Measuring Annual Variation in Reproductive Output Reveals a Key Role of Maternal Body Condition in Determining the Size of Eggs in Snakes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Animal Collection and Care
2.3. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meiri, S.; Feldman, A.; Schwarz, R.; Shine, R. Viviparity does not affect the numbers and sizes of reptile offspring. J. Anim. Ecol. 2020, 89, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Räsänen, K.; Söderman, F.; Laurila, A.; Merilä, J. Geographic variation in maternal investment: Acidity affects egg size and fecundity in Rana arvalis. Ecology 2008, 89, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Sinervo, B. The evolution of maternal investment in lizards: An experimental and comparative analysis of egg size and its effects on offspring performance. Evolution 1990, 44, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D. Intraspecific variation in egg size and egg composition in birds: Effects on offspring fitness. Biol. Rev. 1994, 68, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Fretwell, S.D. The optimal balance between size and number of offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Bashey, F. Competition as a selective mechanism for larger offspring size in guppies. Oikos 2008, 117, 104–113. [Google Scholar] [CrossRef]
- Johnston, T.A.; Leggett, W.C. Maternal and environmental gradients in the egg size of an iteroparous fish. Ecology 2002, 83, 1777–1791. [Google Scholar] [CrossRef]
- Jordan, M.A.; Snell, H.L. Variation in reproductive traits in a population of the lizard Lacerta vivipara. Oecologia 2002, 130, 44–52. [Google Scholar] [CrossRef]
- Swain, R.; Jones, S.M. Facultative placentotrophy: Half-way house or strategic solution? Comp. Biochem. Physiol. A 2000, 127, 441–451. [Google Scholar] [CrossRef]
- Taborsky, B. Mothers determine offspring size in response to own juvenile growth conditions. Biol. Lett. 2006, 2, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Bleu, J.; Massot, M.; Haussy, C.; Meylan, S. An experimental study of the gestation costs in a viviparous lizard: A hormonal manipulation. Physiol. Biochem. Zool. 2013, 86, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caley, M.J.; Schwarzkopf, L.; Shine, R. Does total reproductive effort evolve independently of offspring size? Evolution 2001, 55, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Reznick, D.N.; Bryga, H.; Endler, J.A. Experimentally induced life history evolution in a natural population. Nature 1990, 346, 357–359. [Google Scholar] [CrossRef]
- Rollinson, N.; Brooks, R.J. Optimal offspring provisioning when egg is “constrained”: A case study with the painted turtle Chrysemys picta. Oikos 2008, 117, 144–151. [Google Scholar] [CrossRef]
- Ji, X.; Du, W.-G.; Li, H.; Lin, L.-H. Experimentally reducing clutch size reveals a fixed upper limit to egg size in snakes: Evidence from the king ratsnake (Elaphe carinata). Comp. Biochem. Physiol. A 2006, 144, 474–478. [Google Scholar] [CrossRef]
- Ji, X.; Du, W.-G.; Qu, Y.-F.; Lin, L.-H. Nonlinear continuum of egg size-number trade-offs in a snake: Is egg-size variation fitness related? Oecologia 2009, 159, 689–696. [Google Scholar] [CrossRef]
- Sinervo, B.; Licht, P. Hormonal and physiological control of clutch size, egg size and egg shape in side-blotched lizards (Uta stansburiana): Constraints on the evolution of lizard life histories. J. Exp. Zool. 1991, 257, 252–264. [Google Scholar] [CrossRef]
- Sinervo, B.; Licht, P. Proximate constraints on the evolution of egg size, number, and total clutch mass in lizards. Science 1991, 252, 1300–1302. [Google Scholar] [CrossRef]
- Olsson, M.; Wapstra, E.; Olofsson, C. Offspring size-number strategies: Experimental manipulation of offspring size in a viviparous lizard (Lacerta vivipara). Funct. Ecol. 2002, 16, 135–140. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Y.; Ji, X. Clutch frequency affects the offspring size-number trade-off in lizards. PLoS ONE 2011, 6, e16585. [Google Scholar] [CrossRef]
