Evaluation of a Novel DNA Vaccine Double Encoding Somatostatin and Cortistatin for Promoting the Growth of Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids and Gene Synthesis
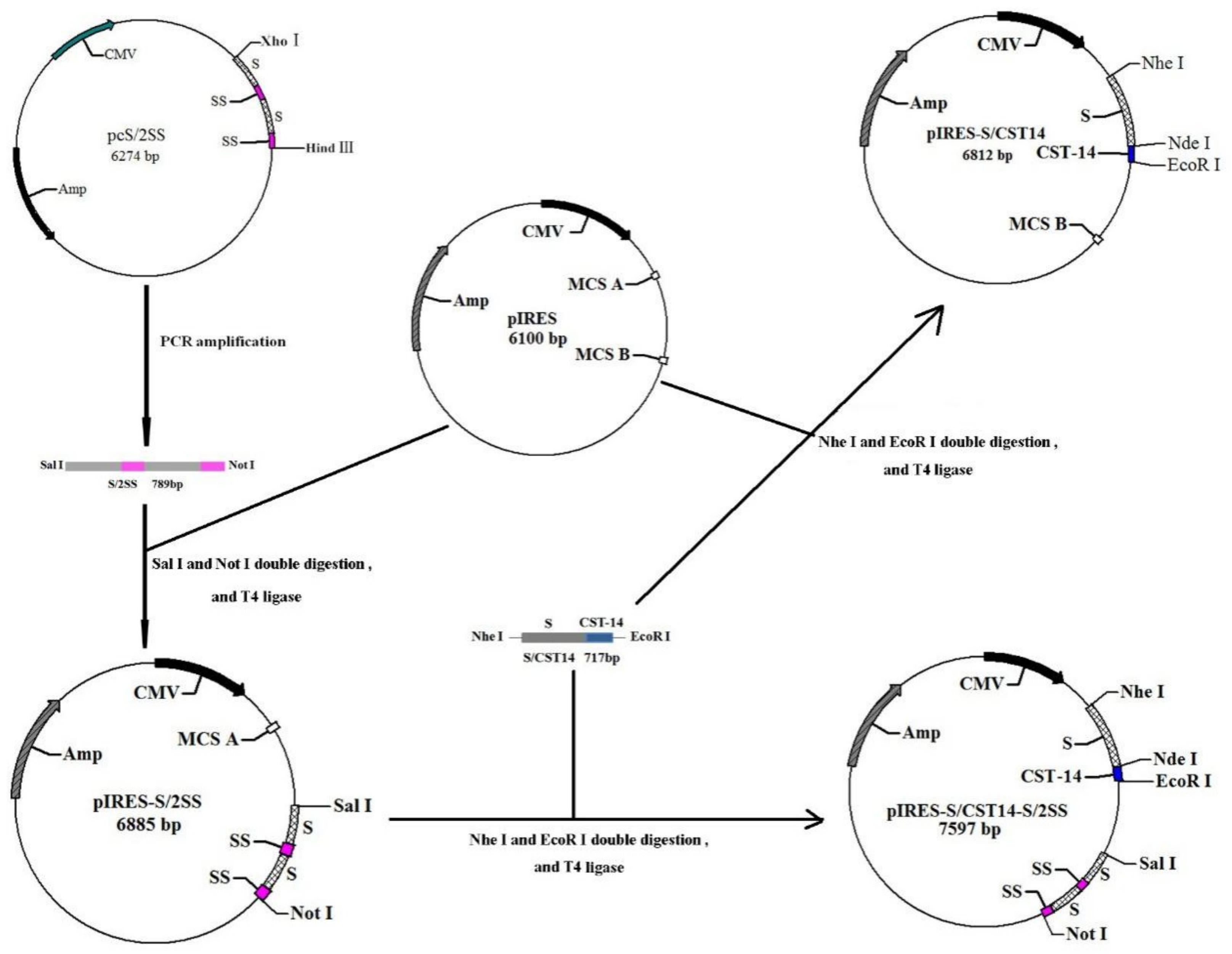
2.2. Construction of pIRES-S/CST14-S/2SS Plasmid
2.3. Cell Culture and Transfection
2.4. Expression of pIRES-S/CST14-S/2SS Plasmid in GH3 Cells
2.5. cAMP Assay after Transfection with Plasmids
2.6. Mice Immunization
2.7. Detection of Hormones and Antibodies
2.8. Statistical Analysis
3. Results
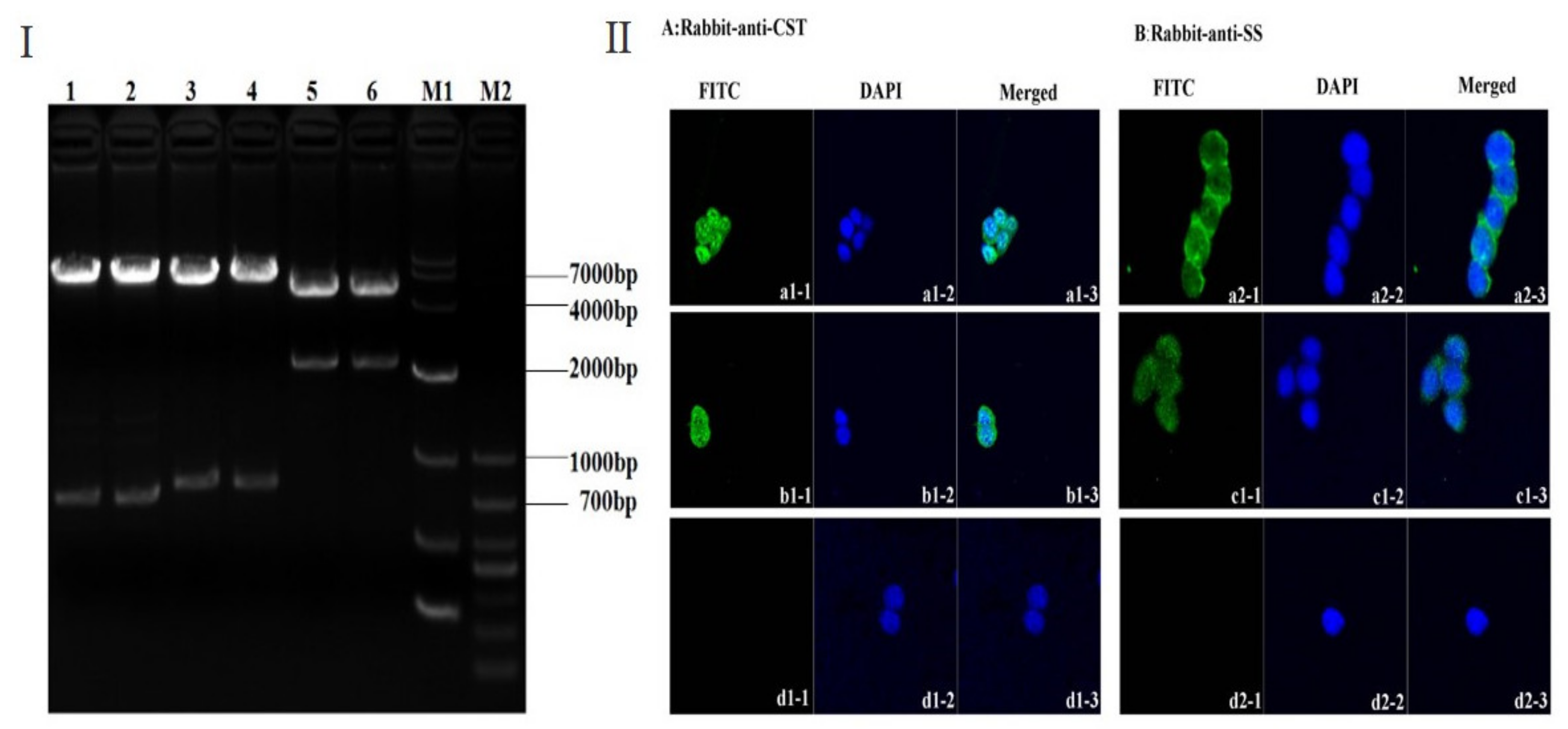
3.1. Construction and Characterization of pIRES-S/CST14-S/2SS Plasmid
3.2. The Biological Effect of Dual Expression Plasmid on Hormone Secretion and cAMP Accumulation
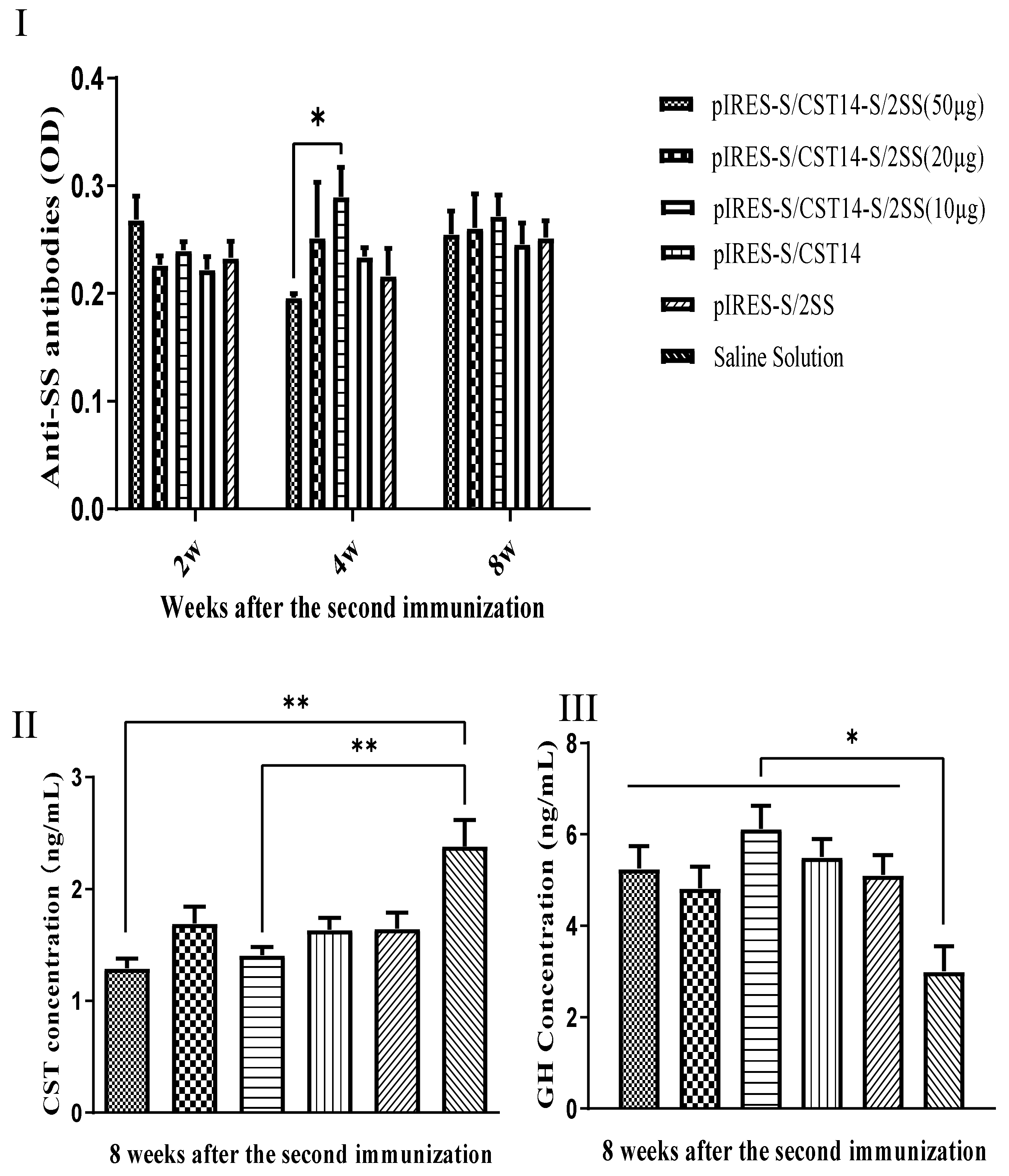
3.3. Immunization Effect of pIRES-S/CST14-S/2SS Plasmid on Hormone Levels and Growth in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tannenbaum, G.S.; Epelbaum, J.; Bowers, C.Y. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 2003, 144, 967–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steyn, F.J.; Tolle, V.; Chen, C.; Epelbaum, J. Neuroendocrine regulation of growth hormone secretion. Compr. Physiol. 2016, 6, 687–735. [Google Scholar] [CrossRef] [PubMed]
- Peverelli, E.; Treppiedi, D.; Giardino, E.; Vitali, E.; Lania, A.G.; Mantovani, G. Dopamine and somatostatin analogues resistance of pituitary tumors: Focus on cytoskeleton involvement. Front. Endocrinol. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, H.; Dong, P.; Shahzad, M.; Yang, L. Constitutive and follicle-stimulating hormone-induced action of somatostatin receptor-2 on regulation of apoptosis and steroidogenesis in bovine granulosa cells. J. Steroid Biochem. Mol. Biol. