar] [CrossRef]
- Muley, N.S.; van Heugten, E.; Moeser, A.J.; Rausch, K.D.; van Kempen, T.A. Nutritional value for swine of extruded corn and corn fractions obtained after dry milling. J. Anim. Sci. 2007, 85, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chi, Y.J. Effects of twin-screw extrusion on soluble dietary fibre and physicochemical properties of soybean residue. Food Chem. 2013, 138, 884–889. [Google Scholar] [CrossRef]
- Wang, P.; Fan, C.G.; Chang, J.; Yin, Q.Q.; Song, A.D.; Dang, X.W.; Lu, F.S. Study on effects of microbial fermented soyabean meal on production performances of sows and suckling piglets and its acting mechanism. J. Anim. Feed Sci. 2016, 25, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lin, C.; Su, W.; Zhang, Y.; Wang, F.; Wang, Y.; Shi, C.; Lu, Z. Effects of supplementing sow diets with fermented corn and soybean meal mixed feed during lactation on the performance of sows and progeny. J. Anim. Sci. 2018, 96, 206–214. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.-J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Che, L.; Wu, C.; Curtasu, M.V.; Wu, F.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Li, J.; et al. Metabolomic profiling reveals the difference on reproductive performance between high and low lactational weight loss sows. Metabolites 2019, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Barrera, M.; Cervantes, M.; Sauer, W.C.; Araiza, A.B.; Torrentera, N.; Cervantes, M. Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. J. Anim. Sci. 2004, 82, 1997–2003. [Google Scholar] [CrossRef] [Green Version]
- de Souza, A.L.P.; Lindemann, M.D.; Cromwell, G.L. Supplementation of dietary enzymes has varying effects on apparent protein and amino acid digestibility in reproducing sows. Livest. Sci. 2007, 109, 122–124. [Google Scholar] [CrossRef]
- Nortey, T.N.; Patience, J.F.; Sands, J.S.; Trottier, N.L.; Zijlstra, R.T. Effects of xylanase supplementation on the apparent digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs. J. Anim. Sci. 2008, 86, 3450–3464. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Song, J.; Deng, Z.; Cheng, L.; Tian, M.; Guan, W. Effects of combined alpha-galactosidase and xylanase supplementation on nutrient digestibility and growth performance in growing pigs. Arch. Anim. Nutr. 2017, 71, 441–454. [Google Scholar] [CrossRef]
