Fasciolosis—An Increasing Challenge in the Sheep Industry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Life Cycle
3. Pathogenesis
4. Clinical Signs
5. Gross Pathology
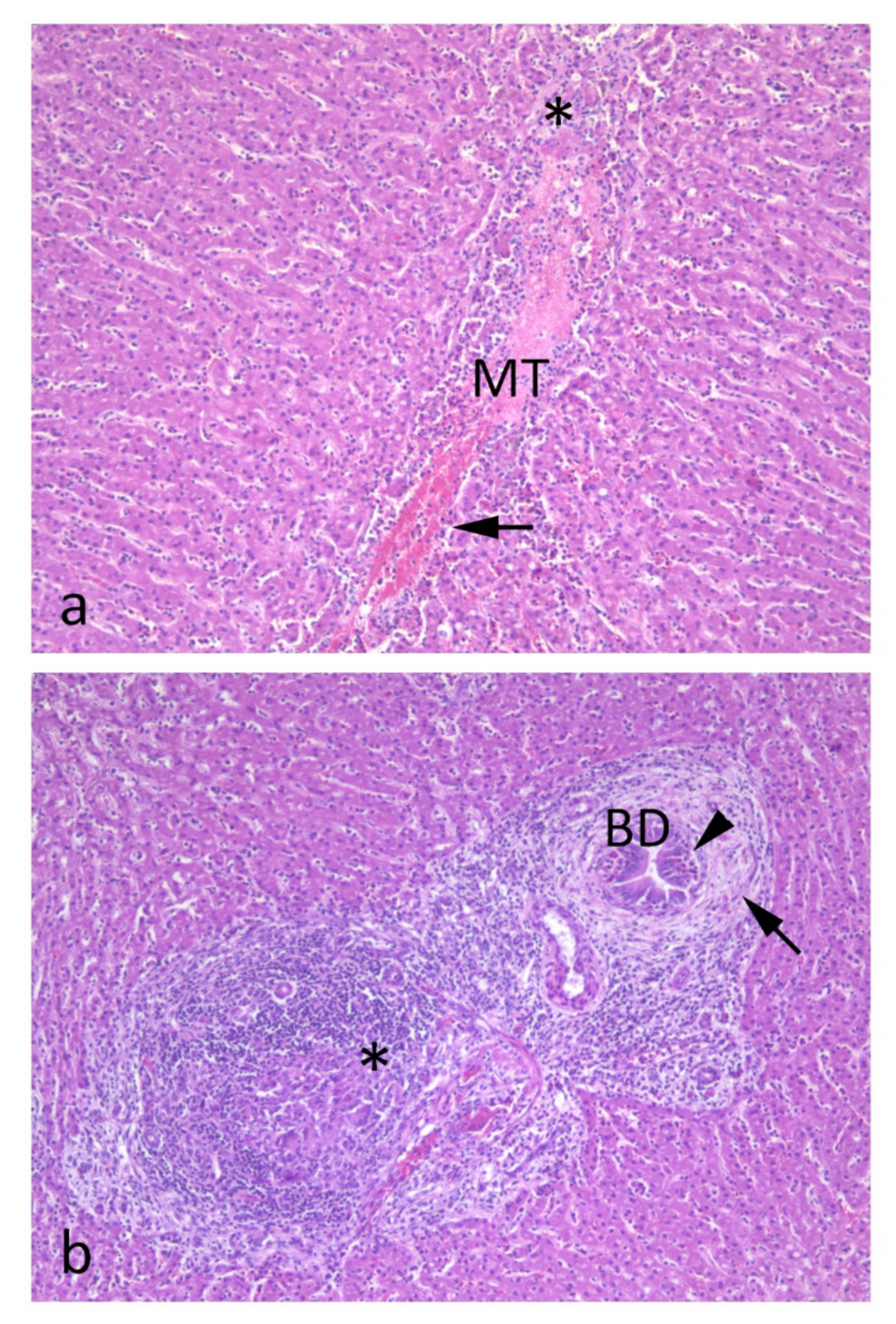
6. Histopathology
7. Diagnosis
8. Treatment and Resistance towards Flukicides
9. Pasture Management
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fascioliasis. Adv. Exp. Med. Biol. 2019, 1154, 71–103. [Google Scholar] [PubMed]
- Boray, J.C. Disease of Domestic Animals Caused by Flukes; Food and Agricultural Organisation of the United Nations: Rome, Italy, 1994; p. 49. [Google Scholar]
- Mehmood, K.; Zhang, H.; Sabir, A.J.; Abbas, R.Z.; Ijaz, M.; Durrani, A.Z.; Saleem, M.H.; Ur Rehman, M.; Iqbal, M.K.; Wang, Y.; et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb. Pathog. 2017, 109, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, I.; Brennan, G.P.; Hanna, R.E.B.; Robinson, M.W.; Skuce, P.J. Drug resistance in liver flukes. Int. J. Parasitol. Drugs Drug. Resist. 2020, 12, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Mazeri, S.; Rydevik, G.; Handel, I.; Bronsvoort, B.M.D.; Sargison, N. Estimation of the impact of Fasciola hepatica infection on time taken for UK beef cattle to reach slaughter weight. Sci. Rep. 2017, 7, 7319. [Google Scholar] [CrossRef]
- Charlier, J.; De Meulemeester, L.; Claerebout, E.; Williams, D.; Vercruysse, J. Qualitative and quantitative evaluation of coprological and serological techniques for the diagnosis of fasciolosis in cattle. Vet. Parasitol. 2008, 153, 44–51. [Google Scholar] [CrossRef]
- Mitchell, G.B.B. Liver fluke. In Diseases of Sheep, 4th ed.; Aitken, I.D., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 195–204. [Google Scholar]
- Mavrogianni, V.S.; Papadopoulos, E.; Spanos, S.A.; Mitsoura, A.; Ptochos, S.; Gougoulis, D.A.; Barbagianni, M.S.; Kyriazakis, I.; Fthenakis, G.C. Trematode infections in pregnant ewes can predispose to mastitis during the subsequent lactation period. Res. Vet. Sci. 2014, 96, 171–179. [Google Scholar] [CrossRef]
- Sargison, N.D.; Scott, P.R. Diagnosis and economic consequences of triclabendazole resistance in Fasciola hepatica in a sheep flock in south-east Scotland. Vet. Rec. 2011, 168, 159. [Google Scholar] [CrossRef]
- Happich, F.A.; Boray, J.C. Quantitative diagnosis of chronic fasciolosis. 2. The estimation of daily total egg production of Fasciola hepatica and the number of adult flukes in sheep by faecal egg counts. Aust. Vet. J. 1969, 45, 329–331. [Google Scholar] [CrossRef]
- Howell, A.K.; Williams, D.J.L. The Epidemiology and Control of Liver Flukes in Cattle and Sheep. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 109–123. [Google Scholar] [CrossRef]