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- McCosh, R.B.; Szeligo, B.M.; Bedenbaugh, M.N.; Lopez, J.A.; Hardy, S.L.; Hileman, S.M.; Lehman, M.N.; Goodman, R.L. Evidence that endogenous somatostatin inhibits episodic, but not surge, secretion of LH in female sheep. Endocrinology 2017, 158, 1827–1837. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Yuan, T.; Qi, R.; He, J.; Fu, Y.; Niu, D.; Li, W. Effect of immunization with a recombinant cholera toxin B subunit/somatostatin fusion protein on immune response and growth hormone levels in mice. Biotechnol. Lett. 2012, 34, 2199–2203. [Google Scholar] [CrossRef]
- Liang, A.; Cao, S.; Han, L.; Yao, Y.; Moaeen-Ud-Din, M.; Yang, L. Construction and evaluation of the eukaryotic expression plasmid encoding two copies of somatostatin genes fused with hepatitis B surface antigen gene S. Vaccine 2008, 26, 2935–2941. [Google Scholar] [CrossRef]
- Spencer, G.S.; Harvey, S.; Audsley, A.R.; Hallett, K.G.; Kestin, S. The effect of immunization against somatostatin on growth rates and growth hormone secretion in the chicken. Comp. Biochem. Physiol. Part A Comp. Physiol. 1986, 85, 553–556. [Google Scholar] [CrossRef]
- Xue, C.L.; Liang, A.X.; Mao, D.G.; Han, L.; Liu, X.B.; Zhang, J.; Yang, L.G. Effect of genetic adjuvants on immune respondance, growth and hormone levels in somatostatin DNA vaccination-induced Hu lambs. Vaccine 2010, 28, 1541–1546. [Google Scholar] [CrossRef]
- Han, Y.; Na, R.; Jiang, X.; Wu, J.; Han, Y.; Zeng, Y.E.G.; Liang, A.; Yang, L.; Zhao, Y.; Huang, Y. Effect of a novel somatostatin-14 DNA vaccine fused to tPA signal peptide and CpG adjuvant on goat lactation and milk composition. Small Rumin. Res. 2020, 187, 106107. [Google Scholar] [CrossRef]