- Ji, X.; Diong, C.-H. Does follicle excision always result in enlargement of offspring size in lizards? J. Comp. Physiol. B 2006, 176, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-G.; Ding, G.-H.; Ji, X. Income breeding and temperature-induced plasticity in reproductive traits in lizards. J. Exp. Biol. 2010, 213, 2073–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.-G.; Ji, X.; Shine, R. Does body-volume constrain reproductive output in lizards? Biol. Lett. 2005, 1, 98–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Du, W.-G.; Lin, Z.-H.; Luo, L.-G. Measuring temporal variation in reproductive output reveals optimal resource allocation to reproduction in the northern grass lizard, Takydromus septentrionalis. Biol. J. Linn. Soc. 2007, 91, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Shine, R. Life-history evolution in reptiles. Annu. Rev. Ecol. Syst. 2005, 36, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.P.; Shine, R. Rain, prey and predators: Climatically driven shifts in frog abundance modify reproductive allometry in a tropical snake. Oecologia 2007, 154, 361–368. [Google Scholar] [CrossRef]
- Tuttle, K.N.; Gregory, P.T. Reproduction of the plains garter snake, Thamnophis radix, near its northern range limit: More evidence for a “fast” life history. Copeia 2014, 2014, 130–135. [Google Scholar] [CrossRef]
- Ma, J.-P. Fauna Sinica Volume 3 (Squamata: Serpentes); Science Press: Beijing, China, 1998; pp. 130–172. [Google Scholar]
- Ji, X.; Sun, P.-Y.; Xu, X.-F.; Du, W.-G. Relationships among body size, clutch size, and egg size in five species of oviparous colubrid snakes from Zhoushan Islands, Zhejiang, China. Acta Zool. Sin. 2000, 46, 138–145. [Google Scholar]
- Ji, X.; Xu, X.-F.; Lin, Z.-H. Influence of incubation temperature on characteristics of Dinodon rufozonatum (Reptilia: Colubridae) hatchlings, with comments on the function of residual yolk. Zool. Res. 1999, 20, 342–346. [Google Scholar]
- Zhang, Y.-P.; Ji, X. Further studies on egg incubation of an oviparous snake, Dinodon rufozonatum (Colubridae), with comments on the influence of hydric environments. Acta Zool. Sin. 2002, 48, 51–59. [Google Scholar]
- Shine, R. Relative clutch mass and body shape in lizards and snakes: It reproductive investment constrained or optimized? Evolution 1992, 46, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Gignac, A.; Gregory, P.T. The effects of body size, age, and food intake during pregnancy on reproductive traits of a viviparous snake, Thamnophis ordinoides. Ecoscience 2005, 12, 236–245. [Google Scholar] [CrossRef]