- Rauw, W.M.; Portolés, O.; Corella, D.; Soler, J.; Reixach, J.; Tibau, J.; Prat, J.M.; Diaz, I.; Gómez-Raya, L. Behaviour influences cholesterol plasma levels in a pig model. Animal 2007, 1, 865–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.J.; Chuang, S.Y.; Chang, H.Y.; Pan, W.H. Energy intake at different times of the day: Its association with elevated total and LDL cholesterol levels. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 390–397. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Q.; Jiao, Z.; Li, H.; Bai, G.; Wang, H. Adipose-derived stem cells reduce liver oxidative stress and autophagy induced by ischemia-reperfusion and hepatectomy injury in swine. Life Sci. 2018, 214, 62–69. [Google Scholar] [CrossRef]
- Su, R.C.; Lard, A.; Breidenbach, J.D.; Kleinhenz, A.L.; Modyanov, N.; Malhotra, D.; Haller, S.T.; Kennedy, D.J. Assessment of diagnostic biomarkers of liver injury in the setting of microcystin-LR (MC-LR) hepatotoxicity. Chemosphere 2020, 257, 127111. [Google Scholar] [CrossRef] [PubMed]
- McKimmie, R.L.; Daniel, K.R.; Carr, J.J.; Bowden, D.W.; Freedman, B.I.; Register, T.C.; Hsu, F.-C.; Lohman, K.K.; Weinberg, R.B.; Wagenknecht, L.E. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: The Diabetes Heart Study. Am. J. Gastroenterol. 2008, 103, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Lipko-Przybylska, J.; Kankofer, M. Antioxidant defence of colostrum and milk in consecutive lactations in sows. Ir. Vet. J. 2012, 65, 4. [Google Scholar] [CrossRef] [Green Version]
- Yigit, A.A.; Cinar, M.; Macun, H.C.; Ozenc, E.; Salar, S. Total oxidant and antioxidant activities in milk with various somatic cell count intervals during discrete cow and buffalo lactation periods. Indian J. Dairy Sci. 2018, 71, 72–77. [Google Scholar]
- Zigo, F.; Elecko, J.; Vasil, M.; Ondrasovicova, S.; Farkasova, Z.; Malova, J.; Takac, L.; Zigova, M.; Bujok, J.; Pecka-Kielb, E.; et al. The occurrence of mastitis and its effect on the milk malondialdehyde concentrations and blood enzymatic antioxidants in dairy cows. Vet. Sci. 2019, 64, 423–432. [Google Scholar] [CrossRef]
- Hu, H.; Wang, M.; Zhan, X.; Li, X.; Zhao, R. Effect of different selenium sources on productive performance, serum and milk Se concentrations, and antioxidant status of sows. Biol. Trace Elem. Res. 2011, 142, 471–480. [Google Scholar] [CrossRef]

| Items | CON | HQ |
|---|---|---|
| Ingredients, kg | ||
| Corn | 524.20 | 296.86 |
| Extruded corn | 200.00 | |
| Wheat bran | 150.00 | 100.00 |