- Han, Y.; Liang, A.; Han, L.; Guo, A.; Jiang, X.; Yang, L. Efficacy and safety of an oral somatostatin DNA vaccine without antibiotic resistance gene in promoting growth of piglets. Scand. J. Immunol. 2014, 79, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Costa, A.; Luque, R.M.; Castaño, J.P. Cortistatin: A new link between the growth hormone/prolactin axis, stress, and metabolism. Growth Horm. IGF Res. 2017, 33, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Spier, A.D.; de Lecea, L. Cortistatin: A member of the somatostatin neuropeptide family with distinct physiological functions. Brain research. Brain Res. Rev. 2000, 33, 228–241. [Google Scholar] [CrossRef]
- Gahete, M.D.; Durán-Prado, M.; Luque, R.M.; Martínez-Fuentes, A.J.; Vázquez-Martínez, R.; Malagón, M.M.; Castaño, J.P. Are somatostatin and cortistatin two siblings in regulating endocrine secretions? In vitro work ahead. Mol. Cell. Endocrinol. 2008, 286, 128–134. [Google Scholar] [CrossRef]
- Cordoba-Chacón, J.; Gahete, M.D.; Pozo-Salas, A.I.; de Lecea, L.; Castaño, J.P.; Luque, R.M. Cortistatin is a key factor regulating the sex-dependent response of the GH and stress axes to fasting in mice. Endocrinology 2016, 157, 2810–2823. [Google Scholar] [CrossRef]
- Cammalleri, M.; Cervia, D.; Dal Monte, M.; Martini, D.; Langenegger, D.; Fehlmann, D.; Feuerbach, D.; Pavan, B.; Hoyer, D.; Bagnoli, P. Compensatory changes in the hippocampus of somatostatin knockout mice: Upregulation of somatostatin receptor 2 and its function in the control of bursting activity and synaptic transmission. Eur. J. Neurosci. 2006, 23, 2404–2422. [Google Scholar] [CrossRef] [Green Version]
- Pedraza-Arévalo, S.; Córdoba-Chacón, J.; Pozo-Salas, A.I.; L-López, F.; de Lecea, L.; Gahete, M.D.; Castaño, J.P.; Luque, R.M. Not so giants: Mice lacking both somatostatin and cortistatin have high GH levels but show no changes in growth rate or IGF-1 levels. Endocrinology 2015, 156, 1958–1964. [Google Scholar] [CrossRef] [Green Version]