- John, B.C.; Davies, D.R.; Howell, A.K.; Williams, D.J.L.; Hodgkinson, J.E. Anaerobic fermentation results in loss of viability of Fasciola hepatica metacercariae in grass silage. Vet. Parasitol. 2020, 285, 109218. [Google Scholar] [CrossRef]
- Enigk, K.; Hildebrandt, J. Zur Lebensdauer Der Metacercarien von Fasciola hepatica im heu. Tierärztl. Umsch. 1964, 19, 592–599. [Google Scholar]
- Ollerenshaw, C.B.J.; Rowlands, W.T. A method of forecasting the incidence of fascioliasis in Anglesey. Vet. Rec. 1959, 71, 591–598. [Google Scholar]
- Novobilský, A.; Engström, A.; Sollenberg, S.; Gustafsson, K.; Morrison, D.A.; Höglund, J. Transmission patterns of Fasciola hepatica to ruminants in Sweden. Vet. Parasitol. 2014, 203, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, N.J.; White, P.C.; McClean, C.J.; Marion, G.; Evans, A.; Hutchings, M.R. Predicting impacts of climate change on Fasciola hepatica risk. PLoS ONE 2011, 6, e16126. [Google Scholar] [CrossRef] [PubMed]
- Lalor, R.; Cwiklinski, K.; Calvani, N.E.D.; Dorey, A.; Hamon, S.; Corrales, J.L.; Dalton, J.P.; De Marco Verissimo, C. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola gigantica that cause the zoonosis fasciolosis. Virulence 2021, 12, 2839–2867. [Google Scholar] [CrossRef]
- Ryan, S.; Shiels, J.; Taggart, C.C.; Dalton, J.P.; Weldon, S. Fasciola hepatica-derived molecules as regulators of the host immune response. Front. Immunol. 2020, 11, 2182. [Google Scholar] [CrossRef]
- López Corrales, J.; Cwiklinski, K.; De Marco Verissimo, C.; Dorey, A.; Lalor, R.; Jewhurst, H.; McEvoy, A.; Diskin, M.; Duffy, C.; Cosby, S.L.; et al. Diagnosis of sheep fasciolosis caused by Fasciola hepatica using cathepsin L enzyme-linked immunosorbent assays (ELISA). Vet. Parasitol. 2021, 298, 109517. [Google Scholar] [CrossRef]
- Armour, J. Liver fluke. In Diseases of Sheep, 3rd ed.; Martin, W.B., Aitken, I.D., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 168–174. [Google Scholar]
- Ruiz-Campillo, M.T.; Molina Hernandez, V.; Escamilla, A.; Stevenson, M.; Perez, J.; Martinez-Moreno, A.; Donnelly, S.; Dalton, J.P.; Cwiklinski, K. Immune signatures of pathogenesis in the peritoneal compartment during early infection of sheep with Fasciola hepatica. Sci. Rep. 2017, 7, 2782. [Google Scholar] [CrossRef]
- Pérez-Caballero, R.; Siles-Lucas, M.; González-Miguel, J.; Martínez-Moreno, F.J.; Escamilla, A.; Pérez, J.; Martínez-Moreno, A.; Buffoni, L. Pathological, immunological and parasitological study of sheep vaccinated with the recombinant protein 14-3-3z and experimentally infected with Fasciola hepatica. Vet. Immunol. Immunopathol. 2018, 202, 115–121. [Google Scholar] [CrossRef]
- Cullen, J.M.; Stalker, M.J. Liver and biliary system. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 258–352. [Google Scholar]