- Shine, R.; Charnov, E.L. Patterns of survival, growth, and maturation in snakes and lizards. Am. Nat. 1992, 139, 1257–1269. [Google Scholar] [CrossRef] [Green Version]
- Waye, H.L. Size and age structure of a population of western terrestrial garter snakes (Thamnophis elegans). Copeia 1999, 1999, 819–823. [Google Scholar] [CrossRef]
- Ford, N.B.; Seigel, R.A. Phenotypic plasticity in reproductive traits: Evidence from a viviparous snake. Ecology 1989, 70, 1768–1774. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Z.-W. Geographic variation in reproductive traits and trade-offs between size and number of eggs of the Chinese cobra (Naja atra). Biol. J. Linn. Soc. 2005, 85, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Seigel, R.A.; Ford, N.B. Effect of energy input on variation in clutch size and offspring size in a viviparous reptile. Funct. Ecol. 1992, 6, 382–385. [Google Scholar] [CrossRef]
- Madsen, T.; Shine, R. The adjustment of reproductive threshold to prey abundance in a capital breeder. J. Anim. Ecol. 1999, 68, 571–580. [Google Scholar] [CrossRef]
- Crespi, B.; Semeniuk, C. Parent-offspring conflict in the evolution of vertebrate reproductive mode. Am. Nat. 2004, 163, 635–653. [Google Scholar] [CrossRef]
- Ma, L.; Liu, P.; Su, S.; Luo, L.-G.; Zhao, W.-G.; Ji, X. Life-history consequences of local adaptation in lizards: Takydromus wolteri (Lacertidae) as a model organism. Biol. J. Linn. Soc. 2019, 127, 88–99. [Google Scholar] [CrossRef]
- Qu, Y.-F.; Li, H.; Gao, J.-F.; Ji, X. Geographical variation in reproductive traits and trade-offs between size and number of eggs in the king ratsnake, Elaphe carinata. Biol. J. Linn. Soc. 2011, 104, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Olsson, M.; Shine, R. The seasonal timing of oviposition in sand lizards (Lacerta agilis): Why early clutches are better. J. Evol. Biol. 1997, 10, 369–381. [Google Scholar] [CrossRef]
- Farrell, T.M.; May, P.G.; Pilgrim, M.A. Reproduction in the rattlesnake, Sistrurus miliarius barbouri, in central Florida. J. Herpetol. 1995, 29, 21–27. [Google Scholar] [CrossRef]