- Córdoba-Chacón, J.; Gahete, M.D.; Culler, M.D.; Castaño, J.P.; Kineman, R.D.; Luque, R.M. Somatostatin dramatically stimulates growth hormone release from primate somatotrophs acting at low doses via somatostatin receptor 5 and cyclic AMP. J. Neuroendocrinol. 2012, 24, 453–463. [Google Scholar] [CrossRef]
- Riaz, H.; Liang, A.; Khan, M.K.; Dong, P.; Han, L.; Shahzad, M.; Chong, Z.; Ahmad, S.; Hua, G.; Yang, L. Somatostatin and its receptors: Functional regulation in the development of mice Sertoli cells. J. Steroid Biochem. Mol. Biol. 2013, 138, 257–266. [Google Scholar] [CrossRef]
- Liang, A.; Riaz, H.; Dong, F.; Luo, X.; Yu, X.; Han, Y.; Chong, Z.; Han, L.; Guo, A.; Yang, L. Evaluation of efficacy, biodistribution and safety of antibiotic-free plasmid encoding somatostatin genes delivered by attenuated Salmonella enterica serovar Choleraesuis. Vaccine 2014, 32, 1368–1374. [Google Scholar] [CrossRef]
- Yang, S.K.; Parkington, H.C.; Blake, A.D.; Keating, D.J.; Chen, C. Somatostatin increases voltage-gated K+ currents in GH3 cells through activation of multiple somatostatin receptors. Endocrinology 2005, 146, 4975–4984. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Deghenghi, R.; Papotti, M.; Ghigo, E.; Muccioli, G. Cortistatin, but not somatostatin, binds to growth hormone secretagogue (GHS) receptors of human pituitary gland. J. Endocrinol. Investig. 2001, 24, RC1–RC3. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Delgado, M. Emergence of cortistatin as a new immunomodulatory factor with therapeutic potential in immune disorders. Mol. Cell Endocrinol. 2008, 286, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Luque, R.M.; Peinado, J.R.; Gracia-Navarro, F.; Broglio, F.; Ghigo, E.; Kineman, R.D.; Malagón, M.M.; Castaño, J.P. Cortistatin mimics somatostatin by inducing a dual, dose-dependent stimulatory and inhibitory effect on growth hormone secretion in somatotropes. J. Mol. Endocrinol. 2006, 36, 547–556. [Google Scholar] [CrossRef] [Green Version]