| Defatted rice bran | 65.50 | 46.00 |
| Extruded full-fat soybean | - | 80.00 |
| Fermented soybean meal | - | 40.00 |
| Soybean meal | 166.00 | 109.00 |
| Imported fish meal | 20.00 | 20.00 |
| Sucrose | 20.00 | 20.00 |
| Glucose | 20.00 | 20.00 |
| Corn oil | - | 25.00 |
| L-Lysine.H2SO4 (78.8%) | 3.56 | 3.87 |
| L-Methionine (99%) | - | 0.51 |
| L-Threonine (98.5%) | 1.21 | 1.48 |
| L-Tryptophan (98%) | 0.32 | 0.38 |
| L-Valine (98%) | 2.00 | 2.27 |
| Calcium hydrophosphate | 4.64 | 15.82 |
| Limestone | 14.18 | 10.41 |
| Premix 1 | 8.39 | 8.39 |
| Total | 1000.00 | 1000.00 |
| Chemical compositions 2 | ||
| Gross energy, MJ/kg | 15.79 | 16.76 |
| Digestible energy, MJ/kg | 13.30 | 14.70 |
| Metabolizable energy, MJ/kg | 12.94 | 13.90 |
| Net energy, MJ/kg | 9.70 | 10.54 |
| Crude protein, % | 16.22 | 17.21 |
| Ether extract, % | 2.64 | 5.65 |
| Standardized ileal digestible amino acids, % | ||
| Lys, % | 0.95 | 1.04 |
| Met, % | 0.23 | 0.27 |
| Met + Cys, % | 0.52 | 0.56 |
| Thr, % | 0.60 | 0.65 |
| Try, % | 0.19 | 0.20 |
| Ile, % | 0.54 | 0.58 |
| Val, % | 0.81 | 0.88 |
| Total calcium, % | 0.78 | 0.85 |
| Total phosphorus, % | 0.80 | 0.96 |
| Standard total tract digestible phosphorus, % | 0.32 | 0.48 |
| Neutral detergent fiber, % | 18.29 | 14.64 |
| Acid detergent fiber, % | 4.41 | 4.17 |
| Crude fiber, % | 3.74 | 3.74 |
| Items | CON | HQ | F + E | SEM | p-Value 1 |
|---|---|---|---|---|---|
| Sows | |||||
| No. of sows | 10 | 10 | 10 | - | - |
| Feed intake | |||||
| Day 2–7, kg/d | 2.22 | 2.41 | 2.40 | 0.212 | 0.77 |
| Day 8–14, kg/d | 4.74 | 5.02 | 4.41 | 0.267 | 0.29 |
| Day 15–21, kg/d | 5.77 | 5.90 | 5.60 | 0.252 | 0.71 |
| Day 2–21, kg/d | 4.34 | 4.54 | 4.22 | 0.197 | 0.51 |
| Live weight | |||||
| Day 2, kg | 198 | 197 | 196 | 4.9 | 0.97 |
| Day 21, kg | 186 | 186 | 183 | 4.0 | 0.78 |
| Loss on day 2–21, kg | 12 | 11 | 14 | 3.1 | 0.83 |
| BFT | |||||
| BFT on day 2, mm | 17.5 | 17.8 | 17.7 | 0.98 | 0.97 |
| BFT on day 21, mm | 15.5 | 15.7 | 15.6 | 1.00 | 0.98 |
| BFT loss on day 2–21, mm | 2.0 | 2.0 | 2.1 | 0.57 | 0.99 |
| Calculated body composition | |||||
| Body lipid loss, kg | 5.18 | 5.04 | 5.71 | 1.118 | 0.90 |
| Body lipid loss, % | 12.99 | 12.97 | 14.26 | 2.689 | 0.93 |
| Body protein loss, kg | 1.38 | 1.24 | 1.65 | 0.525 | 0.85 |
| Body protein loss, % | 4.32 | 3.91 | 5.28 | 1.785 | 0.86 |
| Body energy loss, MJ | 248 | 238 | 277 | 53.5 | 0.87 |