- Munita, M.P.; Rea, R.; Martinez-Ibeas, A.M.; Byrne, N.; Kennedy, A.; Sekiya, M.; Mulcahy, G.; Sayers, R. Comparison of four commercially available ELISA kits for diagnosis of Fasciola hepatica in Irish cattle. BMC Vet. Res. 2019, 15, 414. [Google Scholar] [CrossRef] [Green Version]
- Chauvin, A.; Bouvet, G.; Boulard, C. Humoral and cellular immune responses to Fasciola hepatica experimental primary and secondary infection in sheep. Int. J. Parasitol. 1995, 25, 1227–1241. [Google Scholar] [CrossRef]
- Salimi-Bejestani, M.R.; McGarry, J.W.; Felstead, S.; Ortiz, P.; Akca, A.; Williams, D.J. Development of an antibody-detection ELISA for Fasciola hepatica and its evaluation against a commercially available test. Res. Vet. Sci. 2005, 78, 177–181. [Google Scholar] [CrossRef]
- Knubben-Schweizer, G.; Torgerson, P.R. Bovine fasciolosis: Control strategies based on the location of Galba truncatula habitats on farms. Vet. Parasitol. 2015, 208, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Phiri, A.M.; Phiri, I.K.; Sikasunge, C.S.; Chembensofu, M.; Monrad, J. Comparative fluke burden and pathology in condemned and non-condemned cattle livers from selected abattoirs in Zambia. Onderstepoort J. Vet. Res. 2006, 73, 275–281. [Google Scholar] [PubMed]
- Martínez-Valladares, M.; Rojo-Vázquez, F.A. Loop-mediated isothermal amplification (LAMP) assay for the diagnosis of fasciolosis in sheep and its application under field conditions. Parasit. Vectors 2016, 9, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, S.; Shemshadi, B.; Shirali, S.; Kheirandish, F.; Fallahi, S. Accurate and rapid detection of Fasciola hepatica copro-DNA in sheep using loop-mediated isothermal amplification (LAMP) technique. Vet. Med. Sci. 2021, 7, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Cabada, M.M.; Malaga, J.L.; Castellanos-Gonzalez, A.; Bagwell, K.A.; Naeger, P.A.; Rogers, H.K.; Maharsi, S.; Mbaka, M.; White, A.C., Jr. Recombinase polymerase amplification compared to real-time polymerase chain reaction test for the detection of Fasciola hepatica in human stool. Am. J. Trop. Med. Hyg. 2017, 96, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arifin, M.I.; Höglund, J.; Novobilský, A. Comparison of molecular and conventional methods for the diagnosis of Fasciola hepatica infection in the field. Vet. Parasitol. 2016, 232, 8–11. [Google Scholar] [CrossRef]
- Calvani, N.E.D.; George, S.D.; Windsor, P.A.; Bush, R.D.; Šlapeta, J. Comparison of early detection of Fasciola hepatica in experimentally infected Merino sheep by real-time PCR, coproantigen ELISA and sedimentation. Vet. Parasitol. 2018, 251, 85–89. [Google Scholar] [CrossRef]
- Animalia. 2021. Available online: https://www.animalia.no/no/Dyr/sau/aktuelt---sau/store-leverikter/ (accessed on 10 May 2022). (In Norwegian).