- Madsen, T.; Shine, R. Life-history consequences of nest-site variation in tropical pythons. Ecology 1999, 80, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Shine, R. Reproductive strategies in snakes. Proc. R. Soc. B 2003, 270, 995–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Galliard, J.F.; Massot, M.; Baron, J.P.; Clobert, J. Wildlife Conservation in a Changing Climate; University of Chicago Press: Chicago, IL, USA, 2012; pp. 179–203. [Google Scholar]
- Madsen, T.; Shine, R. Conflicting conclusions from long-term versus short-term studies on growth and reproduction of a tropical snake. Herpetologica 2001, 59, 147–156. [Google Scholar]
- Farrell, T.M.; May, P.G.; Pilgrim, M.A. Repeatability of female reproductive traits in pigmy rattlesnakes (Sistrurus miliarius). J. Herpetol. 2009, 43, 332–335. [Google Scholar] [CrossRef]
- Gao, J.-F.; Qu, Y.-F.; Luo, L.-G.; Ji, X. Evolution of reptilian viviparity: A test of the maternal manipulation hypothesis in a temperate snake, Gloydius brevicaudus (Viperidae). Zool. Sci. 2010, 27, 248–255. [Google Scholar] [CrossRef]
- Seigel, R.A.; Fitch, H.S. Ecological patterns of relative clutch mass in snakes. Oecologia 1984, 61, 293–301. [Google Scholar] [CrossRef]
- Vitt, L.J.; Price, H.J. Ecological and evolutionary determinants of relative clutch mass in lizards. Herpetologica 1982, 38, 237–255. [Google Scholar]
- Lin, Z.-H.; Ji, X. Reproductive output and effects of incubation thermal environments on hatchling phenotypes of mucous ratsnake (Ptyas mucous). Acta Zool. Sin. 2004, 50, 541–550. [Google Scholar]
- Lin, L.-H.; Mao, F.; Chen, C.; Ji, X. Reproductive traits of the gray ratsnake Ptyas korros from three geographically distinct populations. Curr. Zool. 2012, 58, 820–827. [Google Scholar] [CrossRef] [Green Version]
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics; Comstock; Cornell University Press: Ithaca, NY, USA, 2002; Volume 1. [Google Scholar]

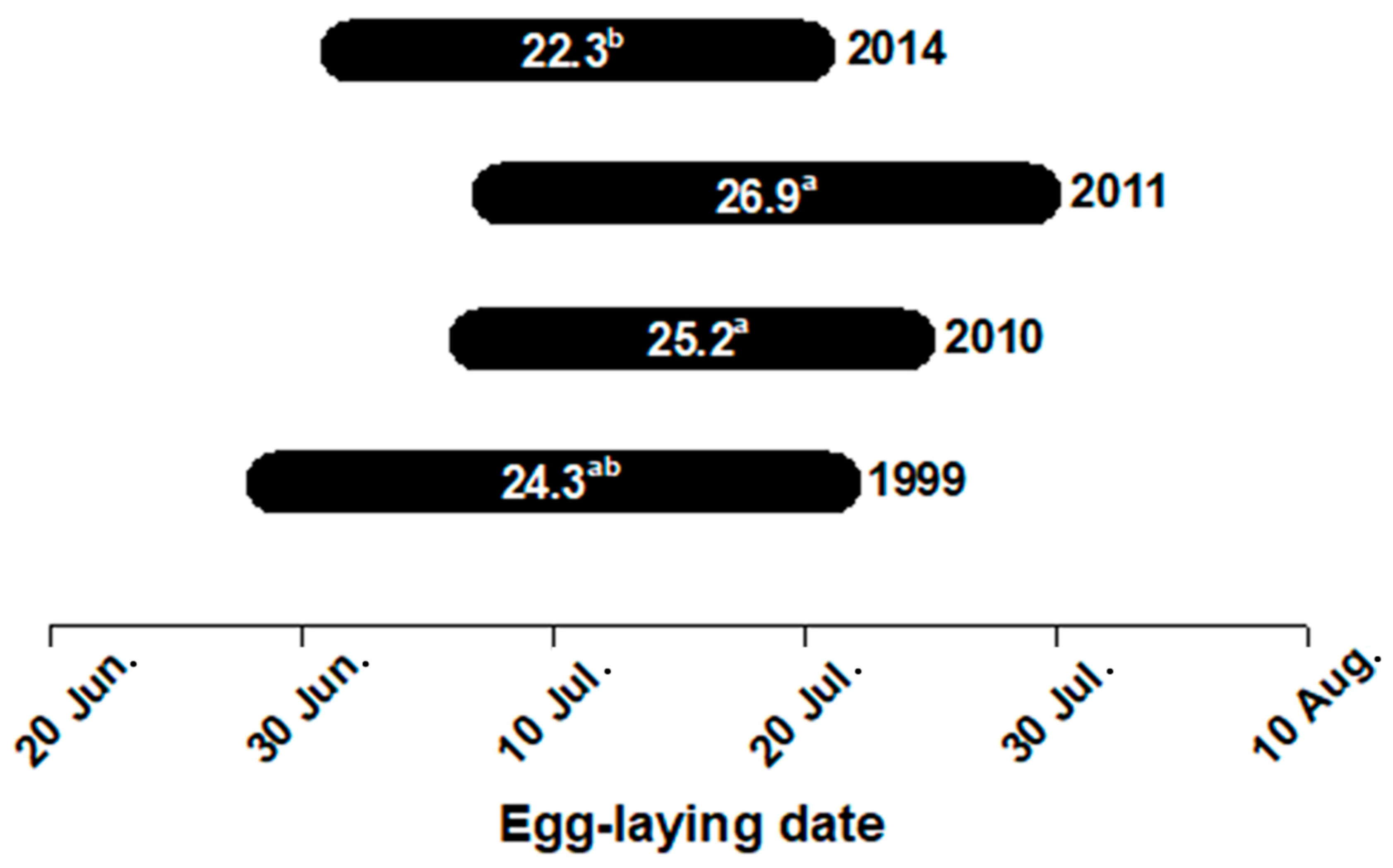
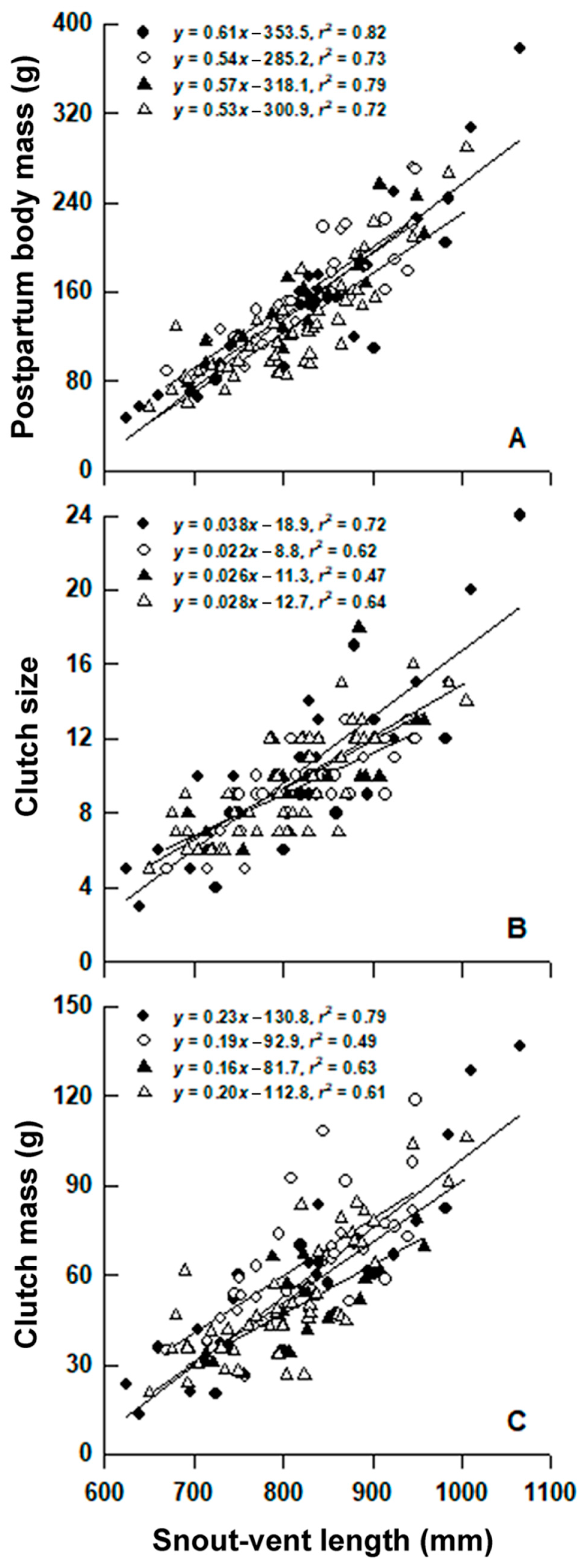
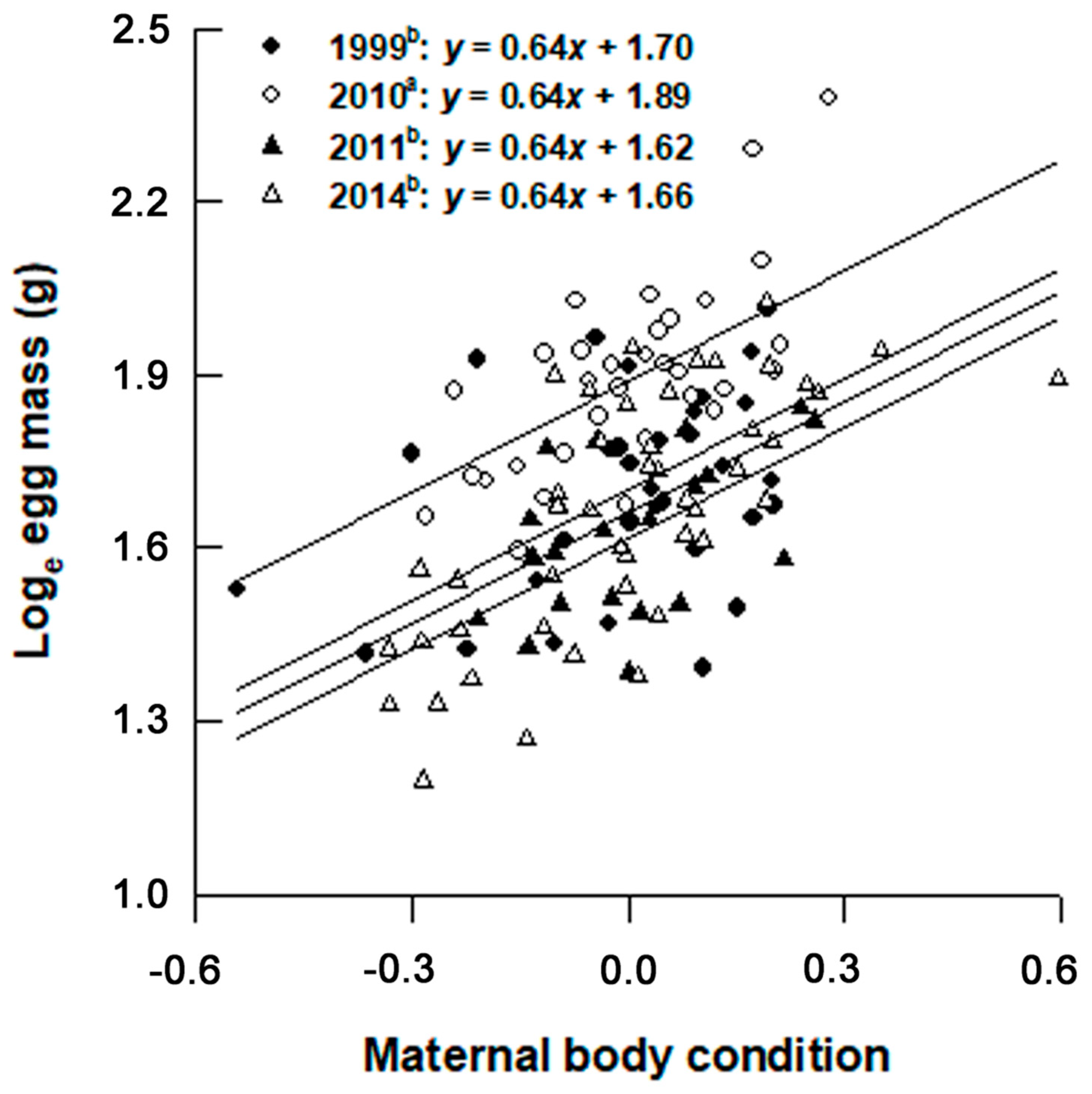
| Year | ANOVA or ANCOVA Results | ||||
|---|---|---|---|---|---|
| 1999 | 2010 | 2011 | 2014 | ||
| N | 32 | 32 | 21 | 46 | |