| Body energy loss, % | 10.30 | 10.24 | 11.55 | 2.184 | 0.89 |
| Items | Treatments | SEM | Time (Week) | SEM | p-Value 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HQ | F + E | 1 | 2 | 3 | Treatment | Time | Treatment × Time | |||
| Litter size, pig | 8.9 | 9.0 | 8.9 | 0.05 | 9.0 | 8.9 | 8.9 | 0.05 | 0.37 | 0.37 | 0.84 |
| Piglet live weight, kg/pig | 3.55 b | 3.77 a | 3.78 a | 0.096 | 2.82 c | 4.32 b | 5.83 a | 0.096 | 0.04 | <0.001 | 0.88 |
| Piglet ADG, g/d | 184 | 206 | 206 | 7.8 | 166b | 214 a | 216 a | 7.8 | 0.07 | <0.001 | 0.93 |
| Litter weight gain, kg/d | 1.64 | 1.86 | 1.82 | 0.072 | 1.54 b | 2.01 a | 1.92 a | 0.072 | 0.08 | <0.001 | 0.95 |
| Weekly milk yield, kg/week | 50.89 | 52.90 | 52.14 | 1.062 | 36.64 c | 57.78 b | 61.52 a | 0.652 | 0.41 | <0.001 | 0.67 |
| Piglet growth:milk yield, kg/kg | 0.21 | 0.24 | 0.24 | 0.008 | 0.24 | 0.23 | 0.22 | 0.008 | 0.07 | 0.05 | 0.84 |
| Items | CON | HQ | F + E | SEM | p-Value 1 |
|---|---|---|---|---|---|
| ATTD, % | |||||
| DM | 89.40 b | 91.53 a | 89.63 b | 0.486 | 0.01 |
| EE | 64.22 b | 83.86 a | 69.44 b | 1.908 | <0.001 |
| GE | 83.88 b | 87.72 a | 84.64 b | 0.575 | <0.01 |
| CP | 85.18 b | 89.00 a | 86.61 b | 0.743 | <0.01 |
| Ash | 42.10 b | 54.35 a | 50.33 a | 1.712 | <0.001 |
| NDF | 57.41 | 62.49 | 61.86 | 1.703 | 0.08 |
| ADF | 42.69 b | 53.24 a | 40.23 b | 2.389 | <0.01 |
| CF | 35.47 b | 52.55 a | 42.39 b | 2.621 | <0.01 |
| Calcium | 44.68 | 53.38 | 53.60 | 3.451 | 0.134 |
| Phosphorus | 51.69 c | 73.25 a | 60.63 b | 2.415 | <0.001 |
| DE 2, MJ/kg | 13.32 | 14.70 | 13.28 | - | - |
| Lactation energy intake, MJ | |||||
| Total DE | 1157.61 b | 1335.82 a | 1121.10 b | 53.360 | 0.02 |
| Daily DE | 57.88 b | 66.79 a | 56.05 b | 2.668 | 0.02 |
| Lactation efficiency, g/MJ DE | 27.33 | 25.7 | 29.14 | 1.517 | 0.28 |
| Items | Treatments | SEM | Time (Day) | SEM | p-Value 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HQ | F + E | 7 | 14 | 21 | Treatment | Time | Treatment × Time | |||
| SOD U/mL | 50.04 | 46.72 | 49.23 | 3.532 | 44.50 b | 46.92 ab | 54.56 a | 1.843 | 0.79 | 0.01 | 0.70 |
| CAT, U/mL | 12.14 | 10.14 | 11.33 | 0.653 | 15.01 a | 8.96 b | 9.65 b | 0.653 | 0.10 | <0.001 | 0.70 |
| GSH-Px, U/mL | 1091 | 1088 | 1102 | 50.4 | 992 b | 1173 a | 1118 a | 38.2 | 0.98 | 0.04 | 0.36 |
| T-AOC, mmol/L | 0.24 | 0.28 | 0.24 | 0.025 | 0.33 a | 0.14 b | 0.28 a | 0.028 | 0.41 | <0.001 | 0.99 |