- Novobilský, A.; Sollenberg, S.; Höglund, J. Distribution of Fasciola hepatica in Swedish dairy cattle and associations with pasture management factors. Geospat. Health 2015, 9, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Beesley, N.J.; Williams, D.J.; Paterson, S.; Hodgkinson, J. Fasciola hepatica demonstrates high levels of genetic diversity, a lack of population structure and high gene flow: Possible implications for drug resistance. Int. J. Parasitol. 2017, 47, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, I.; Boray, J.C. Fasciolicides: Efficacy, actions, resistance and its management. Vet. J. 1999, 158, 81–112. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valladares, M.; Cordero-Pérez, C.; Rojo-Vázquez, F.A. Efficacy of an anthelmintic combination in sheep infected with Fasciola hepatica resistant to albendazole and clorsulon. Exp. Parasitol. 2014, 136, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.E.; McMahon, C.; Ellison, S.; Edgar, H.W.; Kajugu, P.E.; Gordon, A.; Irwin, D.; Barley, J.P.; Malone, F.E.; Brennan, G.P.; et al. Fasciola hepatica: A comparative survey of adult fluke resistance to triclabendazole, nitroxynil and closantel on selected upland and lowland sheep farms in Northern Ireland using faecal egg counting, coproantigen ELISA testing and fluke histology. Vet. Parasitol. 2015, 207, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Novobilský, A.; Höglund, J. First report of closantel treatment failure against Fasciola hepatica in cattle. Int. J. Parasitol. Drug. Drug. Resist. 2015, 5, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Kelley, J.M.; Elliott, T.P.; Beddoe, T.; Anderson, G.; Skuce, P.; Spithill, T.W. Current threat of triclabendazole resistance in Fasciola hepatica. Trends Parasitol. 2016, 32, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Beesley, N.J.; Caminade, C.; Charlier, J.; Flynn, R.J.; Hodgkinson, J.E.; Martinez-Moreno, A.; Martinez-Valladares, M.; Perez, J.; Rinaldi, L.; Williams, D.J.L. Fasciola and fasciolosis in ruminants in Europe: Identifying research needs. Transbound Emerg. Dis. 2018, 65, 199–216. [Google Scholar] [CrossRef]
- Munita, M.P.; Rea, R.; Martinez-Ibeas, A.M.; Byrne, N.; McGrath, G.; Munita-Corbalan, L.E.; Sekiya, M.; Mulcahy, G.; Sayers, R.G. Liver fluke in Irish sheep: Prevalence and associations with management practices and co-infection with rumen fluke. Parasites Vectors 2019, 12, 525. [Google Scholar] [CrossRef]
- Novobilský, A.; Amaya Solis, N.; Skarin, M.; Höglund, J. Assessment of flukicide efficacy against Fasciola hepatica in sheep in Sweden in the absence of a standardised test. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, I. Reducing the future threat from (liver) fluke: Realistic prospect or quixotic fantasy? Vet. Parasitol. 2011, 180, 133–143. [Google Scholar] [CrossRef]
- Novobilský, A.; Averpil, H.B.; Höglund, J. The field evaluation of albendazole and triclabendazole efficacy against Fasciola hepatica by coproantigen ELISA in naturally infected sheep. Vet. Parasitol. 2012, 190, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Moreno, G.; Moreno, L.; Ceballos, L.; Shaw, L.; Fairweather, I.; Lanusse, C. Comparative assessment of albendazole and triclabendazole ovicidal activity on Fasciola hepatica eggs. Vet. Parasitol. 2009, 164, 211–216. [Google Scholar] [CrossRef]