| Group | Initial Weight | Weight of 2 w after the First Immunization | Final Weight | Total Weight Gain of the Second Immunization |
|---|---|---|---|---|
| pIRES-S/CST14-S/2SS (50 μg) | 17.4 ± 0.12 | 27.6 ± 0.26 ab | 36.1 ±0.31 | 8.5 ±0.19 a |
| pIRES-S/CST14-S/2SS (20 μg) | 17.3 ± 0.20 | 26.5 ± 0.32 ab | 35.4 ±0.38 | 8.9 ±0.23 ab |
| pIRES-S/CST14-S/2SS (10 μg) | 17.6 ± 0.13 | 27.3 ± 0.21 ab | 38.1 ±0.25 | 10.8 ±0.16 b |
| pIRES-S/CST14 | 17.3 ± 0.10 | 25.5 ± 0.20 a | 36.1 ±0.35 | 10.6 ±0.23 b |
| pIRES-S/2SS | 18.0 ± 0.11 | 27.7 ± 0.17 b | 37.3 ±0.28 | 9.6 ±0.18 ab |
| Saline Solution | 17.7 ± 0.14 | 26.8 ± 0.20 ab | 35.4 ±0.28 | 8.6 ±0.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Zu, Z.; Riaz, H.; Dan, X.; Yu, X.; Liu, S.; Guo, A.; Wen, Y.; Liang, A.; Yang, L. Evaluation of a Novel DNA Vaccine Double Encoding Somatostatin and Cortistatin for Promoting the Growth of Mice. Animals 2022, 12, 1490. https://doi.org/10.3390/ani12121490
Luo X, Zu Z, Riaz H, Dan X, Yu X, Liu S, Guo A, Wen Y, Liang A, Yang L. Evaluation of a Novel DNA Vaccine Double Encoding Somatostatin and Cortistatin for Promoting the Growth of Mice. Animals. 2022; 12(12):1490. https://doi.org/10.3390/ani12121490
Chicago/Turabian StyleLuo, Xuan, Zhuoxin Zu, Hasan Riaz, Xingang Dan, Xue Yu, Shuanghang Liu, Aizhen Guo, Yilin Wen, Aixin Liang, and Liguo Yang. 2022. "Evaluation of a Novel DNA Vaccine Double Encoding Somatostatin and Cortistatin for Promoting the Growth of Mice" Animals 12, no. 12: 1490. https://doi.org/10.3390/ani12121490
APA StyleLuo, X., Zu, Z., Riaz, H., Dan, X., Yu, X., Liu, S., Guo, A., Wen, Y., Liang, A., & Yang, L. (2022). Evaluation of a Novel DNA Vaccine Double Encoding Somatostatin and Cortistatin for Promoting the Growth of Mice. Animals, 12(12), 1490. https://doi.org/10.3390/ani12121490