| MDA, nmol/mL | 3.12 | 2.69 | 2.69 | 0.314 | 3.06 | 2.84 | 2.60 | 0.258 | 0.54 | 0.37 | 0.89 |
| Items | Treatments | SEM | Time (Day) | SEM | p-Value 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HQ | F + E | 7 | 14 | 21 | Treatment | Time | Treatment × Time | |||
| Protein and lipid metabolism | |||||||||||
| CREA, μmol/L | 143.32 | 143.74 | 146.25 | 5.662 | 154.75 a | 138.35 b | 140.21 b | 3.954 | 0.93 | <0.001 | 0.75 |
| UN, mmol/L | 8.55 a | 8.68 a | 7.27 b | 0.362 | 8.66 | 7.94 | 7.90 | 0.356 | 0.02 | 0.36 | 0.52 |
| β-HBA, mmol/L | 0.026 | 0.019 | 0.017 | 0.0051 | 0.043 a | 0.009 b | 0.010 b | 0.0039 | 0.41 | <0.001 | 0.54 |
| TG, mmol/L | 0.47 | 0.43 | 0.45 | 0.023 | 0.57 a | 0.40 b | 0.39 b | 0.021 | 0.46 | <0.001 | 0.54 |
| NEFA, mmol/L | 0.72 | 0.62 | 0.69 | 0.102 | 1.33 a | 0.33 b | 0.38 b | 0.094 | 0.81 | <0.001 | 0.26 |
| LDL-C, mmol/L | 0.84 | 0.90 | 0.79 | 0.054 | 0.78 | 0.88 | 0.87 | 0.041 | 0.35 | 0.09 | 0.59 |
| HDL-C, mmol/L | 0.65 ab | 0.76 a | 0.60 b | 0.034 | 0.54 b | 0.68 a | 0.79 a | 0.034 | <0.01 | <0.001 | 0.39 |
| TC, mmol/L | 1.85 | 2.17 | 1.73 | 0.098 | 1.62 b | 1.99 a | 2.14 a | 0.098 | 0.07 | <0.001 | 0.21 |
| Liver health and immunity | |||||||||||
| AST, U/L | 34.63 a | 30.07 ab | 29.60 b | 1.442 | 33.77 | 29.5 | 31.03 | 1.442 | 0.03 | 0.11 | 0.83 |
| ALT, U/L | 27.8 | 25.83 | 25.8 | 1.579 | 26.27 a | 24.67 b | 28.50 a | 1.122 | 0.60 | <0.01 | 0.22 |
| GGT, U/L | 35.07 | 38.4 | 38.97 | 2.922 | 38.13 | 37.23 | 37.07 | 1.938 | 0.60 | 0.79 | 0.26 |
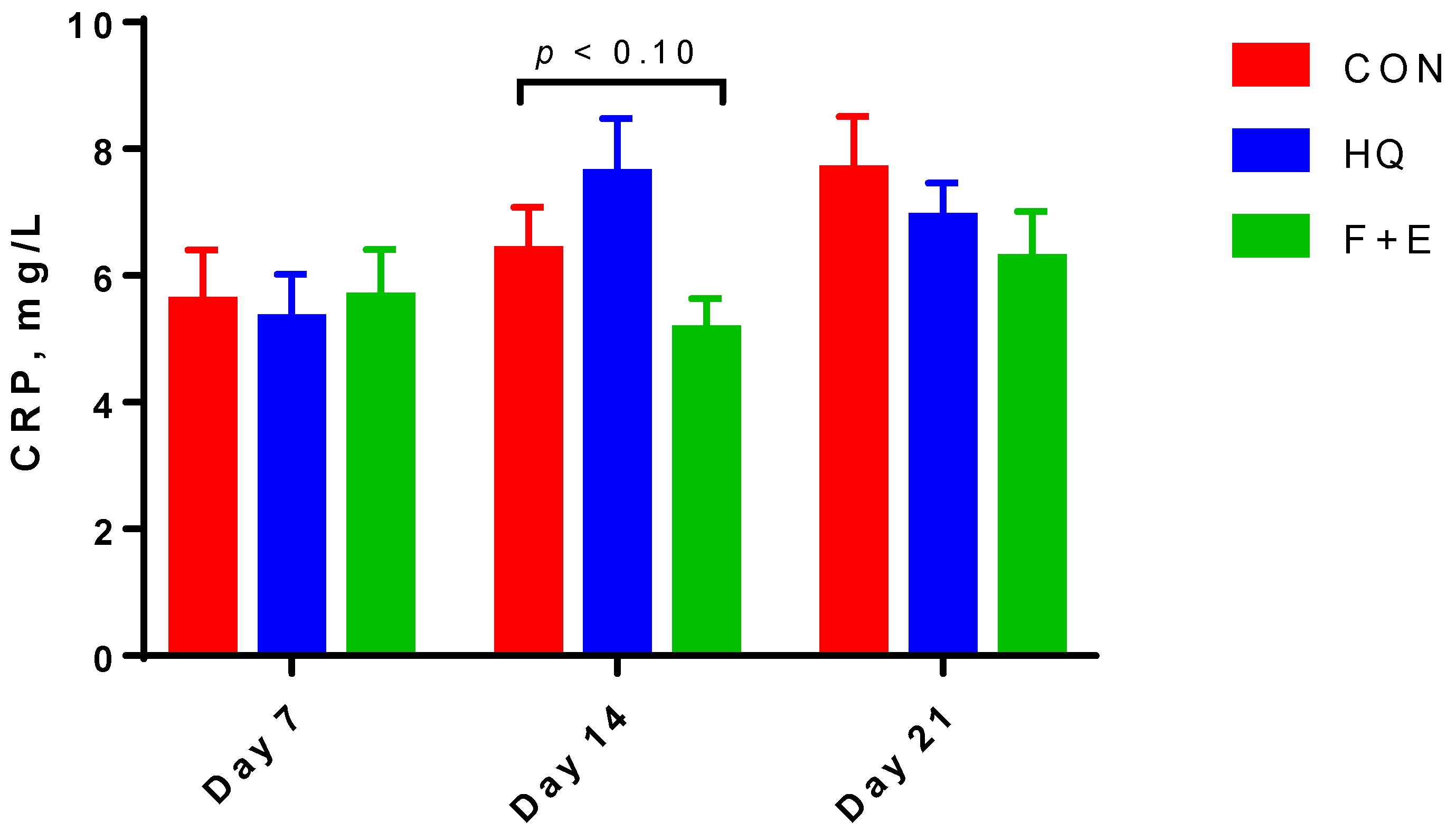
| CRP, mg/L | 6.55 | 6.61 | 5.68 | 0.601 | 5.52 b | 6.38 ab | 6.94 a | 0.426 | 0.49 | 0.02 | 0.03 |
| Items | Treatments | SEM | Time (Day) | SEM | p-Value 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HQ | F + E | 7 | 14 | 21 | Treatment | Time | Treatment × Time | |||
| Milk composition | |||||||||||
| DM, % | 20.7 | 20.8 | 21.0 | 0.52 | 24.1 a | 19.1 b | 19.4 b | 0.58 | 0.90 | <0.001 | 0.43 |
| Fat, % | 8.6 | 8.5 | 8.9 | 0.47 | 11.5 a | 7.2 b | 7.3 b | 0.47 | 0.72 | <0.001 | 0.71 |
| Protein, % | 5.5 | 5.4 | 5.5 | 0.14 | 6.1 a | 5.1 b | 5.2 b | 0.14 | 0.82 | <0.001 | 0.46 |
| Non-fat milk solids, % | 12.1 | 12.4 | 12.0 | 0.19 | 12.5 | 11.9 | 12.1 | 0.19 | 0.47 | 0.11 | 0.35 |
| Lactose, % | 5.4 | 5.7 | 5.3 | 0.16 | 5.1 b | 5.6 a | 5.7 a | 0.14 | 0.21 | <0.01 | 0.58 |
| SCC, ×1000 cells/mL | 1614 | 793 | 1493 | 846.4 | 1998 | 734 | 1168 | 708.6 | 0.33 | 0.53 | 0.45 |