| Snout vent length (mm) | 822.6 ± 19.2 625–1065 | 829.4 ± 13.4 670–948 | 819.0 ± 16.5 693–958 | 807.9 ± 12.0 650–1005 | F3,127 = 0.42, p = 0.742 |
| Postpartum body mass (g) | 148.8 ± 12.9 47.3–378.2 | 161.6 ± 8.5 89.0–271.0 | 148.9 ± 10.5 78.0–256.5 | 128.1 ± 7.5 57.4–289.6 | F3,126 = 4.19, p < 0.01 1999 ab, 2010 a, 2011 ab, 2014 b |
| Clutch size | 10.4 ± 0.8 3–24 | 9.6 ± 0.4 5–13 | 9.8 ± 0.6 6–18 | 9.6 ± 0.4 5–16 | F3,126 = 0.41, p = 0.734 |
| Clutch mass (g) | 58.1 ± 5.0 13.0–136.8 | 65.4 ± 3.7 26.1–118.6 | 50.3 ± 3.3 27.0–79.1 | 52.2 ± 3.1 20.6–106.1 | F3,126 = 4.64, p < 0.01 1999 ab, 2010 a, 2011 b, 2014 b |
| Egg size (g) | 5.5 ± 0.2 4.0–7.5 | 6.8 ± 0.2 4.9–10.8 | 5.1 ± 0.2 4.0–6.3 | 5.4 ± 0.2 3.3–7.6 | F3,126 = 14.78, p < 0.0001 1999 b, 2010 a, 2011 b, 2014 b |
| CV of egg size (%) | 6.8 ± 0.7 2.8–20.1 | 5.3 ± 0.5 1.7–12.8 | 5.0 ± 0.4 1.2–8.9 | 5.4 ± 0.3 2.1–11.0 | F3,127 = 2.38, p = 0.073 |
| Relative clutch mass | 0.40 ± 0.02 0.23–0.64 | 0.41 ± 0.01 0.28–0.61 | 0.34 ± 0.01 0.22–0.47 | 0.40 ± 0.01 0.20–0.73 | F3,126 = 5.29, p < 0.01 1999 a, 2010 a, 2011 b, 2014 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Li, X.-M.; Wu, Y.-Q.; Qu, Y.-F.; Ji, X. Measuring Annual Variation in Reproductive Output Reveals a Key Role of Maternal Body Condition in Determining the Size of Eggs in Snakes. Animals 2022, 12, 1494. https://doi.org/10.3390/ani12121494
Guo K, Li X-M, Wu Y-Q, Qu Y-F, Ji X. Measuring Annual Variation in Reproductive Output Reveals a Key Role of Maternal Body Condition in Determining the Size of Eggs in Snakes. Animals. 2022; 12(12):1494. https://doi.org/10.3390/ani12121494
Chicago/Turabian StyleGuo, Kun, Xiang-Mo Li, Yan-Qing Wu, Yan-Fu Qu, and Xiang Ji. 2022. "Measuring Annual Variation in Reproductive Output Reveals a Key Role of Maternal Body Condition in Determining the Size of Eggs in Snakes" Animals 12, no. 12: 1494. https://doi.org/10.3390/ani12121494
APA StyleGuo, K., Li, X.-M., Wu, Y.-Q., Qu, Y.-F., & Ji, X. (2022). Measuring Annual Variation in Reproductive Output Reveals a Key Role of Maternal Body Condition in Determining the Size of Eggs in Snakes. Animals, 12(12), 1494. https://doi.org/10.3390/ani12121494