- Canevari, J.; Ceballos, L.; Sanabria, R.; Romero, J.; Olaechea, F.; Ortiz, P.; Cabrera, M.; Gayo, V.; Fairweather, I.; Lanusse, C.; et al. Testing albendazole resistance in Fasciola hepatica: Validation of an egg hatch test with isolates from South America and the United Kingdom. J. Helminthol. 2014, 8, 286–292. [Google Scholar] [CrossRef]
- Molina-Hernández, V.; Mulcahy, G.; Pérez, J.; Martínez-Moreno, Á.; Donnelly, S.; O’Neill, S.M.; Dalton, J.P.; Cwiklinski, K. Fasciola hepatica vaccine: We may not be there yet but we’re on the right road. Vet. Parasitol. 2015, 208, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toet, H.; Piedrafita, D.M.; Spithill, T.W. Liver fluke vaccines in ruminants: Strategies, progress and future opportunities. Int. J. Parasitol. 2014, 44, 915–927. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P. Recent progress in the development of liver fluke and blood fluke vaccines. Vaccines 2020, 8, 553. [Google Scholar] [CrossRef]
- Zafra, R.; Buffoni, L.; Pérez-Caballero, R.; Molina-Hernández, V.; Ruiz-Campillo, M.T.; Pérez, J.; Martínez-Moreno, Á.; Martínez Moreno, F.J. Efficacy of a multivalent vaccine against Fasciola hepatica infection in sheep. Vet. Res. 2021, 52, 13. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinski, K.; Dalton, J.P.; Dufresne, P.J.; La Course, J.; Williams, D.J.; Hodgkinson, J.; Paterson, S. The Fasciola hepatica genome: Gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biol. 2015, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Bennema, S.C.; Ducheyne, E.; Vercruysse, J.; Claerebout, E.; Hendrickx, G.; Charlier, J. Relative importance of management, meteorological and environmental factors in the spatial distribution of Fasciola hepatica in dairy cattle in a temperate climate zone. Int. J. Parasitol. 2011, 41, 225–233. [Google Scholar] [CrossRef]
- Caminade, C.; van Dijk, J.; Baylis, M.; Williams, D. Modelling recent and future climatic suitability for fasciolosis in Europe. Geospat. Health 2015, 9, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Gaasenbeek, C.P.; Over, H.J.; Noorman, N.; de Leeuw, W.A. An epidemiological study of Fasciola hepatica in The Netherlands. Vet. Q. 1992, 14, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Brophy, P.M.; Davis, C.N.; Davies, T.E.; Emberson, H.; Rees Stevens, P.; Williams, H.W. Detection of Galba truncatula, Fasciola hepatica and Calicophoron daubneyi environmental DNA within water sources on pasture land, a future tool for fluke control? Parasites Vectors 2018, 11, 342. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuen, S.; Ersdal, C. Fasciolosis—An Increasing Challenge in the Sheep Industry. Animals 2022, 12, 1491. https://doi.org/10.3390/ani12121491
Stuen S, Ersdal C. Fasciolosis—An Increasing Challenge in the Sheep Industry. Animals. 2022; 12(12):1491. https://doi.org/10.3390/ani12121491
Chicago/Turabian StyleStuen, Snorre, and Cecilie Ersdal. 2022. "Fasciolosis—An Increasing Challenge in the Sheep Industry" Animals 12, no. 12: 1491. https://doi.org/10.3390/ani12121491
APA StyleStuen, S., & Ersdal, C. (2022). Fasciolosis—An Increasing Challenge in the Sheep Industry. Animals, 12(12), 1491. https://doi.org/10.3390/ani12121491