| UN, mg/dL | 52.7 | 52.3 | 51.8 | 2.18 | 60.9 a | 43.6 c | 52.3 b | 2.18 | 0.95 | <0.001 | 0.47 |
| Daily output | |||||||||||
| Fat, g/d | 676 | 686 | 719 | 40.7 | 822 a | 630 b | 628 b | 40.7 | 0.73 | 0.001 | 0.81 |
| Protein, g/d | 429 | 439 | 450 | 15.1 | 433 | 438 | 446 | 15.1 | 0.63 | 0.85 | 0.91 |
| Lactose, g/d | 427 | 472 | 442 | 15.5 | 366 b | 483 a | 492 a | 15.5 | 0.12 | <0.001 | 0.85 |
| Items | Treatments | SEM | Time (Day) | SEM | p-Value 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HQ | F + E | 7 | 14 | 21 | Treatment | Time | Treatment × Time | |||
| SOD, U/mL | 90.51 b | 119.73 ab | 132.03 a | 10.283 | 221.01 a | 56.26 b | 64.99 b | 15.898 | 0.02 | <0.001 | <0.01 |
| CAT, U/mL | 2.24 b | 3.26 ab | 4.72 a | 0.46 | 4.31 a | 3.44 ab | 2.47 b | 0.41 | <0.01 | <0.01 | 0.08 |
| GSH-Px, U/mL | 30.54 b | 33.88 b | 44.60 a | 3.05 | 45.43 a | 26.88 b | 36.71 a | 3.21 | <0.01 | <0.01 | 0.65 |
| T-AOC, mmol/L | 0.89 | 0.98 | 0.74 | 0.099 | 0.89 | 0.84 | 0.88 | 0.09 | 0.25 | 0.88 | 0.52 |
| MDA, nmol/mL | 3.59 a | 3.53 a | 2.73 b | 0.198 | 4.39 a | 3.21 b | 2.25 c | 0.23 | <0.01 | <0.001 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhe, L.; Zhou, R.; Theil, P.K.; Krogh, U.; Yang, L.; Zhuo, Y.; Lin, Y.; Xu, S.; Jiang, X.; Huang, L.; et al. The Impact of Enhancing Diet Quality or Dietary Supplementation of Flavor and Multi-Enzymes on Primiparous Lactating Sows. Animals 2022, 12, 1493. https://doi.org/10.3390/ani12121493
Zhe L, Zhou R, Theil PK, Krogh U, Yang L, Zhuo Y, Lin Y, Xu S, Jiang X, Huang L, et al. The Impact of Enhancing Diet Quality or Dietary Supplementation of Flavor and Multi-Enzymes on Primiparous Lactating Sows. Animals. 2022; 12(12):1493. https://doi.org/10.3390/ani12121493
Chicago/Turabian StyleZhe, Li, Rui Zhou, Peter Kappel Theil, Uffe Krogh, Lunxiang Yang, Yong Zhuo, Yan Lin, Shengyu Xu, Xuemei Jiang, Lingjie Huang, and et al. 2022. "The Impact of Enhancing Diet Quality or Dietary Supplementation of Flavor and Multi-Enzymes on Primiparous Lactating Sows" Animals 12, no. 12: 1493. https://doi.org/10.3390/ani12121493
APA StyleZhe, L., Zhou, R., Theil, P. K., Krogh, U., Yang, L., Zhuo, Y., Lin, Y., Xu, S., Jiang, X., Huang, L., Che, L., Feng, B., Wu, D., & Fang, Z. (2022). The Impact of Enhancing Diet Quality or Dietary Supplementation of Flavor and Multi-Enzymes on Primiparous Lactating Sows. Animals, 12(12), 1493. https://doi.org/10.3390/ani12121493







